Intradermal Inoculation of Inactivated Foot-and-Mouth Disease Vaccine Induced Effective Immune Responses Comparable to Conventional Intramuscular Injection in Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Intradermal Jet Injector and Vaccine Information
2.2. Animals and Experimental Design
2.3. General Observation and Sample Preparation
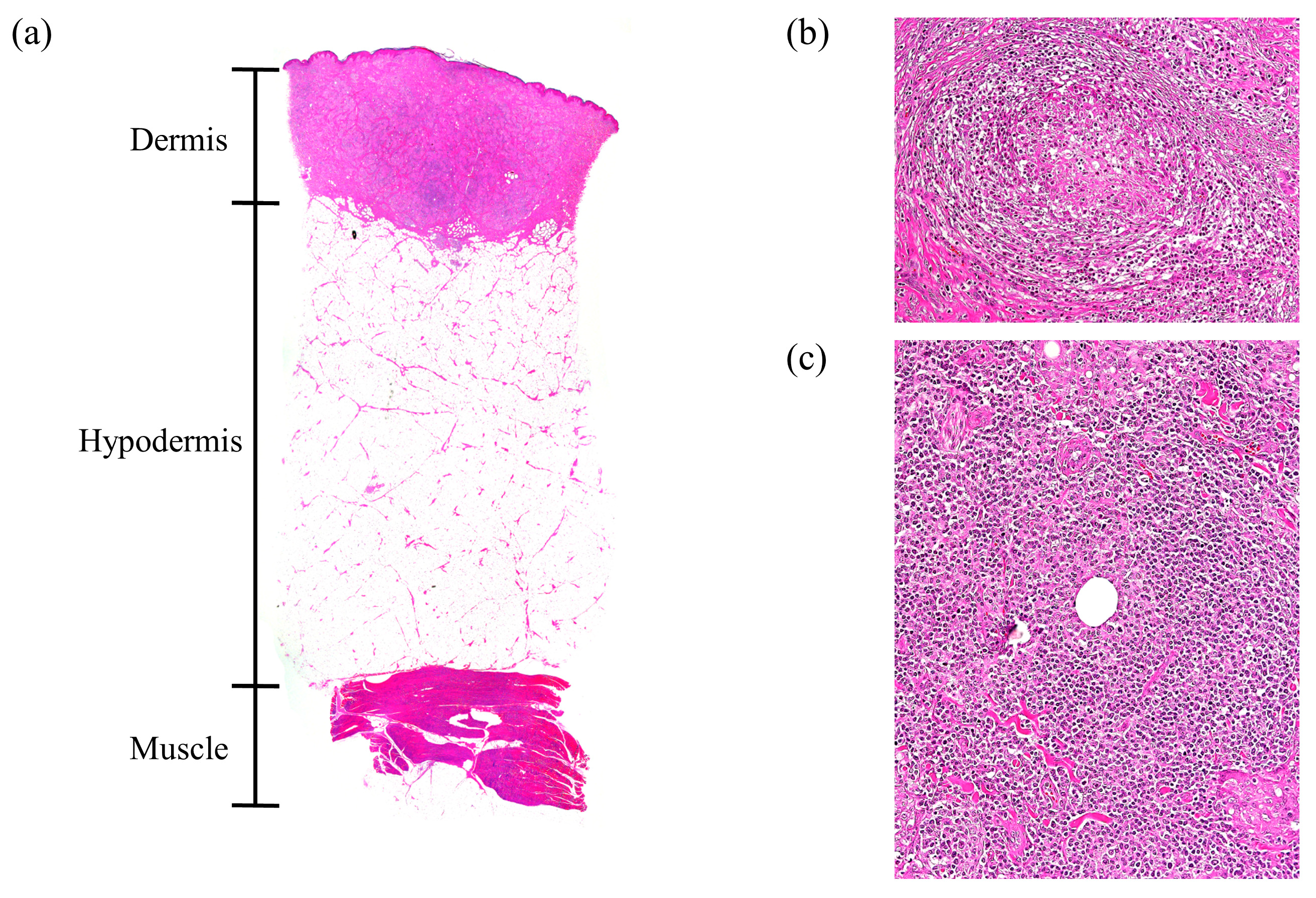
2.4. Histopathology
2.5. ELISA and Virus Neutralizing Test
2.6. Animal Experiment with Conventional Pigs
2.7. Data Analysis and Statistical Methods
3. Results
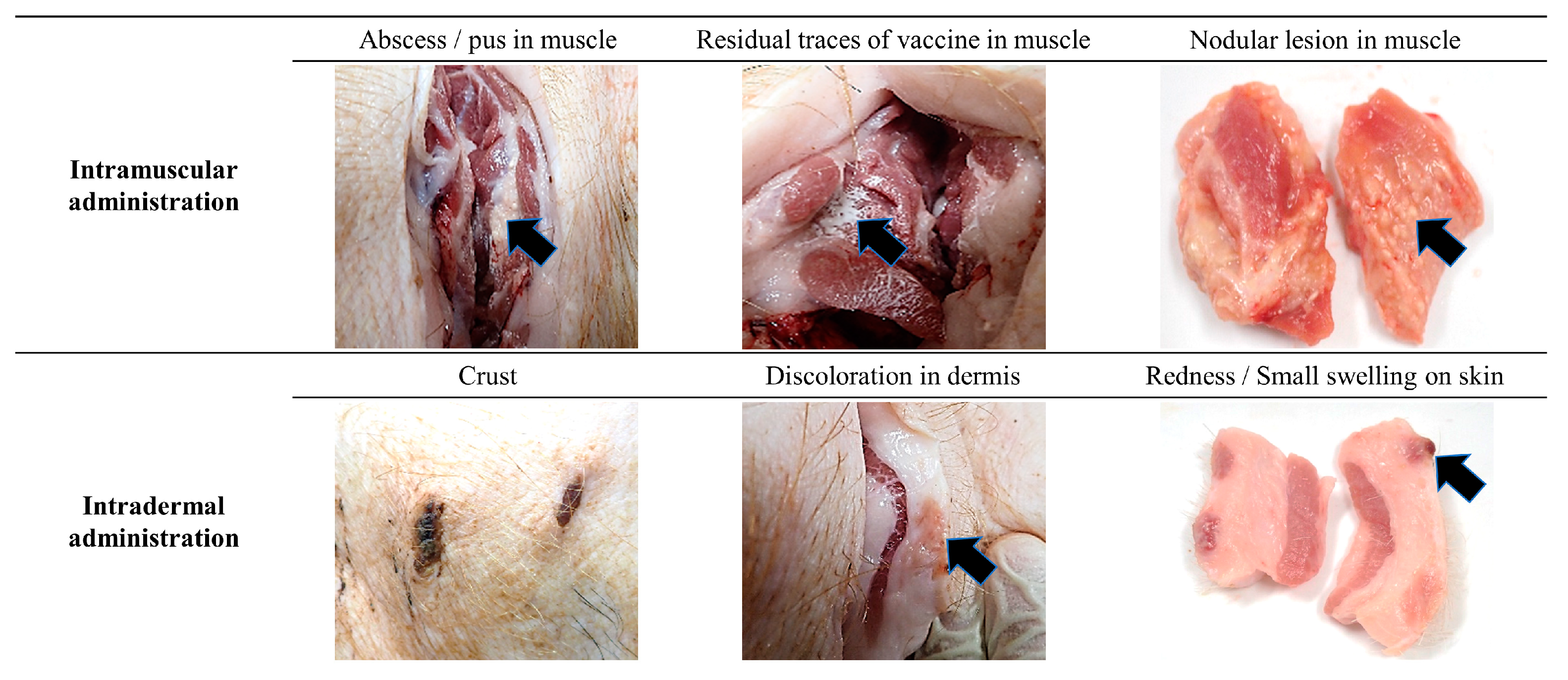
3.1. General Observation
3.2. Local Injection Site Reaction (ISR) Score
3.3. Histopathology
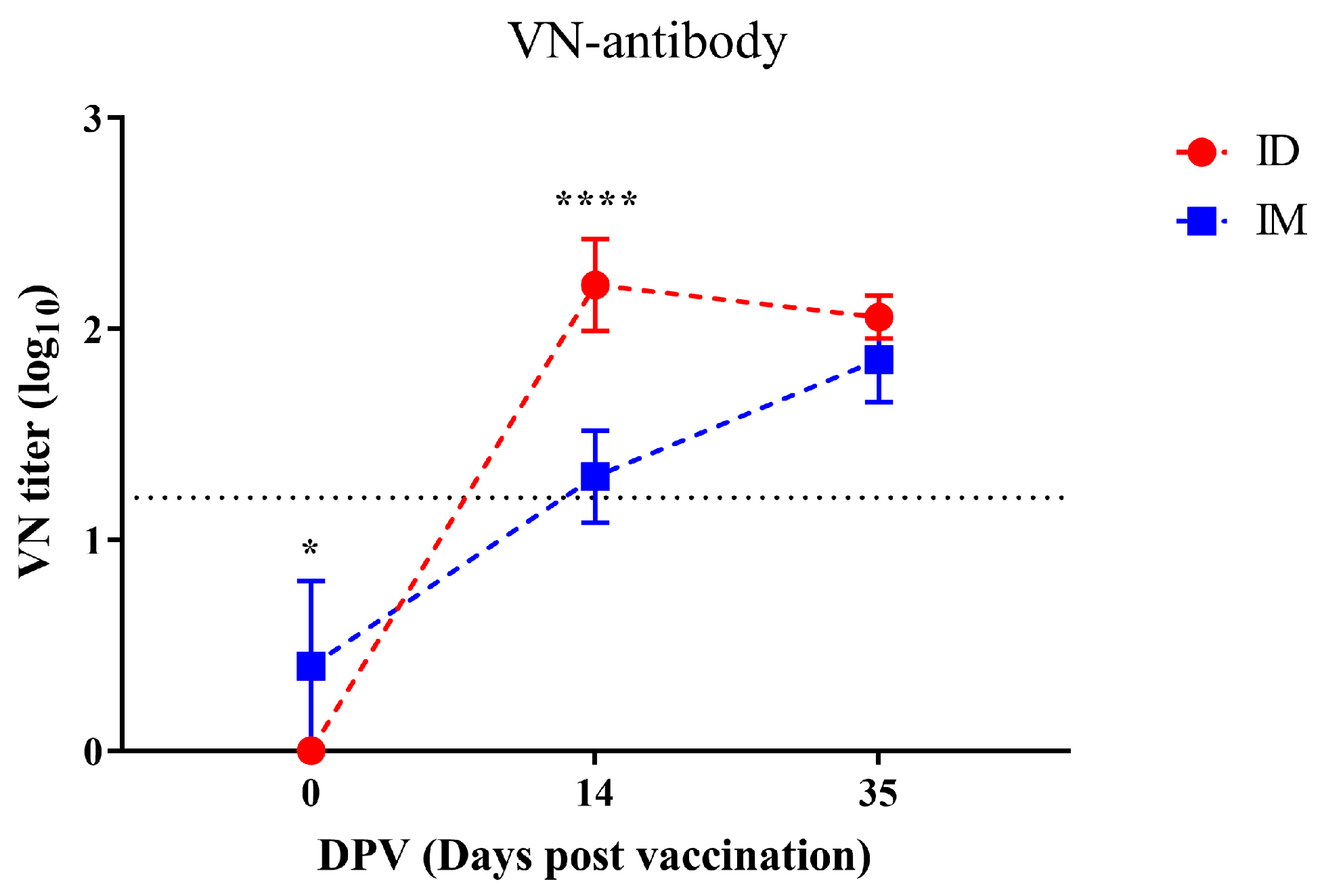
3.4. FMDV-Specific Antibody and Neutralizing Antibody Induction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz-San Segundo, F.; Medina, G.N.; Stenfeldt, C.; Arzt, J.; de Los Santos, T. Foot-and-Mouth Disease Vaccines. Vet. Microbiol. 2017, 206, 102–112. [Google Scholar] [CrossRef]
- Li, P.; Bai, X.; Sun, P.; Li, D.; Lu, Z.; Cao, Y.; Fu, Y.; Bao, H.; Chen, Y.; Xie, B.; et al. Evaluation of a Genetically Modified Foot-and-Mouth Disease Virus Vaccine Candidate Generated by Reverse Genetics. BMC Vet. Res. 2012, 8, 57. [Google Scholar] [CrossRef]
- Arzt, J.; Juleff, N.; Zhang, Z.; Rodriguez, L.L. The Pathogenesis of Foot-and-Mouth Disease I: Viral Pathways in Cattle. Transbound. Emerg. Dis. 2011, 58, 291–304. [Google Scholar] [CrossRef]
- Gim, U.-S.; Choi, S.-H.; Cho, J.-H. An Impact Analysis of FMD News on Pork Demand in Korea. Korean J. Community Living Sci. 2015, 26, 75–85. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, K.-N.; Ko, Y.-J.; Kim, S.-M.; Lee, H.-S.; Shin, Y.-K.; Sohn, H.-J.; Park, J.-Y.; Yeh, J.-Y.; Lee, Y.-H.; et al. Control of Foot-and-Mouth Disease during 2010-2011 Epidemic, South Korea. Emerg. Infect. Dis. 2013, 19, 655–659. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Lee, K.-N.; Kim, S.-M.; Lee, G.; Moon, Y.; Kim, B.; Lee, J.-S.; Park, J.-H. Needleless Intradermal Vaccination for Foot-and-Mouth Disease Induced Granuloma-Free Effective Protection in Pigs. J. Vet. Sci. 2019, 20, e29. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Portuondo, D.; Carlos, I.Z.; Pérez, O. An Approach to Local Immunotoxicity Induced by Adjuvanted Vaccines. Int. Immunopharmacol. 2013, 17, 526–536. [Google Scholar] [CrossRef]
- Ko, E.Y.; Jung, S.; Jeong, H.K.; Han, J.H.; Son, J.H. Effects of Foot-and-Mouth Disease Vaccination Location and Injection Device on the Incidence of Site Lesions in Pork. Korean J. Food Sci. Anim. Resour. 2018, 38, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Animal and Plant Quarantine Agency. Field Study of FMD Vaccination Using Needless Transdermal Device; Ministry of Agriculture, Food and Rural Affairs (MAFRA): Seojong, Republic of Korea, 2016; Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO202000030953 (accessed on 2 February 2023).
- Choi, S.-H.; Pak, S.-I. Economic Burden of Foot-and-Mouth Disease Vaccination-Induced Injection Site Lesions in Slaughtered Pigs and Its Causal Relationship. J. Prev. Vet. Med. 2015, 39, 153–156. [Google Scholar] [CrossRef]
- Lee, S.-I.; Jeong, C.-G.; Ul Salam Mattoo, S.; Nazki, S.; Prasad Aganja, R.; Kim, S.-C.; Khatun, A.; Oh, Y.; Noh, S.-H.; Lee, S.-M.; et al. Protective Immunity Induced by Concurrent Intradermal Injection of Porcine Circovirus Type 2 and Mycoplasma Hyopneumoniae Inactivated Vaccines in Pigs. Vaccine 2021, 39, 6691–6699. [Google Scholar] [CrossRef] [PubMed]
- Galdo Novo, S.; Malirat, V.; Maradei, E.D.; Pedemonte, A.R.; Espinoza, A.M.; Smitsaart, E.; Lee, K.N.; Park, J.H.; Bergmann, I.E. Efficacy of a High Quality O1/Campos Foot-and-Mouth Disease Vaccine upon Challenge with a Heterologous Korean O Mya98 Lineage Virus in Pigs. Vaccine 2018, 36, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.; Naderer, L.; Beffort, L.; Ritzmann, M.; Emrich, D.; Hermanns, W.; Fiebig, K.; Saalmüller, A.; Gerner, W.; Glatthaar-Saalmüller, B.; et al. Safety and Immune Responses after Intradermal Application of Porcilis PRRS in Either the Neck or the Perianal Region. PLoS ONE 2018, 13, e0203560. [Google Scholar] [CrossRef] [PubMed]
- Madapong, A.; Saeng-Chuto, K.; Chaikhumwang, P.; Tantituvanont, A.; Saardrak, K.; Pedrazuela Sanz, R.; Miranda Alvarez, J.; Nilubol, D. Immune Response and Protective Efficacy of Intramuscular and Intradermal Vaccination with Porcine Reproductive and Respiratory Syndrome Virus 1 (PRRSV-1) Modified Live Vaccine against Highly Pathogenic PRRSV-2 (HP-PRRSV-2) Challenge, Either Alone or in Combination with of PRRSV-1. Vet. Microbiol. 2020, 244, 108655. [Google Scholar] [CrossRef]
- Temple, D.; Escribano, D.; Jiménez, M.; Mainau, E.; Cerón, J.J.; Manteca, X. Effect of the Needle-Free “Intra Dermal Application of Liquids” Vaccination on the Welfare of Pregnant Sows. Porc. Health Manag. 2017, 3, 9. [Google Scholar] [CrossRef]
- Pandya, M.; Pacheco, J.M.; Bishop, E.; Kenney, M.; Milward, F.; Doel, T.; Golde, W.T. An Alternate Delivery System Improves Vaccine Performance against Foot-and-Mouth Disease Virus (FMDV). Vaccine 2012, 30, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Kim, Y.M.; Shi, H.; Le Cour Grandmaison, J.; Blanchard, A.; Kim, I.H. Standardized Plant Extract Alleviates the Negative Effects of FMD Vaccination on Animal Performance. Animals 2020, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Valtulini, S.; Macchi, C.; Ballanti, P.; Cherel, Y.; Laval, A.; Theaker, J.M.; Bak, M.; Ferretti, E.; Morvan, H. Aluminium Hydroxide-Induced Granulomas in Pigs. Vaccine 2005, 23, 3999–4004. [Google Scholar] [CrossRef]
- Ackermann, M.R. Inflammation and Healing. In Pathologic Basis of Veterinary Disease; Zachary, J.F., Ed.; Elsevier: Frisco, CO, USA, 2017; pp. 73–131. ISBN 978-0-323-35775-3. [Google Scholar]
- Eblé, P.L.; Weerdmeester, K.; van Hemert-Kluitenberg, F.; Dekker, A. Intradermal vaccination of pigs against FMD with 1/10 dose results in comparable vaccine efficacy as intramuscular vaccination with a full dose. Vaccine 2009, 27, 1272–1278. [Google Scholar] [CrossRef]
- Schnyder, J.L.; De Pijper, C.A.; Garcia Garrido, H.M.; Daams, J.G.; Goorhuis, A.; Stijnis, C.; Schaumburg, F.; Grobusch, M.P. Fractional Dose of Intradermal Compared to Intramuscular and Subcutaneous Vaccination—A Systematic Review and Meta-Analysis. Travel Med. Infect. Dis. 2020, 37, 101868. [Google Scholar] [CrossRef]
- Ludi, A.B.; Morris, A.; Gubbins, S.; Asfor, A.; Afzal, M.; Browning, C.F.; Grazioli, S.; Foglia, E.A.; Wilsden, G.; Burman, A.; et al. Cross-Serotype Reactivity of ELISAs Used to Detect Antibodies to the Structural Proteins of Foot-and-Mouth Disease Virus. Viruses 2022, 14, 1495. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Park, S.H.; Ko, M.-K.; Kim, S.-M.; Kim, B.; Park, J.-H. Advanced Foot-And-Mouth Disease Vaccine Platform for Stimulation of Simultaneous Cellular and Humoral Immune Responses. Vaccines 2020, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-E.; You, S.-H.; Lee, S.-Y.; Lee, K.-N.; Ko, M.-K.; Choi, J.-H.; Kim, B.; Lee, J.-S.; Park, J.-H. Immune Responses in Pigs and Cattle Vaccinated with Half-Volume Foot-and-Mouth Disease Vaccine. J. Vet. Sci. 2017, 18, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, B.; Liard, C. Transcutaneous and Intradermal Vaccination. Hum. Vaccin. 2011, 7, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Habibe, I.; Celis-Giraldo, C.; Patarroyo, M.E.; Avendaño, C.; Patarroyo, M.A. A Comprehensive Review of the Immunological Response against Foot-and-Mouth Disease Virus Infection and Its Evasion Mechanisms. Vaccines 2020, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Jain, B. Antiviral Immunity Evoked Post Foot-and-Mouth Disease Virus (FMDV) Infection and Vaccination. J. Antivir. Antiretrovir. 2019, 11, 185. [Google Scholar] [CrossRef][Green Version]
- Nagendrakumar, S.B.; Srinivasan, V.A.; Madhanmohan, M.; Yuvaraj, S.; Parida, S.; Di Nardo, A.; Horsington, J.; Paton, D.J. Evaluation of Cross-Protection between O1 Manisa and O1 Campos in Cattle Vaccinated with Foot-and-Mouth Disease Virus Vaccine Incorporating Different Payloads of Inactivated O1 Manisa Antigen. Vaccine 2011, 29, 1906–1912. [Google Scholar] [CrossRef]
- Choi, J.-H.; You, S.-H.; Ko, M.-K.; Jo, H.E.; Shin, S.H.; Jo, H.; Lee, M.J.; Kim, S.-M.; Kim, B.; Lee, J.-S.; et al. Improved Immune Responses and Safety of Foot-and-Mouth Disease Vaccine Containing Immunostimulating Components in Pigs. J. Vet. Sci. 2020, 21, e74. [Google Scholar] [CrossRef]
- Khorasani, A.; Madadgar, O.; Soleimanjahi, H.; Keyvanfar, H.; Mahravani, H. Evaluation of the Efficacy of a New Oil-Based Adjuvant ISA 61 VG FMD Vaccine as a Potential Vaccine for Cattle. Iran. J. Vet. Res. 2016, 17, 8–12. [Google Scholar]
- Richter, V.; Muche, R.; Mayer, B. How much confidence do we need in animal experiments? Statistical assumptions in sample size estimation. J. Appl. Anim. Welf Sci. 2018, 21, 325–333. [Google Scholar] [CrossRef]


| Group | No. | Redness | Size of Redness | Swelling | Pain during Palpation | Necrosis/Ulcer | Induration | Abscess/Pus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Mean ± SEM | Score | Mean ± SEM | Score | Mean ± SEM | Score | Mean ± SEM | Score | Mean ± SEM | Score | Mean ± SEM | Score | Mean ± SEM | ||
| IM | 1 | 0 | 0.33 ± 0.33 | 0 | 0.33 ± 0.33 | 0 | 0 | 1 | 0.67 ± 0.33 | 0 | 0 | 1 | 0.67 ± 0.33 | 2 | 2 ± 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | ||||||||
| 3 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | ||||||||
| ID | 1 | 0 | 0 | 0 | 0 | 1 | 0.33 ± 0.33 | 0 | 0 | 0 | 0 | 1 | 0.33 ± 0.33 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Mattoo, S.u.S.; Jeong, C.-G.; Kim, S.-C.; Nazki, S.; Lee, G.; Park, Y.-S.; Park, S.Y.; Yang, M.-S.; Kim, B.; et al. Intradermal Inoculation of Inactivated Foot-and-Mouth Disease Vaccine Induced Effective Immune Responses Comparable to Conventional Intramuscular Injection in Pigs. Vaccines 2024, 12, 190. https://doi.org/10.3390/vaccines12020190
Lee S, Mattoo SuS, Jeong C-G, Kim S-C, Nazki S, Lee G, Park Y-S, Park SY, Yang M-S, Kim B, et al. Intradermal Inoculation of Inactivated Foot-and-Mouth Disease Vaccine Induced Effective Immune Responses Comparable to Conventional Intramuscular Injection in Pigs. Vaccines. 2024; 12(2):190. https://doi.org/10.3390/vaccines12020190
Chicago/Turabian StyleLee, Simin, Sameer ul Salam Mattoo, Chang-Gi Jeong, Seung-Chai Kim, Salik Nazki, Gyehan Lee, Yong-Soo Park, Sun Young Park, Myeon-Sik Yang, Bumseok Kim, and et al. 2024. "Intradermal Inoculation of Inactivated Foot-and-Mouth Disease Vaccine Induced Effective Immune Responses Comparable to Conventional Intramuscular Injection in Pigs" Vaccines 12, no. 2: 190. https://doi.org/10.3390/vaccines12020190
APA StyleLee, S., Mattoo, S. u. S., Jeong, C.-G., Kim, S.-C., Nazki, S., Lee, G., Park, Y.-S., Park, S. Y., Yang, M.-S., Kim, B., Lee, S.-M., & Kim, W.-I. (2024). Intradermal Inoculation of Inactivated Foot-and-Mouth Disease Vaccine Induced Effective Immune Responses Comparable to Conventional Intramuscular Injection in Pigs. Vaccines, 12(2), 190. https://doi.org/10.3390/vaccines12020190







