Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5–10-Year-Old Burkinabe Children Naturally Exposed to Malaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design, Site, and Participants
2.3. Anti-SE36 IgG Antibody Assessment
2.4. Statistical Methods
3. Results
3.1. Study Site and Population
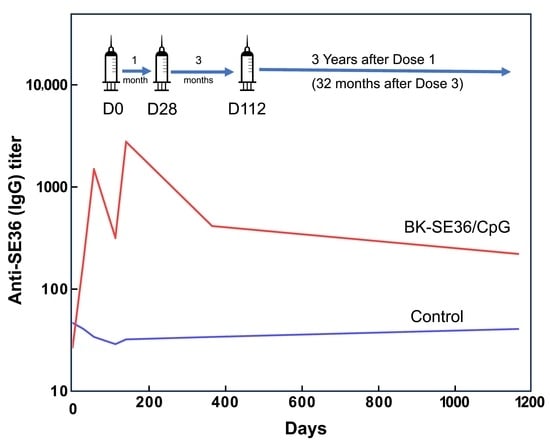
3.2. Persistence of Anti-SE36 Antibody Titres
3.3. Factors Affecting Post Immunisation Immune Responses
3.3.1. Baseline Anti-SE36 IgG Antibodies in the BK-SE36/CpG Arm
3.3.2. Participants’ Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report; WHO: Geneva, Switzerland, 2023. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (accessed on 18 January 2024).
- McCall, M.B.B.; Kremsner, P.G.; Mordmüller, B. Correlating efficacy and immunogenicity in malaria vaccine trials. Semin. Immunol. 2018, 39, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.I.; Good, M.F. Malaria Vaccines: Progress to Date. BioDrugs 2023, 37, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Takashima, E.; Otsuki, H.; Morita, M.; Ito, D.; Nagaoka, H.; Yuguchi, T.; Hassan, I.; Tsuboi, T. The need for novel asexual blood-stage malaria vaccine candidates for Plasmodium falciparum. Biomolecules 2024, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Technical Strategy for Malaria 2016–2030, 2021 Update. 2021. Available online: https://www.who.int/publications/i/item/9789240031357 (accessed on 18 January 2024).
- El-Moamly, A.A.; El-Sweify, M.A. Malaria vaccines: The 60-year journey of hope and final success-lessons learned and future prospects. Trop. Med. Health 2023, 51, 29. [Google Scholar] [CrossRef] [PubMed]
- Devi, S. 12 countries to get first doses of malaria vaccine. Lancet 2023, 402, 172. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: Current Status of Two Adjuvanted Malaria Vaccines and the World Health Organization (WHO) Strategy to Eradicate Malaria by 2030. Med. Sci. Monit. 2023, 29, e939357. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Highlights from the Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization of 25–29 September 2023. 2023. Available online: https://www.who.int/publications/m/item/highlights-from-the-meeting-of-the-strategic-advisory-group-ofexperts-(sage)-on-immunization25-29-september-2023 (accessed on 19 January 2024).
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: A randomised controlled trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, H.M.; Somé, A.; Bellamy, D.; Traoré, O.; Rouamba, T.; Tahita, M.C.; Ido, N.F.A.; Yameogo, P.; Valia, D.; et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: A phase 1/2b randomised controlled trial. Lancet Infect. Dis. 2022, 22, 1728–1736. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Ramos-Lopez, F.; Magloire Natama, H.; Weston, S.; et al. A Phase III Randomised Controlled Trial Evaluating the Malaria Vaccine Candidate R21/Matrix-M™ in African Children. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4584076 (accessed on 18 January 2024).
- Tsoumani, M.E.; Voyiatzaki, C.; Efstathiou, A. Malaria vaccines: From the past towards the mRNA vaccine era. Vaccines 2023, 11, 1452. [Google Scholar] [CrossRef]
- Zarocostas, J. Gavi unveils malaria vaccine plans. Lancet 2023, 401, 1485. [Google Scholar] [CrossRef]
- Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Gupta, R.P.; Singh, A.K.; Gautam, V.; Kumar, R. Advancements and challenges in developing malaria vaccines: Targeting multiple stages of the parasite life cycle. ACS Infect. Dis. 2023, 9, 1795–1814. [Google Scholar] [CrossRef] [PubMed]
- Calle, C.L.; Mordmüller, B.; Singh, A. Immunosuppression in malaria: Do Plasmodium falciparum parasites hijack the host? Pathogens 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Adjei, N.; Portugal, S.; Tran, T.M.; Yazew, T.B.; Skinner, J.; Li, S.; Jain, A.; Felgner, P.L.; Doumbo, O.K.; Kayentao, K.; et al. Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep. 2015, 13, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Ruybal-Pesántez, S.; Tiedje, K.E.; Pilosof, S.; Tonkin-Hill, G.; He, Q.; Rask, T.S.; Amenga-Etego, L.; Oduro, A.R.; Koram, K.A.; Pascual, M.; et al. Age-specific patterns of DBLα var diversity can explain why residents of high malaria transmission areas remain susceptible to Plasmodium falciparum blood stage infection throughout life. Int. J. Parasitol. 2022, 52, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ogutu, B.R.; Apollo, O.J.; McKinney, D.; Okoth, W.; Siangla, J.; Dubovsky, F.; Tucker, K.; Waitumbi, J.N.; Diggs, C.; Wittes, J.; et al. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 2009, 4, e4708. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.D.; Sagara, I.; Doumbo, O.; Wu, Y. Blood stage vaccines for Plasmodium falciparum: Current status and the way forward. Hum. Vaccines 2010, 6, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.D.; Tang, W.K.; Tolia, N.H. Blood-stage malaria parasite antigens: Structure, function, and vaccine potential. J. Mol. Biol. 2019, 431, 4259–4280. [Google Scholar] [CrossRef]
- Dassah, S.; Adu, B.; Sirima, S.B.; Mordmüller, B.; Ngoa, U.A.; Atuguba, F.; Arthur, F.K.N.; Mensah, B.A.; Kaddumukasa, M.; Bang, P.; et al. Extended follow-up of children in a phase2b trial of the GMZ2 malaria vaccine. Vaccines 2021, 39, 4314–4319. [Google Scholar] [CrossRef]
- Alves, K.C.S.; Guimarães, J.M.; Almeida, M.E.M.; Mariúba, L.A.M. Plasmodium falciparum merozoite surface protein 3 as a vaccine candidate: A brief review. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e23. [Google Scholar] [CrossRef]
- Patel, S.D.; Ahouidi, A.D.; Bei, A.K.; Dieye, T.N.; Mboup, S.; Harrison, S.C.; Duraisingh, M.T. Plasmodium falciparum merozoite surface antigen, PfRH5, elicits detectable levels of invasion-inhibiting antibodies in humans. J. Infect. Dis. 2013, 208, 1679–1687. [Google Scholar] [CrossRef]
- Minassian, A.M.; Silk, S.E.; Barrett, J.R.; Nielsen, C.M.; Miura, K.; Diouf, A.; Loos, C.; Fallon, J.K.; Michell, A.R.; White, M.T.; et al. Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination. Medicine 2021, 2, 701–719.e19. [Google Scholar] [CrossRef]
- Silk, S.E.; Kalinga, W.F.; Mtaka, I.M.; Lilolime, N.S.; Mpina, M.; Milando, F.; Ahmed, S.; Diouf, A.; Mkwepu, F.; Simon, B.; et al. Superior antibody immunogenicity of a viral-vectored RH5 blood-stage malaria vaccine in Tanzanian infants as compared to adults. Medicine 2023, 4, 668–686.e7. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, S.; O’Donnell, R.A.; Koussis, K.; Dluzewski, A.R.; Ansell, K.H.; Osborne, S.A.; Hackett, F.; Withers-Martinez, C.; Mitchell, G.H.; Bannister, L.H.; et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 2007, 131, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, R.; Kavishwar, M.; Withers-Martinez, C.; Hackett, F.; Collins, C.R.; Howell, S.A.; Yeoh, S.; Knuepfer, E.; Atid, A.J.; Holder, A.A.; et al. Plasmodium falciparum SERA5 plays a non-enzymatic role in the malarial asexual blood-stage lifecycle. Mol. Microbiol. 2015, 96, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Tougan, T.; Edula, J.R.; Takashima, E.; Morita, M.; Shinohara, M.; Shinohara, A.; Tsuboi, T.; Horii, T. Molecular camouflage of Plasmodium falciparum merozoites by binding of host vitronectin to P47 fragment of SERA5. Sci. Rep. 2018, 8, 5052. [Google Scholar] [CrossRef] [PubMed]
- Palacpac, N.M.Q.; Ishii, K.J.; Arisue, N.; Tougan, T.; Horii, T. Immune tolerance caused by repeated P. falciparum infection against SE36 malaria vaccine candidate antigen and the resulting limited polymorphism. Parasitol. Int. 2024, 99, 102845. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Palacpac, N.M.; Ito, K.; Oishi, Y.; Itagaki, S.; Balikagala, B.; Ntege, E.H.; Yeka, A.; Kanoi, B.N.; Katuro, O.; et al. Antibody titres and boosting after natural malaria infection in BK-SE36 vaccine responders during a follow-up study in Uganda. Sci. Rep. 2016, 6, 34363. [Google Scholar] [CrossRef] [PubMed]
- Tougan, T.; Aoshi, T.; Coban, C.; Katakai, Y.; Kai, C.; Yasutomi, Y.; Ishii, K.J.; Horii, T. TLR9 adjuvants enhance immunogenicity and protective efficacy of the SE36/AHG malaria vaccine in nonhuman primate models. Hum. Vaccines Immunother. 2013, 9, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bougouma, E.C.; Palacpac, N.M.Q.; Tiono, A.B.; Nebie, I.; Ouédraogo, A.; Houard, S.; Yagi, M.; Coulibaly, S.A.; Diarra, A.; Tougan, T.; et al. Safety and immunogenicity of BK-SE36 in a blinded, randomized, controlled, age de-escalating phase Ib clinical trial in Burkinabe children. Front. Immunol. 2022, 13, 978591. [Google Scholar] [CrossRef]
- Ezoe, S.; Palacpac, N.M.Q.; Tetsutani, K.; Yamamoto, K.; Okada, K.; Taira, M.; Nishida, S.; Hirata, H.; Ogata, A.; Yamada, T.; et al. First-in-human randomised trial and follow-up study of Plasmodium falciparum blood-stage malaria vaccine BK-SE36 with CpG-ODN(K3). Vaccine 2020, 38, 7246–7257. [Google Scholar] [CrossRef]
- Verthelyi, D.; Ishii, K.J.; Gursel, M.; Takeshita, F.; Klinman, D.M. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J. Immunol. 2001, 166, 2372–2377. [Google Scholar] [CrossRef]
- Verthelyi, D.; Kenney, R.T.; Seder, R.A.; Gam, A.A.; Friedag, B.; Klinman, D.M. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 2002, 168, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Verthelyi, D.; Klinman, D.M. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin. Immunol. 2003, 109, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, A.; Bougouma, E.C.; Palacpac, N.M.Q.; Houard, S.; Nebie, I.; Sawadogo, J.; Berges, G.D.; Soulama, I.; Diarra, A.; Hien, D.; et al. Safety and immunogenicity of BK-SE36/CpG malaria vaccine in healthy Burkinabe adults and children: A phase 1b randomised, controlled, double-blinded, age de-escalation trial. Front. Immunol. 2023, 14, 1267372. [Google Scholar] [CrossRef] [PubMed]
- Institut National de la Statistique et de la Démographie et ICF. Enquête Démographique et de Santé du Burkina Faso 2021 (2022). Ouagadougou, Burkina Faso et Rockville, Maryland, USA. Available online: https://dhsprogram.com/pubs/pdf/PR139/PR139.pdf (accessed on 2 February 2024).
- Issaka, Z.; Nagaonle, S.E.; Alassane, H.; Bosco, O.J. Seasonal malaria chemoprevention coverage in Burkina Faso in 2018: Descriptive analysis of mothers’ knowledge and attitude during cross sectional survey. J. Public Health Epidemiol. 2022, 14, 166–172. [Google Scholar] [CrossRef]
- Esen, M.; Kremsner, P.G.; Schleucher, R.; Gässler, M.; Imoukhuede, E.B.; Imbault, N.; Leroy, O.; Jepsen, S.; Knudsen, B.W.; Schumm, M.; et al. Safety and immunogenicity of GMZ2—A MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine 2009, 27, 6862–6868. [Google Scholar] [CrossRef]
- Mordmüller, B.; Szywon, K.; Greutelaers, B.; Esen, M.; Mewono, L.; Treut, C.; Mürbeth, R.E.; Chilengi, R.; Noor, R.; Kilama, W.L.; et al. Safety and immunogenicity of the malaria vaccine candidate GMZ2 in malaria-exposed, adult individuals from Lambaréné, Gabon. Vaccine 2010, 28, 6698–6703. [Google Scholar] [CrossRef] [PubMed]
- Bélard, S.; Issifou, S.; Hounkpatin, A.B.; Schaumburg, F.; Ngoa, U.A.; Esen, M.; Fendel, R.; de Salazar, P.M.; Mürbeth, R.E.; Milligan, P.; et al. A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PLoS ONE 2011, 6, e22525. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Mordmüller, B.; Milligan, P.; Ngoa, U.A.; Kironde, F.; Atuguba, F.; Tiono, A.B.; Issifou, S.; Kaddumukasa, M.; Bangre, O.; et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine 2016, 34, 4536–4542. [Google Scholar] [CrossRef]
- Ouedraogo, B.; Inoue, Y.; Kambiré, A.; Sallah, K.; Dieng, S.; Tine, R.; Rouamba, T.; Herbreteau, V.; Sawadogo, Y.; Ouedraogo, L.S.L.W.; et al. Spatio-temporal dynamic of malaria in Ouagadougou, Burkina Faso, 2011–2015. Malar. J. 2018, 17, 138. [Google Scholar] [CrossRef]
- Genton, B.; Al-Yaman, F.; Betuela, I.; Anders, R.F.; Saul, A.; Baea, K.; Mellombo, M.; Taraika, J.; Brown, G.V.; Pye, D.; et al. Safety and immunogenicity of a three-component blood-stage malaria vaccine (MSP1, MSP2, RESA) against Plasmodium falciparum in Papua New Guinean children. Vaccine 2003, 22, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hartgers, F.C.; Yazdanbakhsh, M. Co-infection of helminths and malaria: Modulation of the immune responses to malaria. Parasite Immunol. 2006, 28, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kinyanjui, S.M.; Conway, D.J.; Lanar, D.E.; Marsh, K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar. J. 2007, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Dobaño, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Tiono, A.B.; Palacpac, N.M.Q.; Bougouma, E.C.; Nebie, I.; Ouédraogo, A.; Houard, S.; Arisue, N.; D’Alessio, F.; Horii, T.; Sirima, S.B. Plasmodium falciparum infection coinciding with the malaria vaccine candidate BK-SE36 administration interferes with the immune responses in Burkinabe children. Front. Immunol. 2023, 14, 1119820. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef]
- Okada, H.; Takahashi, K.; Yaku, H.; Kobiyama, K.; Iwaisako, K.; Zhao, X.; Shiokawa, M.; Uza, N.; Kodama, Y.; Ishii, K.J.; et al. In situ vaccination using unique TLR9 ligand K3-SPG induces long-lasting systemic immune response and synergizes with systemic and local immunotherapy. Sci. Rep. 2022, 12, 2132. [Google Scholar] [CrossRef]
- Otsuka, T.; Nishida, S.; Shibahara, T.; Temizoz, B.; Hamaguchi, M.; Shiroyama, T.; Kimura, K.; Miyake, K.; Hirata, H.; Mizuno, Y.; et al. CpG ODN (K3)-toll-like receptor 9 agonist-induces Th1-type immune response and enhances cytotoxic activity in advanced lung cancer patients: A phase I study. BMC Cancer 2022, 22, 744. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef]
- Beeson, J.G.; Kurtovic, L.; Valim, C.; Asante, K.P.; Boyle, M.J.; Mathanga, D.; Dobano, C.; Moncunill, G. The RTS,S malaria vaccine: Current impact and foundation for the future. Sci. Transl. Med. 2022, 14, eabo6646. [Google Scholar] [CrossRef]



| D0 * | Y3 | |||
|---|---|---|---|---|
| BK-SE36/CpG | Control | BK-SE36/CpG | Control | |
| N = 30 | N = 15 | N = 29 | N = 15 | |
| Mean age (SD) | 7.4 (1.1) | 8.1 (1.3) | 10.6 (1.1) | 11.3 (1.3) |
| Sex (male/female, % male) | 15/30 (50) | 8/15 (53) | 15/29 (52) | 8/15 (53) |
| Proportion of participants with malaria positive slides, n/N (%) | 1/30 (3) | 1/15 (7) | 0/29 | 0/15 |
| Reported use of treated bed net, n/N (%) | ||||
| Last night | 15/29 (52) | 9/15 (60) | ||
| Last 3 months | 20/29 (69) | 10/15 (67) | ||
| Reported use of aerosol/repellent, n/N (%) | ||||
| Last night | 1/29 (3) | 0/15 | ||
| Last 3 months | 4/29 (14) | 3/15 (20) | ||
| Reported use of mosquito coil, n/N (%) | ||||
| Last night | 11/29 (38) | 3/15 (20) | ||
| Last 3 months | 14/29 (48) | 7/15 (47) | ||
| Reported burning of herbs for malaria control, n/N (%) | ||||
| Last night | 1/29 (3) | 0/15 | ||
| Last 3 months | 1/29 (3) | 0/15 | ||
| Reported use of prophylactic medicine, n/N (%) | ||||
| Last night | 0/29 | 0/15 | ||
| Last 3 months | 0/29 | 0/15 | ||
| Timepoints | BK-SE36/CpG n/N (%) | Control n/N (%) | p-Value * |
|---|---|---|---|
| D0 (before Dose 1) | 15 (50) | 9 (60) | |
| D365 (1 year after Dose 1) | 30 (100) | 7 (47) | <0.001 |
| Y3 (3 years after Dose 1) | 24 (83) | 7 (47) | 0.039 |
| SE36 Antibody Titres at Day 0 (prior to Dose 1) | ||||
|---|---|---|---|---|
| Value = 8 * | Value > 8 | Total | ||
| Timepoints | N = 15 | N = 15 | N = 30 | p-Value ** |
| Day 140 vs. Day 0 | ||||
| Geometric mean titre (95% CI), D140 | 2070 (1268, 3378) | 2405 (1789, 3233) | 2231 (1704, 2922) | 0.9674 |
| Geometric mean fold change in titre (95% CI) | 258.7 (158.5, 422.3) | 64.5 (43.4, 95.8) | 129.2 (86.9, 191.9) | 0.0002 |
| Day 365 vs. Day 0 | ||||
| Geometric mean titre (95% CI), D365 | 231.1 (143.9, 370.9) | 300.7 (168.2, 537.6) | 263.6 (184.9, 375.8) | 0.6827 |
| Geometric mean fold change in titre (95% CI) | 28.9 (18.0, 46.4) | 8.1 (4.8, 13.5) | 15.3 (10.2, 22.9) | 0.0007 |
| N = 15 | N = 14 | N = 29 | ||
| Year 3 vs. Day 0 | ||||
| Geometric mean titre (95% CI), Y3 | 59.5 (30.4, 116.4) | 119.2 (45.8, 310.7) | 83.2 (47.7, 145.1) | 0.2690 |
| Geometric mean fold change in titre (95% CI) | 7.4 (3.8, 14.6) | 3.2 (1.3, 8.2) | 5.0 (2.8, 8.6) | 0.1223 |
| Sex | ||||
|---|---|---|---|---|
| Male (95% CI) | Female (95% CI) | Total | ||
| Timepoints | N = 15 | N = 15 | N = 30 | p-Value * |
| D0 (before Dose 1) | ||||
| Geometric mean titre (95% CI) | 16.1 (9.5, 27.1) | 18.6 (11.7, 29.6) | 17.3 (12.4, 24) | 0.7261 |
| Proportion of subjects with detectable titres | 40% | 60% | 50% | |
| D140 (28 days after Dose 3) | ||||
| Geometric mean titre (95% CI) | 2293 (1342, 3918) | 2171 (1762, 2676) | 2231 (1704, 2922) | 0.5393 |
| Geometric mean fold change from D0 (95% CI) | 142.7 (73.6, 276.5) | 116.9 (69.6, 196.6) | 129.2 (86.9, 191.9) | 0.6827 |
| Proportion of subjects with detectable titres | 100% | 100% | 100% | |
| D365 (1 year after Dose 1) | ||||
| Geometric mean titre (95% CI) | 320.1 (187.4, 546.8) | 217.1 (130.2, 361.9) | 263.6 (184.9, 375.8) | 0.1607 |
| Geometric mean fold change from D0 (95% CI) | 19.9 (10.0, 39.8) | 11.7 (7.2, 18.9) | 15.3 (10.2, 22.9) | 0.1736 |
| Proportion of subjects with detectable titres | 100% | 100% | 100% | |
| N = 15 | N = 14 | N = 29 | ||
| Y3 (3 years after Dose 1) | ||||
| Geometric mean titre (95% CI) | 94.7 (41.6, 215.7) | 72.4 (30.9, 170.1) | 83.2 (47.7, 145.1) | 0.8801 |
| Geometric mean fold change from D0 (95% CI) | 5.9 (2.5, 14.1) | 4.1 (1.9, 9.1) | 5.0 (2.8, 8.6) | 0.5611 |
| Proportion of subjects with detectable titres | 87% | 79% | 83% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebie, I.; Palacpac, N.M.Q.; Bougouma, E.C.; Diarra, A.; Ouédraogo, A.; D’Alessio, F.; Houard, S.; Tiono, A.B.; Cousens, S.; Horii, T.; et al. Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5–10-Year-Old Burkinabe Children Naturally Exposed to Malaria. Vaccines 2024, 12, 166. https://doi.org/10.3390/vaccines12020166
Nebie I, Palacpac NMQ, Bougouma EC, Diarra A, Ouédraogo A, D’Alessio F, Houard S, Tiono AB, Cousens S, Horii T, et al. Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5–10-Year-Old Burkinabe Children Naturally Exposed to Malaria. Vaccines. 2024; 12(2):166. https://doi.org/10.3390/vaccines12020166
Chicago/Turabian StyleNebie, Issa, Nirianne Marie Q. Palacpac, Edith Christiane Bougouma, Amidou Diarra, Alphonse Ouédraogo, Flavia D’Alessio, Sophie Houard, Alfred B. Tiono, Simon Cousens, Toshihiro Horii, and et al. 2024. "Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5–10-Year-Old Burkinabe Children Naturally Exposed to Malaria" Vaccines 12, no. 2: 166. https://doi.org/10.3390/vaccines12020166
APA StyleNebie, I., Palacpac, N. M. Q., Bougouma, E. C., Diarra, A., Ouédraogo, A., D’Alessio, F., Houard, S., Tiono, A. B., Cousens, S., Horii, T., & Sirima, S. B. (2024). Persistence of Anti-SE36 Antibodies Induced by the Malaria Vaccine Candidate BK-SE36/CpG in 5–10-Year-Old Burkinabe Children Naturally Exposed to Malaria. Vaccines, 12(2), 166. https://doi.org/10.3390/vaccines12020166