Bladder Cancer: Immunotherapy and Pelvic Lymph Node Dissection
Abstract
:1. Introduction
2. Current Status of Immunotherapy for Bladder Cancer
| Immunotherapy for Bladder Cancer | Clinical Trial | Type of Therapy | Results |
|---|---|---|---|
| Immunotherapy of BCG vaccine | Intravesical BCG therapy (Morales et al., 1976 [13]) | Biological immunotherapy | Effective in preventing postoperative recurrence |
| BCG injections and TURBT | Standard treatment for intermediate- to high-risk NMIBC | Decreases tumor recurrence and enhances patient survival rates | |
| VPM1002B | Genetically engineered BCG variant | Better effectiveness compared to ordinary BCG. Only 7 of 40 high-risk NMIBC patients progressed, indicating improved efficacy | |
| Immunotherapy of immune checkpoint inhibitors (ICIs) | Radical cystectomy (RC) with neoadjuvant chemotherapy (NAC) | Surgery + neoadjuvant chemotherapy | Effective in improving patient survival rates and preventing local recurrence and distant metastasis in MIBC [23,24] |
| Immunocheckpoint inhibitors (ICIs) in neoadjuvant immunotherapy | Monotherapy with ICIs | Improved T cell activity, inhibited immune escape. Progress in reducing tumor size and stage in MIBC [38] | |
| Double immune combination in neoadjuvant immunotherapy | Combination of two ICIs | Synergistic effect in enhancing immune response. Further reduction in tumor size and stage in MIBC | |
| Immune combined with chemotherapy in neoadjuvant immunotherapy | ICIs + chemotherapy | Improved response rates compared to platinum-based NAC alone. Enhanced efficacy in neoadjuvant setting for MIBC | |
| Neoadjuvant immunotherapy | PURE-01 (NCT02736266)—pembrolizumab | Neoadjuvant immunotherapy (single-arm, phase II) | Pathologic complete response (pCR) rate: 37%—Pathological descending response (pDR) rate: 55%—Most common adverse event (AE): hyperthyroidism (23%)—Grade 3 AE: 7% |
| KEYNOTE-057—pembrolizumab for BCG-resistant in situ cancer (NCT02625961) | Pembrolizumab monotherapy | Complete response at 3 months for BCG-unresponsive bladder carcinoma in situ: 41%—Grade 3 or 4 AEs: 13% (including arthralgia and hyponatremia) | |
| ABACUS (NCT02662309)—atezolizumab | Neoadjuvant immunotherapy (phase II) | pCR rate after treatment: 31%—1-year recurrence-free survival (RFS) rate: 79%—Incidence of grade 3–4 AEs: 11% | |
| Atezolizumab for non-BCG-reactive, high-risk NMIBC (NCT02108652) | Atezolizumab treatment | pCR rate for carcinoma in situ: 27%—1-year pCR lasting rate: 48.9%—Only 12 out of 129 patients developed MIBC or metastatic diseases | |
| Neoadjuvant double immunotherapy | NABUCCO (NCT03387761) | Double immunotherapy with ipilimumab and nivolumab | Pathologic complete response (pCR): 46%—Pathological descending rate: 58%—Grade 3–4 immune-related adverse events: 41% |
| CHECKMate-032 (NCT01928394) | Combination of ipilimumab with nivolumab vs. nivolumab alone | Combo group (high PD-L1 expression): median overall survival (mOS) of 15.3 months—Monotherapy group: mOS of 9.9 months—Grade 3–4 adverse events: combo (39%) vs. monotherapy (27%) | |
| Neoadjuvant immunotherapy combined with chemotherapy | BLASST-1 (NCT03294304) | Neoadjuvant immunotherapy (nivolumab) with gemcitabine plus cisplatin (GC) | Pathologic complete response (pCR): 49%—Pathological descending rate: 66%—Grade 3–4 adverse events: renal failure and neutropenia in 20% of participants |
| NCT02989584 | Neoadjuvant immunotherapy (atezolizumab) with gemcitabine plus cisplatin (GC) | Pathological descending phase: 69%—Adverse reactions did not affect subsequent radical cystectomy (RC) | |
| HCRN GU14-188 (NCT02365766) | Neoadjuvant immunotherapy (pembrolizumab) with gemcitabine plus cisplatin (GC) | pCR: 40%—Pathological descending rate: 61%—Incidence of grade 3–4 adverse events (blood-related): 44% |
3. Effect of Pelvic Lymph Node Dissection on Immunotherapy for Bladder Cancer
| Immunotherapy for Bladder Cancer | Clinical Trial | Type of Therapy | Results |
|---|---|---|---|
| Lymphatic drainage of the bladder | Lymph node dissection studies | Surgical procedure | Abol-Enien et al. [61]: Found that approximately 39% of patients with prostate cancer had lymph nodes on both sides, and bilateral lymph node metastases were common. Up to 52% of lymph nodes were situated outside the reconstructed pelvic cavity |
| Leissner et al. [68]: Identified 12 anatomical sites with varying probabilities of metastatic deposits, with the obturator groups being the most frequently afflicted. Higher probabilities of metastases were observed in the interaortocaval and precaval areas (2.9%) and above the common iliac bifurcation (6.9%) | |||
| Tarin et al. [69]: Studied 591 individuals with radical cystectomy, finding LN involvement in 114 cases. The percentage of patients with LN involvement ranged from 6% to 40%, depending on tumor stage (pT2, pT3, and pT4). Identified cases with negative lymph node testing in the pelvis, possibly due to skip lesions or mislabeling | |||
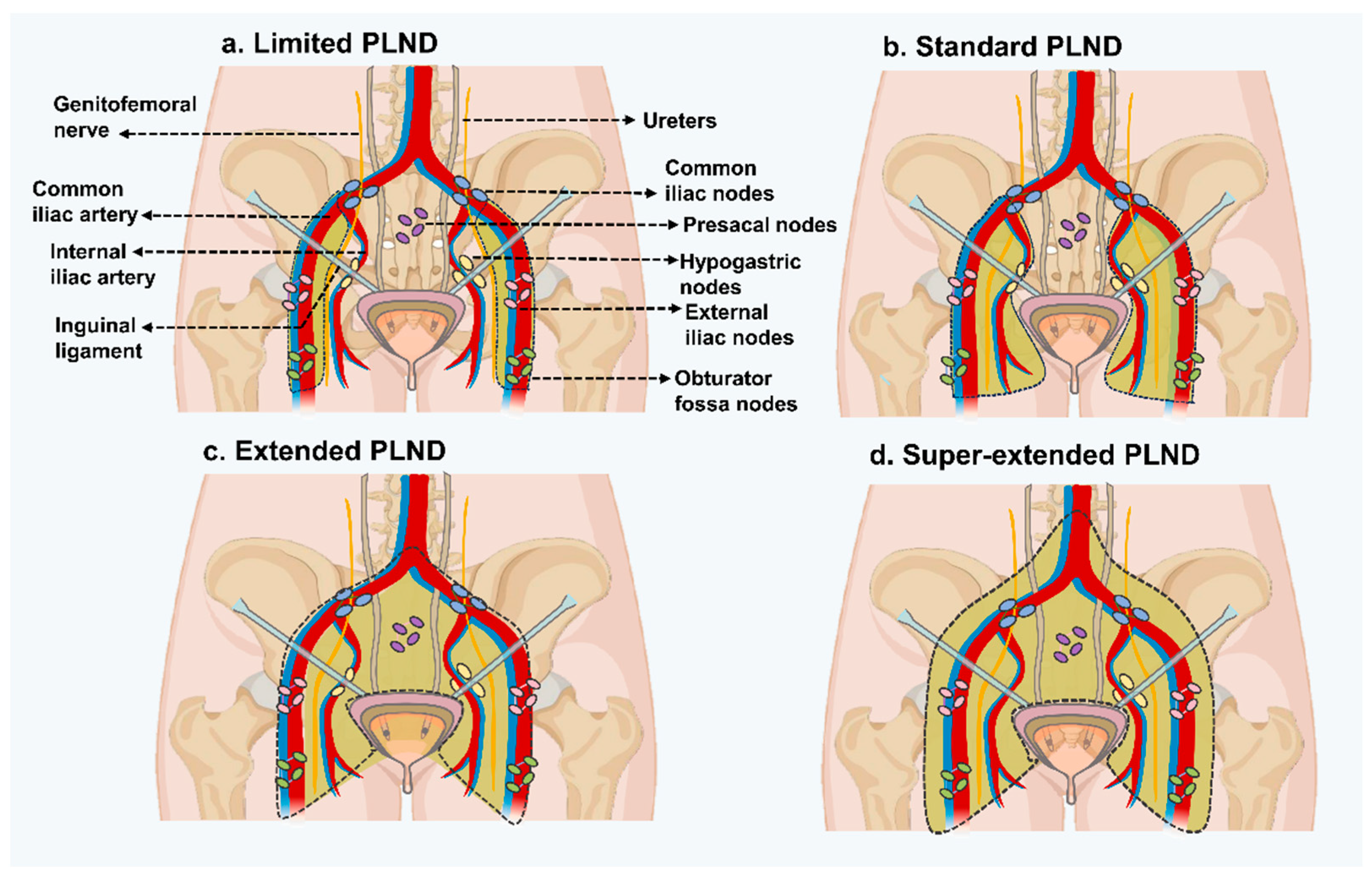
| Templates of pelvic lymph node dissection | EAU Working Group | Limited PLND | Dissects only bilateral obturator fossa |
| Standard PLND | Dissects medial and lateral walls of the bladder, lymph nodes between the lower division of the inguinal ligament and the genitofemoral nerve, upper division of the common iliac artery, and the inguinal ligament. Includes distal common iliac, obturator, external iliac, and hypogastric nodes on either side | ||
| Extended PLND | Excises nodes from the area that includes the aortic bifurcation, internal iliac arteries, circumflex iliac vein, common iliac vessels, and genitofemoral nerve | ||
| Super-extended PLND | Dissection proceeds proximally to the inferior mesenteric artery root |
4. Conclusions and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e119. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.; Jackson, A.M.; Hawkyard, S.J.; Alexandroff, A.B.; James, K. Mechanisms of action of intravesical bacille Calmette-Guérin: Local immune mechanisms. Clin. Infect. Dis. 2000, 31 (Suppl. 3), S91–S93. [Google Scholar] [CrossRef]
- Fuge, O.; Vasdev, N.; Allchorne, P.; Green, J.S.A. Immunotherapy for bladder cancer. Res. Rep. Urol. 2015, 7, 65–79. [Google Scholar] [CrossRef]
- Jokisch, J.F.; Karl, A.; Stief, C. Intravesical immunotherapy in nonmuscle invasive bladder cancer. Indian J. Urol. 2015, 31, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Dejima, T.; Yamada, H.; Shibata, K.; Nakamura, R.; Eto, M.; Nakatani, T.; Naito, S.; Yoshikai, Y. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur. J. Immunol. 2011, 41, 246–251. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 2017, 197, S142–S145. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Buckley, D.; Fu, R.; Gore, J.L.; Gustafson, K.; Griffin, J.; Grusing, S.; Selph, S. AHRQ Comparative Effectiveness Reviews. In Emerging Approaches to Diagnosis and Treatment of Non–Muscle-Invasive Bladder Cancer; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2015. [Google Scholar]
- Jiang, S.; Redelman-Sidi, G. BCG in Bladder Cancer Immunotherapy. Cancers 2022, 14, 3073. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Meeks, J.J. T1 bladder cancer: Current considerations for diagnosis and management. Nat. Rev. Urol. 2019, 16, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Malmström, P.U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Nasser Eddine, A.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef]
- Rentsch, C.A.; Bosshard, P.; Mayor, G.; Rieken, M.; Püschel, H.; Wirth, G.; Cathomas, R.; Parzmair, G.P.; Grode, L.; Eisele, B.; et al. Results of the phase I open label clinical trial SAKK 06/14 assessing safety of intravesical instillation of VPM1002BC, a recombinant mycobacterium Bacillus Calmette Guérin (BCG), in patients with non-muscle invasive bladder cancer and previous failure of conventional BCG therapy. Oncoimmunology 2020, 9, 1748981. [Google Scholar] [CrossRef]
- Brincks, E.L.; Risk, M.C.; Griffith, T.S. PMN and anti-tumor immunity—The case of bladder cancer immunotherapy. Semin. Cancer Biol. 2013, 23, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.; Choudhury, A.; Alam, N. Metastatic bladder cancer: A review of current management. ISRN Urol. 2011, 2011, 545241. [Google Scholar] [CrossRef]
- Ghoneim, M.A.; Abdel-Latif, M.; el-Mekresh, M.; Abol-Enein, H.; Mosbah, A.; Ashamallah, A.; el-Baz, M.A. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J. Urol. 2008, 180, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Calò, B.; Marchioni, M.; Sanguedolce, F.; Falagario, U.G.; Chirico, M.; Carrieri, G.; Cormio, L. Neoadjuvant Chemotherapy Before Radical Cystectomy: Why We Must Adhere? Curr. Drug Targets 2021, 22, 14–21. [Google Scholar] [CrossRef]
- Motterle, G.; Andrews, J.R.; Morlacco, A.; Karnes, R.J. Predicting Response to Neoadjuvant Chemotherapy in Bladder Cancer. Eur. Urol. Focus 2020, 6, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Yang, S.T.; Liu, C.H. Neoadjuvant therapy. J. Chin. Med. Assoc. 2023, 86, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, N.N.; Yuno, A.; Trepel, J.B.; Apolo, A.B. Immunotherapy: A new treatment paradigm in bladder cancer. Curr. Opin. Oncol. 2017, 29, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef]
- Chapman, N.M.; Boothby, M.R.; Chi, H. Metabolic coordination of T cell quiescence and activation. Nat. Rev. Immunol. 2020, 20, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, X. Study and analysis of antitumor resistance mechanism of PD1/PD-L1 immune checkpoint blocker. Cancer Med. 2020, 9, 8086–8121. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2022, 19, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.; Mount, C.; Omar, K.; Nair, R.; Thurairaja, R.; Khan, M.S. Landmarks in the treatment of muscle-invasive bladder cancer. Nat. Rev. Urol. 2017, 14, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Powles, T.; Vogelzang, N.J. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: The future is now. Cancer Treat. Rev. 2017, 54, 58–67. [Google Scholar] [CrossRef]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M.; et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Szabados, B.; Rodriguez-Vida, A.; Durán, I.; Crabb, S.J.; Van Der Heijden, M.S.; Pous, A.F.; Gravis, G.; Herranz, U.A.; Protheroe, A.; Ravaud, A.; et al. Toxicity and Surgical Complication Rates of Neoadjuvant Atezolizumab in Patients with Muscle-invasive Bladder Cancer Undergoing Radical Cystectomy: Updated Safety Results from the ABACUS Trial. Eur. Urol. Oncol. 2021, 4, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Black, P.C.; Tangen, C.M.; Singh, P.; McConkey, D.J.; Lucia, M.S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.J.; Kassouf, W.; et al. Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin-unresponsive High-risk Non-muscle-invasive Bladder Cancer: SWOG S1605. Eur. Urol. 2023, 84, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Hoffman-Censits, J.H.; Viterbo, R.; Trabulsi, E.J.; Ross, E.A.; Greenberg, R.E.; Chen, D.Y.; Lallas, C.D.; Wong, Y.N.; Lin, J.; et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J. Clin. Oncol. 2014, 32, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Hato, S.V.; Khong, A.; de Vries, I.J.; Lesterhuis, W.J. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef]
- Zhou, Y.; Bastian, I.N.; Long, M.D.; Dow, M.; Li, W.; Liu, T.; Ngu, R.K.; Antonucci, L.; Huang, J.Y.; Phung, Q.T.; et al. Activation of NF-κB and p300/CBP potentiates cancer chemoimmunotherapy through induction of MHC-I antigen presentation. Proc. Natl. Acad. Sci. USA 2021, 118, e2025840118. [Google Scholar] [CrossRef]
- Gupta, S.; Sonpavde, G.; Weight, C.J.; McGregor, B.A.; Gupta, S.; Maughan, B.L.; Wei, X.X.; Gibb, E.; Thyagarajan, B.; Einstein, D.J.; et al. Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J. Clin. Oncol. 2020, 38, 439. [Google Scholar] [CrossRef]
- Funt, S.A.; Lattanzi, M.; Whiting, K.; Al-Ahmadie, H.A.; Quinlan, C.; Teo, M.Y.; Kamradt, J.; Khalil, M.F.; Ostrovnaya, I.; McCoy, A.S.; et al. Neoadjuvant atezolizumab (A) with gemcitabine and cisplatin (GC) in patients (pts) with muscle-invasive bladder cancer (MIBC): A multicenter, single-arm, phase 2 trial. J. Clin. Oncol. 2021, 39, 4517. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Adra, N.; Fleming, M.T.; Kaimakliotis, H.Z.; Picus, J.; Smith, Z.L.; Walling, R.; Trabulsi, E.J.; Hoffman-Censits, J.H.; Koch, M.O.; et al. Phase Ib/II neoadjuvant (N-)pembrolizumab (P) and chemotherapy for locally advanced urothelial cancer (laUC): Final results from the cisplatin (C)-eligible cohort of HCRN GU14-188. J. Clin. Oncol. 2020, 38, 5047. [Google Scholar] [CrossRef]
- Funt, S.A.; Rosenberg, J.E. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat. Rev. Clin. Oncol. 2017, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Buscarini, M.; Josephson, D.Y.; Stein, J.P. Lymphadenectomy in bladder cancer: A review. Urol. Int. 2007, 79, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.H.; Lerner, S.P. Utility of lymphadenectomy in bladder cancer: Where do we stand? Curr. Opin. Urol. 2020, 30, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Papalia, R.; Simone, G.; Grasso, R.; Augelli, R.; Faiella, E.; Guaglianone, S.; Cazzato, R.; Del Vescovo, R.; Ferriero, M.; Zobel, B.; et al. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: Detection rate of pelvic lymph node metastases. BJU Int. 2012, 109, 1031–1036. [Google Scholar] [CrossRef]
- Crozier, J.; Papa, N.; Perera, M.; Ngo, B.; Bolton, D.; Sengupta, S.; Lawrentschuk, N. Comparative sensitivity and specificity of imaging modalities in staging bladder cancer prior to radical cystectomy: A systematic review and meta-analysis. World J. Urol. 2019, 37, 667–690. [Google Scholar] [CrossRef]
- Jeong, I.G.; Hong, S.; You, D.; Hong, J.H.; Ahn, H.; Kim, C.S. FDG PET-CT for lymph node staging of bladder cancer: A prospective study of patients with extended pelvic lymphadenectomy. Ann. Surg. Oncol. 2015, 22, 3150–3156. [Google Scholar] [CrossRef]
- Bruins, H.M.; Veskimae, E.; Hernandez, V.; Imamura, M.; Neuberger, M.M.; Dahm, P.; Stewart, F.; Lam, T.B.; N’Dow, J.; van der Heijden, A.G.; et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: A systematic review. Eur. Urol. 2014, 66, 1065–1077. [Google Scholar] [CrossRef]
- Suttmann, H.; Kamradt, J.; Lehmann, J.; Stöckle, M. Improving the prognosis of patients after radical cystectomy. Part I: The role of lymph node dissection. BJU Int. 2007, 100, 1221–1224. [Google Scholar] [CrossRef]
- Abol-Enein, H.; El-Baz, M.; Abd El-Hameed, M.A.; Abdel-Latif, M.; Ghoneim, M.A. Lymph node involvement in patients with bladder cancer treated with radical cystectomy: A patho-anatomical study—A single center experience. J. Urol. 2004, 172, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.H.; Cho, D.; Herr, H.W.; Donat, M.; Kattan, M.W.; Dalbagni, G. Prospectively packaged lymph node dissections with radical cystectomy: Evaluation of node count variability and node mapping. J. Urol. 2004, 172, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Motterle, G.; Zattoni, F.; Morlacco, A.; Dal Moro, F. The Role of Lymph Node Dissection in the Treatment of Bladder Cancer. Front. Surg. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.; Wissmeyer, M.P.; Zehnder, P.; Birkhäuser, F.D.; Thalmann, G.N.; Krause, T.M.; Studer, U.E. A new multimodality technique accurately maps the primary lymphatic landing sites of the bladder. Eur. Urol. 2010, 57, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.; Zehnder, P.; Birkhäuser, F.D.; Burkhard, F.C.; Thalmann, G.N.; Studer, U.E. Is bilateral extended pelvic lymphadenectomy necessary for strictly unilateral invasive bladder cancer? J. Urol. 2012, 187, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Vazina, A.; Dugi, D.; Shariat, S.F.; Evans, J.; Link, R.; Lerner, S.P. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J. Urol. 2004, 171, 1830–1834. [Google Scholar] [CrossRef]
- Leissner, J.; Ghoneim, M.A.; Abol-Enein, H.; Thüroff, J.W.; Franzaring, L.; Fisch, M.; Schulze, H.; Managadze, G.; Allhoff, E.P.; el-Baz, M.A.; et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: Results of a prospective multicenter study. J. Urol. 2004, 171, 139–144. [Google Scholar] [CrossRef]
- Perera, M.; McGrath, S.; Sengupta, S.; Crozier, J.; Bolton, D.; Lawrentschuk, N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat. Rev. Urol. 2018, 15, 686–692. [Google Scholar] [CrossRef]
- Tarin, T.V.; Power, N.E.; Ehdaie, B.; Sfakianos, J.P.; Silberstein, J.L.; Savage, C.J.; Sjoberg, D.; Dalbagni, G.; Bochner, B.H. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: Effect of the level of node positivity. Eur. Urol. 2012, 61, 1025–1030. [Google Scholar] [CrossRef]
- Jensen, J.B.; Ulhøi, B.P.; Jensen, K.M. Extended versus limited lymph node dissection in radical cystectomy: Impact on recurrence pattern and survival. Int. J. Urol. 2012, 19, 39–47. [Google Scholar] [CrossRef]
- Herr, H.; Lee, C.; Chang, S.; Lerner, S. Standardization of radical cystectomy and pelvic lymph node dissection for bladder cancer: A collaborative group report. J. Urol. 2004, 171, 1823–1828; discussion 1827–1828. [Google Scholar] [CrossRef] [PubMed]
- Weisbach, L.; Dahlem, R.; Simone, G.; Hansen, J.; Soave, A.; Engel, O.; Chun, F.K.; Shariat, S.F.; Fisch, M.; Rink, M. Lymph node dissection during radical cystectomy for bladder cancer treatment: Considerations on relevance and extent. Int. Urol. Nephrol. 2013, 45, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Dorin, R.P.; Daneshmand, S.; Eisenberg, M.S.; Chandrasoma, S.; Cai, J.; Miranda, G.; Nichols, P.W.; Skinner, D.G.; Skinner, E.C. Lymph node dissection technique is more important than lymph node count in identifying nodal metastases in radical cystectomy patients: A comparative mapping study. Eur. Urol. 2011, 60, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Huang, H.; Fan, X.; Li, K.; Xu, K.; Jiang, C.; Liu, H.; Dong, W.; Zhang, S.; Yang, X.; et al. Extended vs non-extended pelvic lymph node dissection and their influence on recurrence-free survival in patients undergoing radical cystectomy for bladder cancer: A systematic review and meta-analysis of comparative studies. BJU Int. 2014, 113, E39–E48. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H. Editorial comment to stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int. J. Urol. 2013, 20, 398. [Google Scholar] [CrossRef] [PubMed]
- Vieweg, J.; Gschwend, J.E.; Herr, H.W.; Fair, W.R. The impact of primary stage on survival in patients with lymph node positive bladder cancer. J. Urol. 1999, 161, 72–76. [Google Scholar] [CrossRef]
- Bruins, H.M.; Huang, G.J.; Cai, J.; Skinner, D.G.; Stein, J.P.; Penson, D.F. Clinical outcomes and recurrence predictors of lymph node positive urothelial cancer after cystectomy. J. Urol. 2009, 182, 2182–2187. [Google Scholar] [CrossRef]
- Herr, H.W. Superiority of ratio based lymph node staging for bladder cancer. J. Urol. 2003, 169, 943–945. [Google Scholar] [CrossRef]
- Karl, A.; Carroll, P.R.; Gschwend, J.E.; Knüchel, R.; Montorsi, F.; Stief, C.G.; Studer, U.E. The impact of lymphadenectomy and lymph node metastasis on the outcomes of radical cystectomy for bladder cancer. Eur. Urol. 2009, 55, 826–835. [Google Scholar] [CrossRef]
- Konety, B.R.; Joslyn, S.A.; O’Donnell, M.A. Extent of pelvic lymphadenectomy and its impact on outcome in patients diagnosed with bladder cancer: Analysis of data from the Surveillance, Epidemiology and End Results Program data base. J. Urol. 2003, 169, 946–950. [Google Scholar] [CrossRef]
- Stein, J.P.; Cai, J.; Groshen, S.; Skinner, D.G. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: Concept of lymph node density. J. Urol. 2003, 170, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Papalia, R.; Ferriero, M.; Guaglianone, S.; Castelli, E.; Collura, D.; Muto, G.; Gallucci, M. Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int. J. Urol. 2013, 20, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Larcher, A.; Sun, M.; Schiffmann, J.; Tian, Z.; Shariat, S.F.; McCormack, M.; Saad, F.; Fossati, N.; Abdollah, F.; Briganti, A.; et al. Differential effect on survival of pelvic lymph node dissection at radical cystectomy for muscle invasive bladder cancer. Eur. J. Surg. Oncol. 2015, 41, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bruins, H.M.; Skinner, E.C.; Dorin, R.P.; Ahmadi, H.; Djaladat, H.; Miranda, G.; Cai, J.; Daneshmand, S. Incidence and location of lymph node metastases in patients undergoing radical cystectomy for clinical non-muscle invasive bladder cancer: Results from a prospective lymph node mapping study. Urol. Oncol. 2014, 32, 24.e13–24.e19. [Google Scholar] [CrossRef]
- Brössner, C.; Pycha, A.; Toth, A.; Mian, C.; Kuber, W. Does extended lymphadenectomy increase the morbidity of radical cystectomy? BJU Int. 2004, 93, 64–66. [Google Scholar] [CrossRef]
- Morgan, T.M.; Barocas, D.A.; Penson, D.F.; Chang, S.S.; Ni, S.; Clark, P.E.; Smith, J.A., Jr.; Cookson, M.S. Lymph node yield at radical cystectomy predicts mortality in node-negative and not node-positive patients. Urology 2012, 80, 632–640. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Herrmann, E.; Bolenz, C.; Brookman-May, S.; Tiemann, A.; Moritz, R.; Fritsche, H.M.; Burger, M.; Trojan, L.; Michel, M.S.; et al. Association between the number of dissected lymph nodes during pelvic lymphadenectomy and cancer-specific survival in patients with lymph node-negative urothelial carcinoma of the bladder undergoing radical cystectomy. Ann. Surg. Oncol. 2011, 18, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Burger, M.; Mulders, P.; Witjes, W. Use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is low among major European centres: Results of a feasibility questionnaire. Eur. Urol. 2012, 61, 1070–1071. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef]
- Gore, J.L.; Lai, J.; Setodji, C.M.; Litwin, M.S.; Saigal, C.S. Mortality increases when radical cystectomy is delayed more than 12 weeks: Results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2009, 115, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Nakajima, S.; Kobayashi, D.; Tomii, K.; Li, N.J.; Watarai, T.; Suzuki, R.; Watanabe, S.; Kanda, Y.; Takeuchi, A.; et al. Micro- and Macro-Anatomical Frameworks of Lymph Nodes Indispensable for the Lymphatic System Filtering Function. Front. Cell Dev. Biol. 2022, 10, 902601. [Google Scholar] [CrossRef] [PubMed]
- Anang, V.; Singh, A.; Kottarath, S.K.; Verma, C. Receptors of immune cells mediates recognition for tumors. Prog. Mol. Biol. Transl. Sci. 2023, 194, 219–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Anang, V.; Kumari, K.; Kottarath, S.K.; Verma, C. Role of lymphocytes, macrophages and immune receptors in suppression of tumor immunity. Prog. Mol. Biol. Transl. Sci. 2023, 194, 269–310. [Google Scholar] [CrossRef] [PubMed]
- Fear, V.S.; Forbes, C.A.; Neeve, S.A.; Fisher, S.A.; Chee, J.; Waithman, J.; Ma, S.K.; Lake, R.; Nowak, A.K.; Creaney, J.; et al. Tumour draining lymph node-generated CD8 T cells play a role in controlling lung metastases after a primary tumour is removed but not when adjuvant immunotherapy is used. Cancer Immunol. Immunother. CII 2021, 70, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- The general rules for The gastric cancer study in surgery. Jpn. J. Surg. 1973, 3, 61–71. [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Monrabal Lezama, M.; Murdoch Duncan, N.S.; Bertona, S.; Schlottmann, F. Current standards of lymphadenectomy in gastric cancer. Updates Surg. 2023, 75, 1751–1758. [Google Scholar] [CrossRef]
- Gupta, A.; Moore, J.A. Lymphedema. JAMA Oncol. 2018, 4, 755. [Google Scholar] [CrossRef]
- Rahim, M.K.; Okholm, T.L.H.; Jones, K.B.; McCarthy, E.E.; Liu, C.C.; Yee, J.L.; Tamaki, S.J.; Marquez, D.M.; Tenvooren, I.; Wai, K.; et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 2023, 186, 1127–1143.e1118. [Google Scholar] [CrossRef] [PubMed]
- Clinton, T.N.; Huang, C.; Goh, A.C. Is there an oncological benefit to extended lymphadenectomy for muscle-invasive bladder cancer? Transl. Androl. Urol. 2020, 9, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Salam, M.A.; Smith, C.E.P.; Pan, C.-X. Insights on recent innovations in bladder cancer immunotherapy. Cancer Cytopathol. 2022, 130, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Ham, W.S. Perioperative immunotherapy in muscle-invasive bladder cancer. Transl. Cancer Res. 2020, 9, 6546–6553. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Deng, J.; Wang, Y.; Fan, X.; Xie, J. Bladder Cancer: Immunotherapy and Pelvic Lymph Node Dissection. Vaccines 2024, 12, 150. https://doi.org/10.3390/vaccines12020150
Fan Z, Deng J, Wang Y, Fan X, Xie J. Bladder Cancer: Immunotherapy and Pelvic Lymph Node Dissection. Vaccines. 2024; 12(2):150. https://doi.org/10.3390/vaccines12020150
Chicago/Turabian StyleFan, Zhongru, Junpeng Deng, Yutao Wang, Xin Fan, and Jianjun Xie. 2024. "Bladder Cancer: Immunotherapy and Pelvic Lymph Node Dissection" Vaccines 12, no. 2: 150. https://doi.org/10.3390/vaccines12020150






