Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structural Investigation
2.2. Modeling and Refinement of Tertiary Structure
2.3. Estimation of B-Cell Epitopes
2.4. Molecular Docking Study
2.5. Molecular Dynamic Simulations
2.6. Immunogenicity Evaluation of Construct
2.7. Cell Lines, Virus, and Vectors
2.8. Construction of Plasmid
2.9. Formulation of Inactivated PED Vaccine
2.10. Recombinant Protein Expression and Purification
2.11. Analysis of Recombinant Protein
2.12. Immunization Schedule and Vaccine Preparation
2.13. ELISA
2.14. Lymphocyte Proliferation Assay
2.15. Virus Neutralization Test (VNT)
2.16. Statistical Analysis
3. Results
3.1. Physiochemical and Immunogenic Profiling
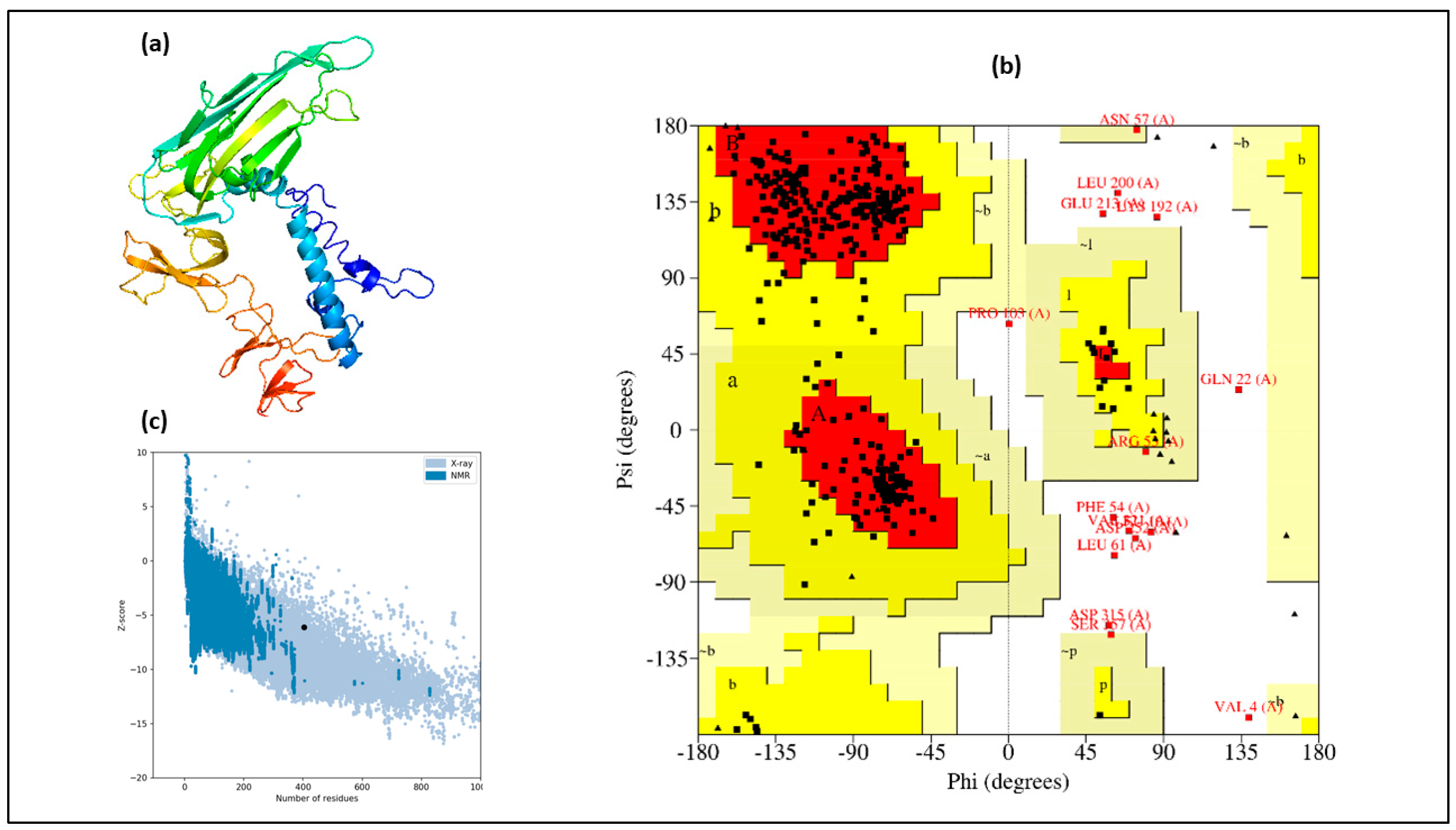
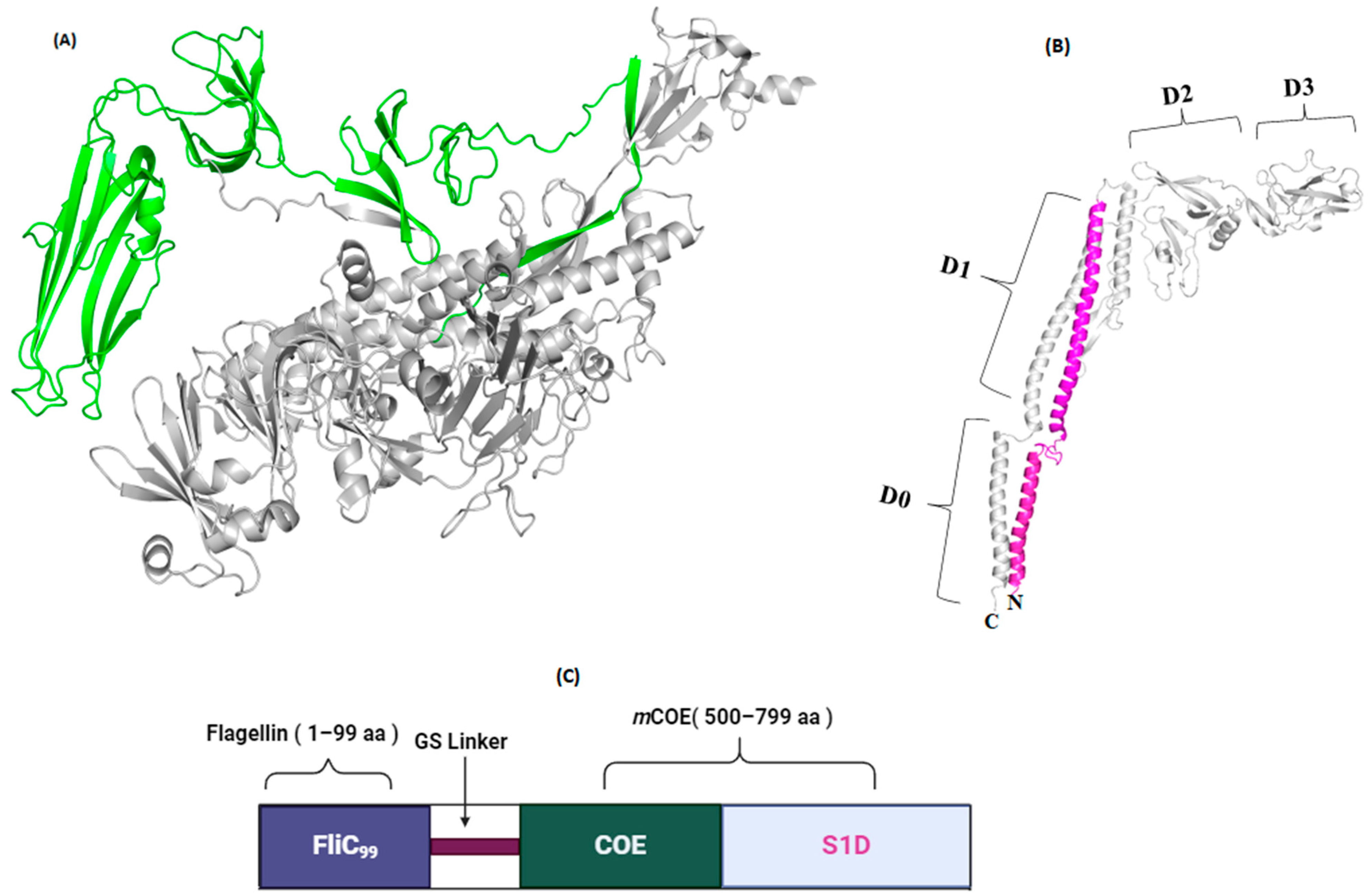
3.2. Structural Assessment
3.3. Modeling of Tertiary Structure
3.4. Selection of B-Cell Epitopes
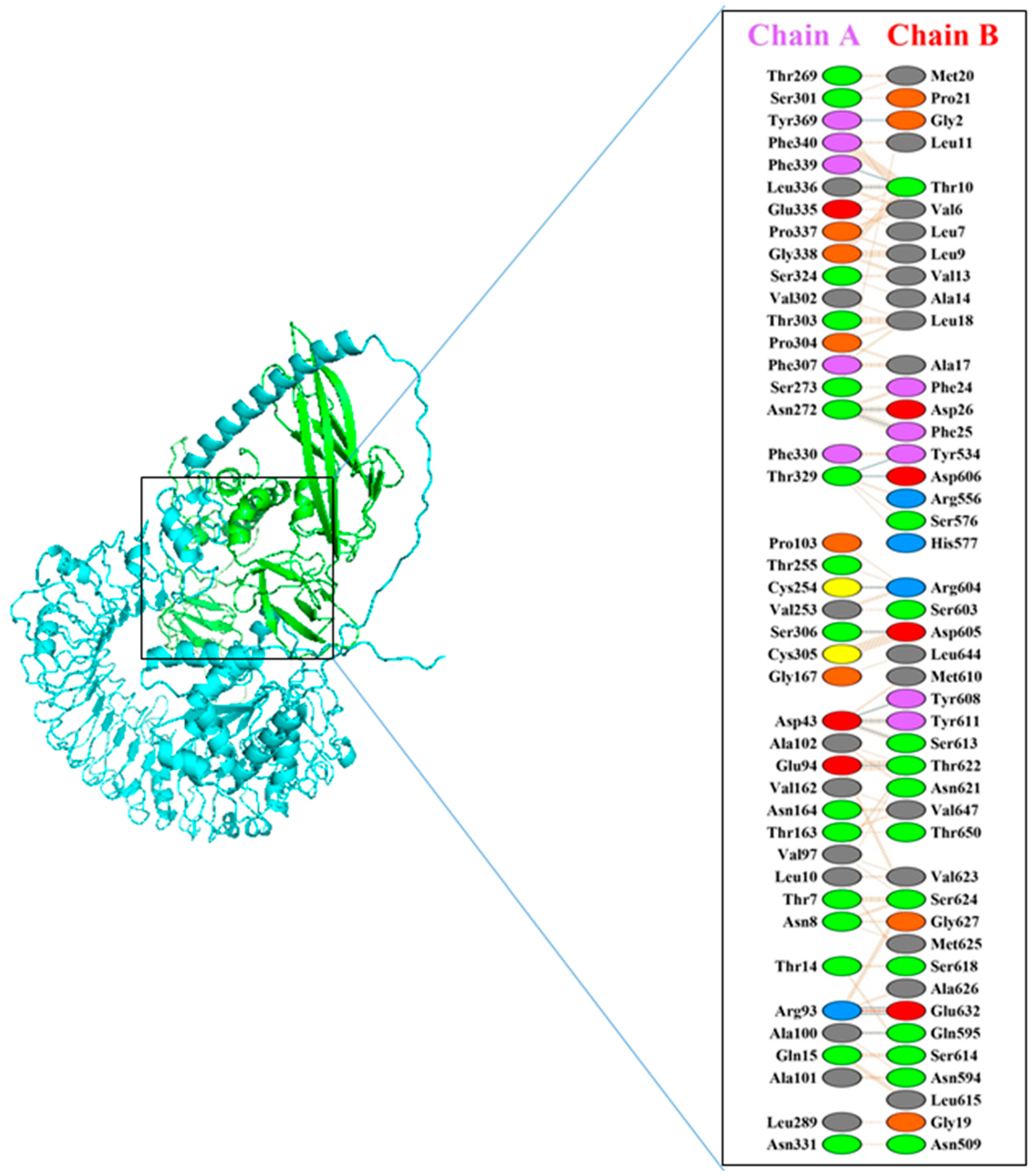
3.5. Molecular Docking
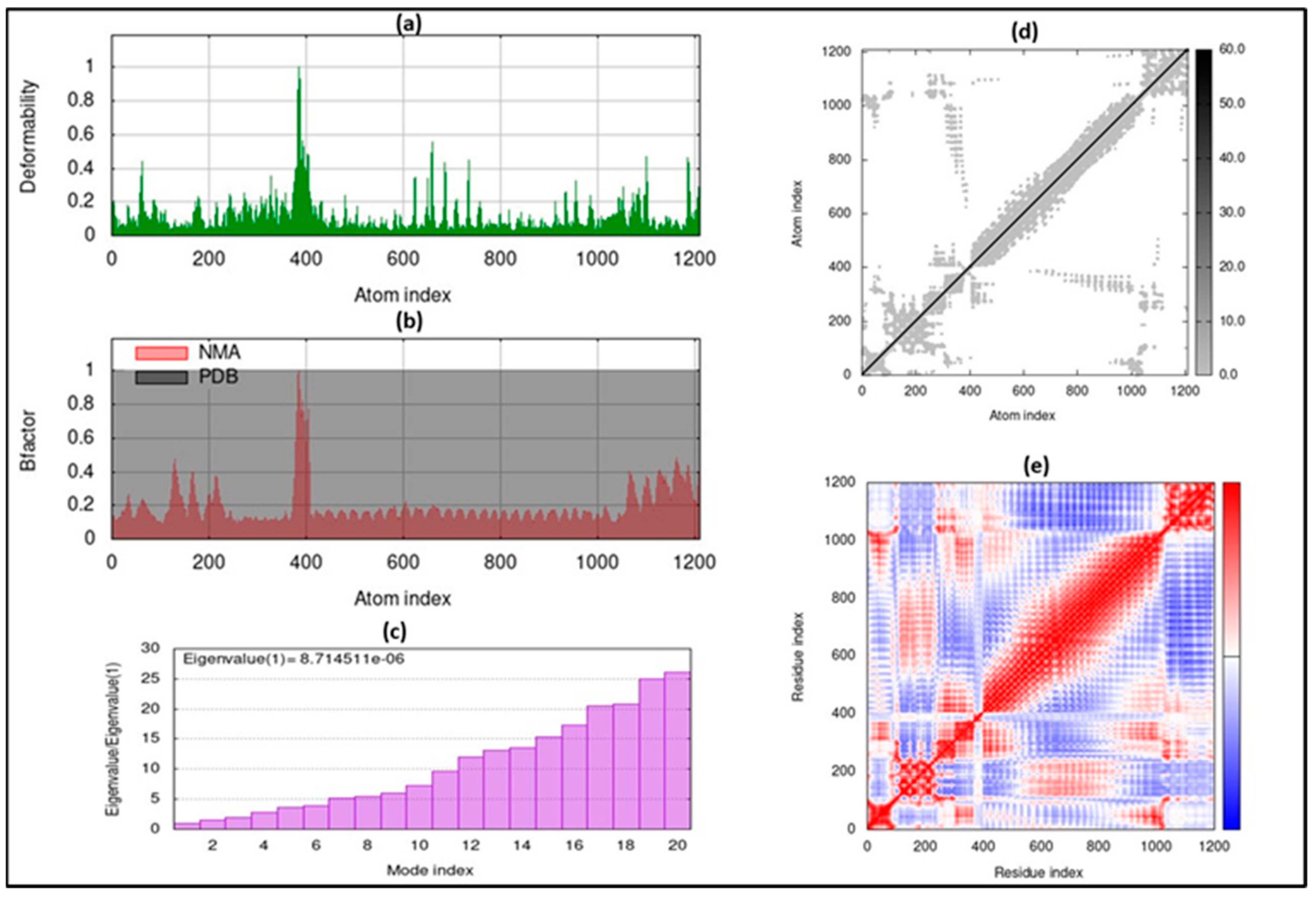
3.6. Molecular Dynamic Simulation
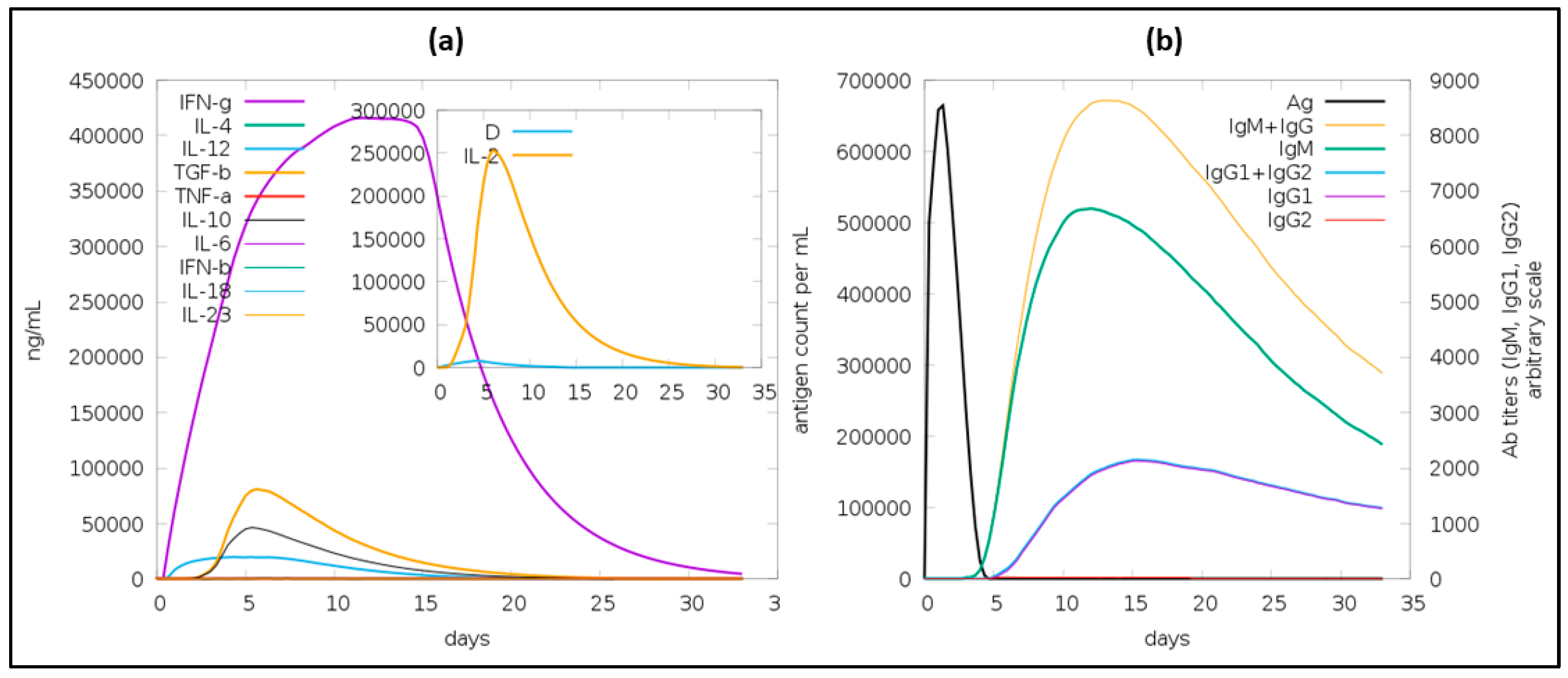
3.7. Immune Simulation
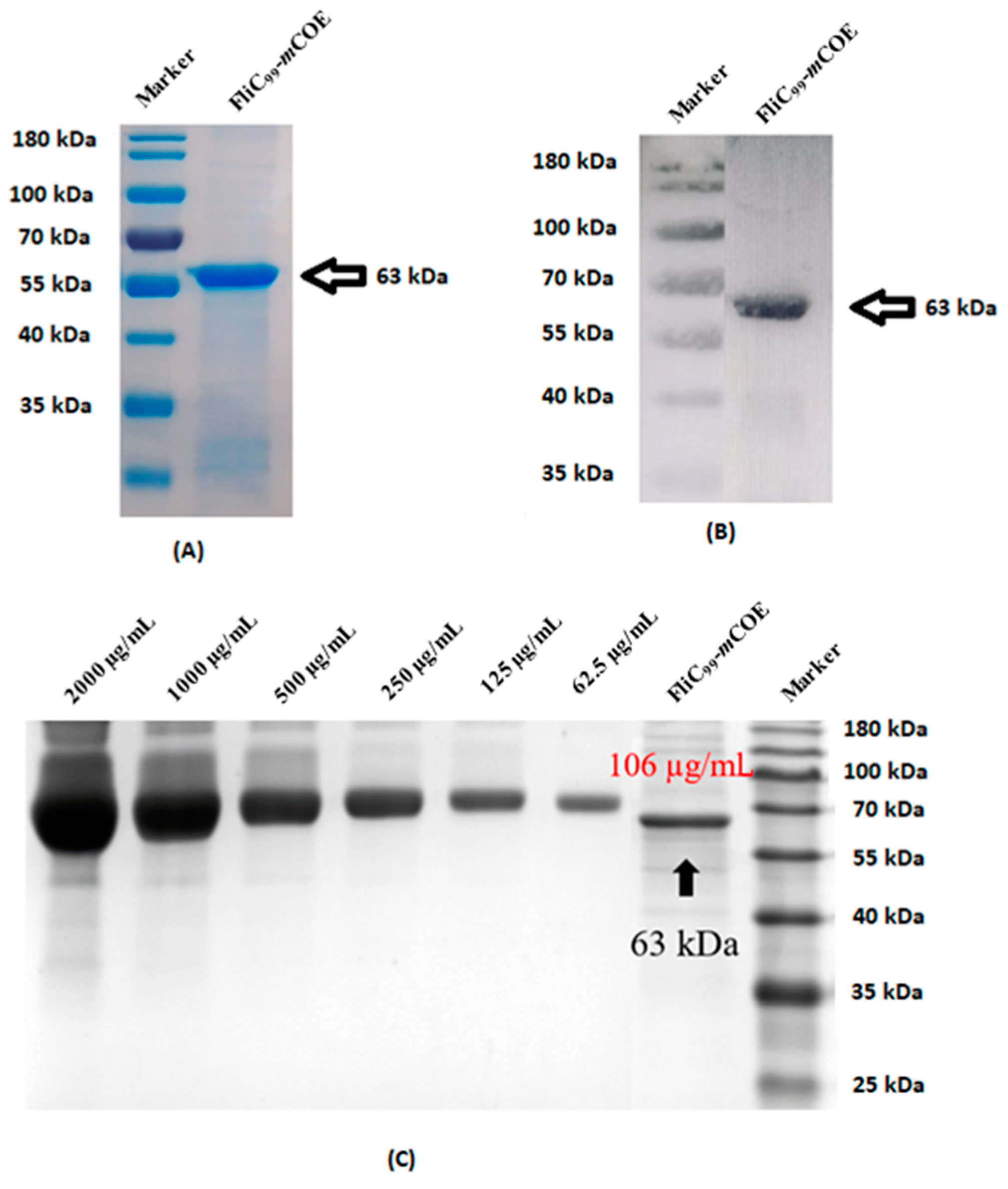
3.8. Generation of Subunit Vaccine and Characterization
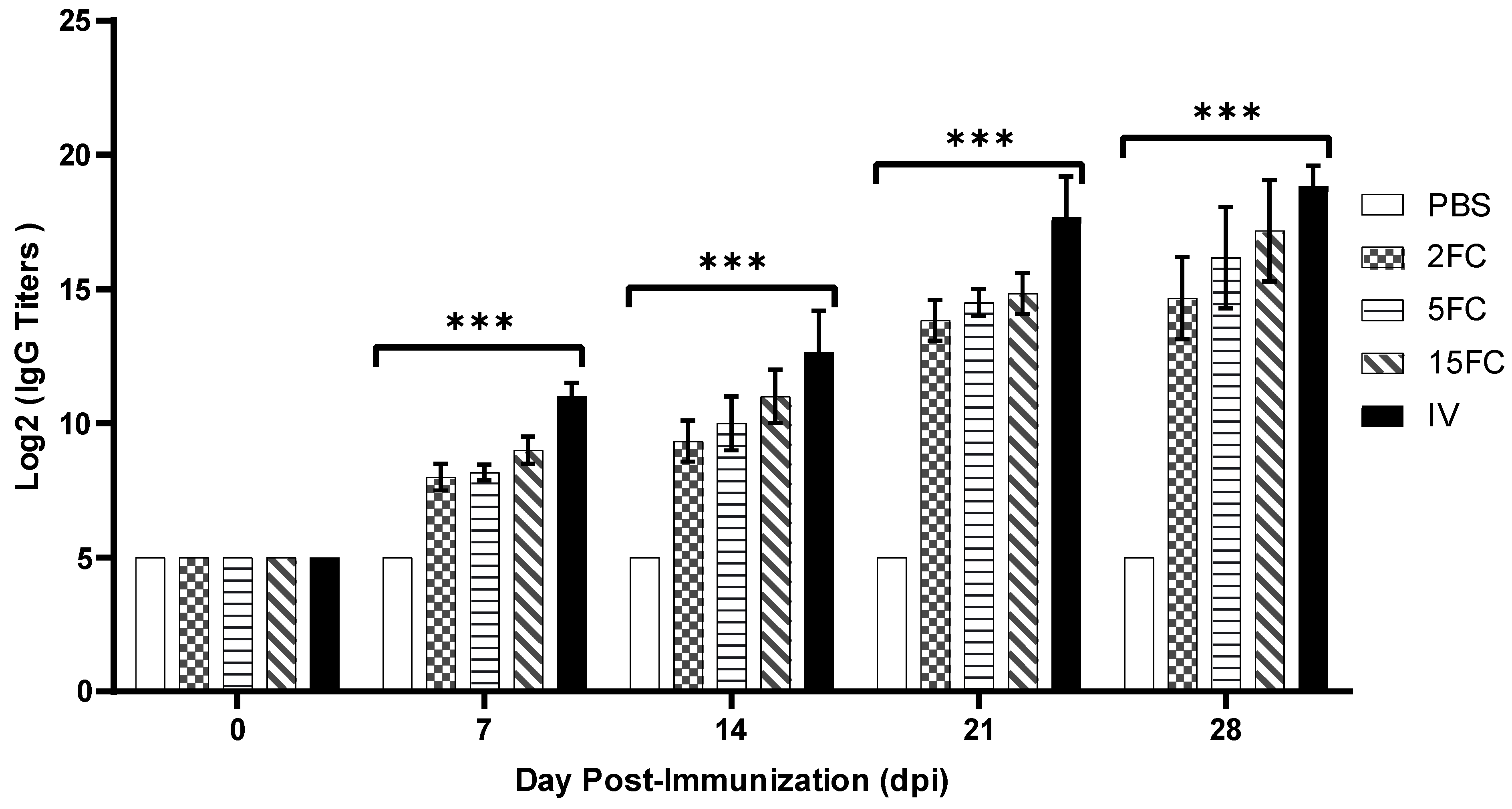
3.9. PEDV-Specific Antibody Titers Increased by Flagellin-Adjuvanted and Inactivated PED Vaccines
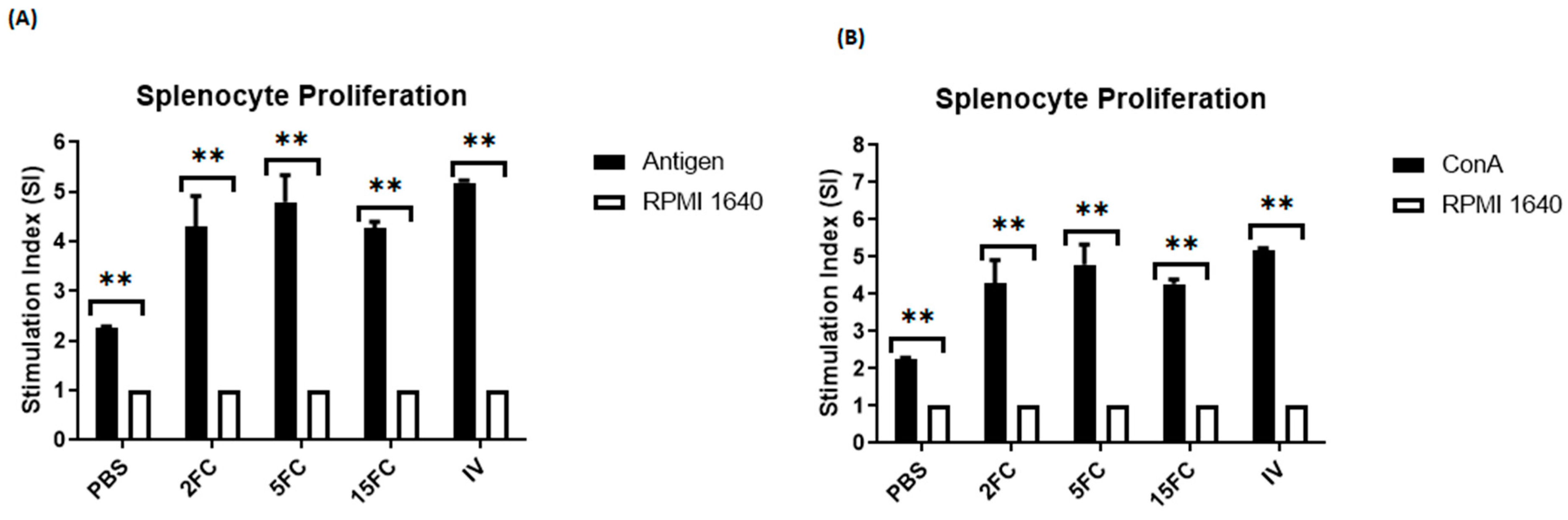
3.10. Both Flagellin-Adjuvanted and PED Vaccine Candidates Proliferate the Lymphocytes In Vitro
3.11. Flagellin-Adjuvanted and Inactivated PED Vaccine Groups Elicited Neutralizing Antibodies in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Diep, N.; Norimine, J.; Sueyoshi, M.; Lan, N.T.; Yamaguchi, R. Novel Porcine Epidemic Diarrhea Virus (PEDV) Variants with Large Deletions in the Spike (S) Gene Coexist with PEDV Strains Possessing an Intact S Gene in Domestic Pigs in Japan: A New Disease Situation. PLoS ONE 2017, 12, e0170126. [Google Scholar]
- Opriessnig, T. Re-Emergence of Porcine Epidemic Diarrhea Virus in the Global Pig Population. Vet. J. 2015, 204, 131. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, J.; Zhen, J.; Yin, L.; Li, Q.; Xue, C.; Cao, Y. Rapid Quantitation of Porcine Epidemic Diarrhea Virus (PEDV) by Virus Counter. J. Virol. Methods 2015, 223, 1–4. [Google Scholar] [CrossRef]
- Hao, J.; Xue, C.; He, L.; Wang, Y.; Cao, Y. Bioinformatics Insight into the Spike Glycoprotein Gene of Field Porcine Epidemic Diarrhea Strains during 2011–2013 in Guangdong, China. Virus Genes 2014, 49, 58–67. [Google Scholar] [CrossRef]
- Chattha, K.S.; Roth, J.A.; Saif, L.J. Strategies for Design and Application of Enteric Viral Vaccines. Annu. Rev. Anim. Biosci. 2015, 3, 375–395. [Google Scholar] [CrossRef]
- Okda, F.A.; Lawson, S.; Singrey, A.; Nelson, J.; Hain, K.S.; Joshi, L.R.; Christopher-Hennings, J.; Nelson, E.A.; Diel, D.G. The S2 Glycoprotein Subunit of Porcine Epidemic Diarrhea Virus Contains Immunodominant Neutralizing Epitopes. Virology 2017, 509, 185–194. [Google Scholar] [CrossRef]
- Oh, J.; Lee, K.-W.; Choi, H.-W.; Lee, C. Immunogenicity and Protective Efficacy of Recombinant S1 Domain of the Porcine Epidemic Diarrhea Virus Spike Protein. Arch. Virol. 2014, 159, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Peng, O.; Wu, T.; Xu, Z.; Huang, L.; Zhang, Y.; Xue, C.; Wen, Z.; Zhou, Q.; Cao, Y. PED Subunit Vaccine Based on COE Domain Replacement of Flagellin Domain D3 Improved Specific Humoral and Mucosal Immunity in Mice. Vaccine 2018, 36, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.-X.; Kim, S.-H.; Yang, M.-S.; Kim, T.-G. Immunogenicity of a Neutralizing Epitope from Porcine Epidemic Diarrhea Virus: M Cell Targeting Ligand Fusion Protein Expressed in Transgenic Rice Calli. Plant Cell Rep. 2012, 31, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Afzal, H.; Doan, T.-D.; Ke, G.-M.; Cheng, L.-T. Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines 2022, 10, 1780. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.D.; Wang, H.Y.; Ke, G.M.; Cheng, L.T. N-Terminus of Flagellin Fused to an Antigen Improves Vaccine Efficacy against Pasteurella Multocida Infection in Chickens. Vaccines 2020, 8, 283. [Google Scholar] [CrossRef]
- Truong, T.-N.; Cheng, L.-T. Development of a Subunit Vaccine against Duck Hepatitis A Virus Serotype 3. Vaccines 2022, 10, 523. [Google Scholar] [CrossRef]
- Chuekwon, K.; Chu, C.-Y.; Cheng, L.-T. N-Terminus of Flagellin Enhances Vaccine Efficacy against Actinobacillus Pleuropneumoniae. BMC Vet. Res. 2022, 18, 279. [Google Scholar] [CrossRef]
- Xu, X.; Du, L.; Fan, B.; Sun, B.; Zhou, J.; Guo, R.; Yu, Z.; Shi, D.; He, K.; Li, B. A Flagellin-Adjuvanted Inactivated Porcine Epidemic Diarrhea Virus (PEDV) Vaccine Provides Enhanced Immune Protection against PEDV Challenge in Piglets. Arch. Virol. 2020, 165, 1299–1309. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kao, C.-F.; Chang, C.-Y.; Jeng, C.-R.; Tsai, P.-S.; Pang, V.F.; Chiou, H.-Y.; Peng, J.-Y.; Cheng, I.-C.; Chang, H.-W. Evaluation and Comparison of the Pathogenicity and Host Immune Responses Induced by a G2b Taiwan Porcine Epidemic Diarrhea Virus (Strain Pintung 52) and Its Highly Cell-Culture Passaged Strain in Conventional 5-Week-Old Pigs. Viruses 2017, 9, 121. [Google Scholar] [CrossRef]
- Kao, C.-F.; Chiou, H.-Y.; Chang, Y.-C.; Hsueh, C.-S.; Jeng, C.-R.; Tsai, P.-S.; Cheng, I.-C.; Pang, V.F.; Chang, H.-W. The Characterization of Immunoprotection Induced by a CDNA Clone Derived from the Attenuated Taiwan Porcine Epidemic Diarrhea Virus Pintung 52 Strain. Viruses 2018, 10, 543. [Google Scholar] [CrossRef]
- Mahram, A.; Herbordt, M.C. Fast and Accurate NCBI BLASTP: Acceleration with Multiphase FPGA-Based Prefiltering. In Proceedings of the 24th International Conference on Supercomputing, Tsukuba, Japan, 2–4 June 2010; pp. 73–82. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server BT—The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. ISBN 978-1-59259-890-8. [Google Scholar]
- McGuffin, L.J.; Bryson, K.; Jones, D.T. The PSIPRED Protein Structure Prediction Server. Bioinformatics 2000, 16, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, W.; Li, Y.; Pearce, R.; Zhang, C.; Bell, E.W.; Zhang, G.; Zhang, Y. I-TASSER-MTD: A Deep-Learning-Based Platform for Multi-Domain Protein Structure and Function Prediction. Nat. Protoc. 2022, 17, 2326–2353. [Google Scholar] [CrossRef] [PubMed]
- Lovell, S.C.; Davis, I.W.; Arendall III, W.B.; de Bakker, P.I.W.; Word, J.M.; Prisant, M.G.; Richardson, J.S.; Richardson, D.C. Structure Validation by Cα Geometry: ϕ, ψ and Cβ Deviation. Proteins Struct. Funct. Bioinform. 2003, 50, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Dym, O.; Eisenberg, D.; Yeates, T.O. Detection of Errors in Protein Models. In International Tables for Crystallography, Set; OnlineMRW; International Union of Crystallography: Cheshire, UK, 2012; Volumes A–G, pp. 677–683. ISBN 9780470685754. Available online: https://onlinelibrary.wiley.com/iucr/itc/Fa/ch21o3v0001/ (accessed on 22 January 2024).
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive Web Service for the Recognition of Errors in Three-Dimensional Structures of Proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.-H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A New Structure-Based Tool for the Prediction of Antibody Epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef]
- Malik, A.A.; Ojha, S.C.; Schaduangrat, N.; Nantasenamat, C. ABCpred: A Webserver for the Discovery of Acetyl- and Butyryl-Cholinesterase Inhibitors. Mol. Divers. 2022, 26, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.H.; Bhatti, R.; Ali, N.F.; Ashfaq, U.A.; Shahid, F.; Almatroudi, A.; Khurshid, M. Rational Design of Chimeric Multiepitope Based Vaccine (MEBV) against Human T-Cell Lymphotropic Virus Type 1: An Integrated Vaccine Informatics and Molecular Docking Based Approach. PLoS ONE 2021, 16, e0258443. [Google Scholar] [CrossRef]
- de Vries, S.J.; van Dijk, M.; Bonvin, A.M.J.J. The HADDOCK Web Server for Data-Driven Biomolecular Docking. Nat. Protoc. 2010, 5, 883–897. [Google Scholar] [CrossRef]
- Laskowski, R.A. PDBsum: Summaries and Analyses of PDB Structures. Nucleic Acids Res. 2001, 29, 221–222. [Google Scholar] [CrossRef] [PubMed]
- López-Yglesias, A.H.; Zhao, X.; Quarles, E.K.; Lai, M.A.; VandenBos, T.; Strong, R.K.; Smith, K.D. Flagellin Induces Antibody Responses through a TLR5- and Inflammasome-Independent Pathway. J. Immunol. 2014, 192, 1587–1596. [Google Scholar] [CrossRef]
- Rapin, N.; Lund, O.; Bernaschi, M.; Castiglione, F. Computational Immunology Meets Bioinformatics: The Use of Prediction Tools for Molecular Binding in the Simulation of the Immune System. PLoS ONE 2010, 5, e9862. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Sharma, G.; Agrawal, P. Chapter 20—Computational Approaches for Vaccine Designing; Singh, D.B., Pathak, R.K.B.T.-B., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 317–335. ISBN 978-0-323-89775-4. [Google Scholar]
- Kulkarni-Kale, U.; Waman, V.; Raskar, S.; Mehta, S.; Saxena, S. Genome To Vaccinome: Role of Bioinformatics, Immunoinformatics & Comparative Genomics. Curr. Bioinform. 2012, 8, 454–466. [Google Scholar] [CrossRef]
- Panda, P.K.; Arul, M.N.; Patel, P.; Verma, S.K.; Luo, W.; Rubahn, H.-G.; Mishra, Y.K.; Suar, M.; Ahuja, R. Structure-Based Drug Designing and Immunoinformatics Approach for SARS-CoV-2. Sci. Adv. 2023, 6, eabb8097. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Sibuyi, N.R.S.; Martin, D.R.; Goboza, M.; Klein, A.; Madiehe, A.M.; Meyer, M. Immunoinformatics Design of a Novel Epitope-Based Vaccine Candidate against Dengue Virus. Sci. Rep. 2021, 11, 19707. [Google Scholar] [CrossRef]
- Ahmad, F.; Albutti, A.; Tariq, M.H.; Din, G.; Tahir ul Qamar, M.; Ahmad, S. Discovery of Potential Antiviral Compounds against Hendra Virus by Targeting Its Receptor-Binding Protein (G) Using Computational Approaches. Molecules 2022, 27, 554. [Google Scholar] [CrossRef] [PubMed]
- Alamri, M.A.; Tariq, M.H.; Tahir ul Qamar, M.; Alabbas, A.B.; Alqahtani, S.M.; Ahmad, S. Discovery of Potential Phytochemicals as Inhibitors of TcdB, a Major Virulence Factors of Clostridioides Difficile. J. Biomol. Struct. Dyn. 2023, 41, 12768–12776. [Google Scholar] [CrossRef]
- Tahir ul Qamar, M.; Ahmad, S.; Fatima, I.; Ahmad, F.; Shahid, F.; Naz, A.; Abbasi, S.W.; Khan, A.; Mirza, M.U.; Ashfaq, U.A.; et al. Designing Multi-Epitope Vaccine against Staphylococcus Aureus by Employing Subtractive Proteomics, Reverse Vaccinology and Immuno-Informatics Approaches. Comput. Biol. Med. 2021, 132, 104389. [Google Scholar] [CrossRef] [PubMed]
- IKAI, A. Thermostability and Aliphatic Index of Globular Proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [PubMed]
- Chan, J.; Mehta, S.; Bharrhan, S.; Chen, Y.; Achkar, J.M.; Casadevall, A.; Flynn, J. The Role of B Cells and Humoral Immunity in Mycobacterium Tuberculosis Infection. Semin. Immunol. 2014, 26, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H. V Antigenicity and Immunogenicity of Synthetic Peptides. Biologicals 2001, 29, 209–213. [Google Scholar] [CrossRef]
- Koyama, S.; Ishii, K.J.; Coban, C.; Akira, S. Innate Immune Response to Viral Infection. Cytokine 2008, 43, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Haque, A.; Alghamdi, Y.S.; Mashraqi, M.M.; Rehman, A.; Shahid, F.; Khurshid, M.; Ashfaq, U.A. Development of a Candidate Multi-Epitope Subunit Vaccine against Klebsiella Aerogenes: Subtractive Proteomics and Immuno-Informatics Approach. Vaccines 2021, 9, 1373. [Google Scholar] [CrossRef]
- Naveed, M.; Yaseen, A.R.; Khalid, H.; Ali, U.; Rabaan, A.A.; Garout, M.; Halwani, M.A.; Al Mutair, A.; Alhumaid, S.; Al Alawi, Z.; et al. Execution and Design of an Anti HPIV-1 Vaccine with Multiple Epitopes Triggering Innate and Adaptive Immune Responses: An Immunoinformatic Approach. Vaccines 2022, 10, 869. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Wu, T.; Peng, O.; Huang, L.; Zhang, Y.; Xue, C.; Wen, Z.; Zhou, Q.; Cao, Y. A Flagellin-Adjuvanted PED Subunit Vaccine Improved Protective Efficiency against PEDV Variant Challenge in Pigs. Vaccine 2018, 36, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Helm, E.T.; Groeltz-Thrush, J.M.; Gabler, N.K.; Burrough, E.R. Epithelial-Mesenchymal Transition of Absorptive Enterocytes and Depletion of Peyer’s Patch M Cells after PEDV Infection. Virology 2021, 552, 43–51. [Google Scholar] [CrossRef] [PubMed]
- de Arriba, M.L.; Carvajal, A.; Pozo, J.; Rubio, P. Mucosal and Systemic Isotype-Specific Antibody Responses and Protection in Conventional Pigs Exposed to Virulent or Attenuated Porcine Epidemic Diarrhoea Virus. Vet. Immunol. Immunopathol. 2002, 85, 85–97. [Google Scholar] [CrossRef] [PubMed]


| Target | Sequence (5′-3′) | RE | Gene- | DNA |
|---|---|---|---|---|
| Gene | Site | Length (bp) | Templates | |
| FliC99 | F 1 gggaattcatggcacaagtcattaatacaaac | EcoRI | 312 | FliC |
| R 2 gctgccgcccccgccagactgaaccgccagttc | - | |||
| mCOE | F 2 ggcgggggcggcagctccttcgtcaccttgcc | - | 915 | Modified S1S2 |
| R aactcgagcagctgcaagtactccgt | Xhol | |||
| FliC99-mCOE | F ggatccaacccgctgcagaaaattg | EcoRI | 1212 | FliC99 and mCOE |
| R ctcgagcgtaacgacagaccctgt | Xhol | |||
| T7 Promoter | F taatacgactcactataggg | - | 705 | |
| R gctagttattgctcagcgg | - |
| Parameter | Value |
|---|---|
| HADDOCK score | 480.6 ± 22.4 |
| Cluster size | 5 |
| RMSD from overall lowest energy structure | 12.9 ± 0.4 |
| Van der Waals energy | −137.6 ± 4.3 |
| Electrostatic energy | −255.8 ± 55.4 |
| Desolvation energy | −3.8 ± 15.1 |
| Restraints violation energy | 6655.6 ± 182.22 |
| Buried surface area | 4504.2 ± 41.1 |
| Z-score | −1.2 |
| Hydrogen Bond Number | Vaccine | TLR5 | Distance (Å) | ||
|---|---|---|---|---|---|
| Amino Acid Residue | Residue Number | Amino Acid Residue | Residue Number | ||
| 1 | ASP | 43 | TYR | 608 | 2.92 |
| 2 | ASP | 43 | SER | 613 | 2.65 |
| 3 | ASP | 43 | TYR | 611 | 2.71 |
| 4 | ARG | 93 | GLU | 632 | 2.68 |
| 5 | ARG | 93 | GLU | 632 | 2.70 |
| 6 | GLU | 94 | THR | 622 | 3.01 |
| 7 | ALA | 100 | GLN | 595 | 3.31 |
| 8 | CYS | 254 | ARG | 604 | 2.75 |
| 9 | ASN | 272 | PHE | 25 | 3.20 |
| 10 | ASN | 272 | ASP | 26 | 2.77 |
| 11 | SER | 306 | ASP | 605 | 2.70 |
| 12 | THR | 329 | TYR | 534 | 2.95 |
| 13 | THR | 329 | ASP | 606 | 2.92 |
| 14 | LEU | 336 | THR | 10 | 2.87 |
| 15 | PHE | 339 | THR | 10 | 2.86 |
| 16 | TYR | 369 | GLY | 2 | 3.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtaza, A.; Hoa, N.-T.; Dieu-Huong, D.; Afzal, H.; Tariq, M.H.; Cheng, L.-T.; Chung, Y.-C. Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines. Vaccines 2024, 12, 139. https://doi.org/10.3390/vaccines12020139
Murtaza A, Hoa N-T, Dieu-Huong D, Afzal H, Tariq MH, Cheng L-T, Chung Y-C. Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines. Vaccines. 2024; 12(2):139. https://doi.org/10.3390/vaccines12020139
Chicago/Turabian StyleMurtaza, Asad, Nguyen-Thanh Hoa, Do Dieu-Huong, Haroon Afzal, Muhammad Hamza Tariq, Li-Ting Cheng, and Yao-Chi Chung. 2024. "Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines" Vaccines 12, no. 2: 139. https://doi.org/10.3390/vaccines12020139





