Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review
Abstract
:1. Introduction
2. Case Report
3. Discussion
3.1. Rheumatic Fever (RF) and Myocarditis
3.2. Viral Carditis
3.3. Vaccination against COVID-19 and Myocarditis
3.4. Multisystem Inflammatory Syndrome (MIS)
3.5. Diagnostic Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tunuguntla, H.; Jeewa, A.; Denfield, S.W. Acute Myocarditis and Pericarditis in Children. Pediatr. Rev. 2019, 40, 14–25. [Google Scholar] [CrossRef]
- Durani, Y.; Giordano, K.; Goudie, B.W. Myocarditis and Pericarditis in Children. Pediatr. Clin. North Am. 2010, 57, 1281–1303. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Pieroni, M.; Rapezzi, C.; Olivotto, I. The Spectrum of Myocarditis: From Pathology to the Clinics. Virchows Arch. 2019, 475, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Maiese, A.; Frati, P.; Del Duca, F.; Santoro, P.; Manetti, A.C.; La Russa, R.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. Myocardial Pathology in COVID-19-Associated Cardiac Injury: A Systematic Review. Diagnostics 2021, 11, 1647. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Giannotta, G.; Murrone, A.; Giannotta, N. COVID-19 mRNA Vaccines: The Molecular Basis of Some Adverse Events. Vaccines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Oi, S.S.P.; Muniz, M.P.R.; Faria, I.M.; Filho, N.S.; de Brito, D.J.A.; Lages, J.S.; Lauande, L.P.; Oliveira, T.K.M.; de Araujo Cunha, K.; de Meneze Neves, P.D.M.; et al. Multisystemic Inflammatory Syndrome and Thrombotic Microangiopathy as Complications of COVID-19 in a Child: A Case Report. Front. Pediatr. 2021, 9, 659069. [Google Scholar] [CrossRef]
- Ouldali, N.; Bagheri, H.; Salvo, F.; Antona, D.; Pariente, A.; Leblanc, C.; Tebacher, M.; Micallef, J.; Levy, C.; Cohen, R.; et al. Hyper Inflammatory Syndrome Following COVID-19 mRNA Vaccine in Children: A National Post-Authorization Pharmacovigilance Study. Lancet Reg. Health Eur. 2022, 17, 100393. [Google Scholar] [CrossRef]
- Gill, J.R.; Tashjian, R.; Duncanson, E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch. Pathol. Lab. Med. 2022, 146, 925–929. [Google Scholar] [CrossRef]
- Hansen, T.; Titze, U.; Kulamadayil-Heidenreich, N.S.A.; Glombitza, S.; Tebbe, J.J.; Röcken, C.; Schulz, B.; Weise, M.; Wilkens, L. First Case of Postmortem Study in a Patient Vaccinated against SARS-CoV-2. Int. J. Infect. Dis. 2021, 107, 172–175. [Google Scholar] [CrossRef]
- Ilonze, O.J.; Guglin, M.E. Myocarditis Following COVID-19 Vaccination in Adolescents and Adults: A Cumulative Experience of 2021. Heart Fail. Rev. 2022, 27, 2033–2043. [Google Scholar] [CrossRef]
- De Faria Pereira, B.Á.; Belo, A.R.; Silva, N.A. da Febre reumática: Atualização dos critérios de Jones à luz da revisão da American Heart Association—2015. Rev. Bras. Reumatol. 2017, 57, 364–368. [Google Scholar] [CrossRef]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; Sable, C.; Steer, A.; Wilson, N.; Wyber, R.; et al. Acute Rheumatic Fever and Rheumatic Heart Disease. Nat. Rev. Dis. Prim. 2016, 2, 15084. [Google Scholar] [CrossRef]
- Cunningham, M.W. Rheumatic Fever, Autoimmunity and Molecular Mimicry: The Streptococcal Connection. Int. Rev. Immunol. 2014, 33, 314–329. [Google Scholar] [CrossRef]
- Dinkla, K.; Rohde, M.; Jansen, W.T.M.; Kaplan, E.L.; Chhatwal, G.S.; Talay, S.R. Rheumatic Fever-Associated Streptococcus Pyogenes Isolates Aggregate Collagen. J. Clin. Invest. 2003, 111, 1905–1912. [Google Scholar] [CrossRef]
- Roberts, S.; Kosanke, S.; Dunn, S.T.; Jankelow, D.; Duran, C.M.G.; Cunningham, M.W. Pathogenic Mechanisms in Rheumatic Carditis: Focus on Valvular Endothelium. J. Infect. Dis. 2001, 183, 507–511. [Google Scholar] [CrossRef]
- Tandon, R.; Sharma, M.; Chandrashekhar, Y.; Kotb, M.; Yacoub, M.H.; Narula, J. Revisiting the Pathogenesis of Rheumatic Fever and Carditis. Nat. Rev. Cardiol. 2013, 10, 171–177. [Google Scholar] [CrossRef]
- Spina, G.S.; Sampaio, R.O.; Branco, C.E.; Miranda, G.B.; Rosa, V.E.E.; Tarasoutchi, F. Incidental Histological Diagnosis of Acute Rheumatic Myocarditis: Case Report and Review of the Literature. Front. Pediatr. 2014, 2, 126. [Google Scholar] [CrossRef]
- Badorff, C.; Lee, G.H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral Protease 2A Cleaves Dystrophin: Evidence of Cytoskeletal Disruption in an Acquired Cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [CrossRef]
- Chen, C.; Fu, F.; Ding, L.; Fang, J.; Xiao, J. Booster Dose of COVID-19 mRNA Vaccine Does Not Increase Risks of Myocarditis and Pericarditis Compared with Primary Vaccination: New Insights from the Vaccine Adverse Event Reporting System. Front. Immunol. 2022, 13, 938322. [Google Scholar] [CrossRef]
- Almamlouk, R.; Kashour, T.; Obeidat, S.; Bois, M.C.; Maleszewski, J.J.; Omrani, O.A.; Tleyjeh, R.; Berbari, E.; Chakhachiro, Z.; Zein-Sabatto, B.; et al. COVID-19–Associated Cardiac Pathology at the Postmortem Evaluation: A Collaborative Systematic Review. Clin. Microbiol. Infect. 2022, 28, 1066–1075. [Google Scholar] [CrossRef]
- Mele, D.; Flamigni, F.; Rapezzi, C.; Ferrari, R. Myocarditis in COVID-19 Patients: Current Problems. Intern. Emerg. Med. 2021, 16, 1123–1129. [Google Scholar] [CrossRef]
- Freire, B.M.; de Melo, F.M.; Basso, A.S. Adrenergic Signaling Regulation of Macrophage Function: Do We Understand It Yet? Immunother. Adv. 2022, 2, ltac010. [Google Scholar] [CrossRef]
- Disruption of a Self-Amplifying Catecholamine Loop Reduces Cytokine Release Syndrome|Nature. Available online: https://www.nature.com/articles/s41586-018-0774-y (accessed on 18 September 2023).
- Yang, D.; Dai, X.; Xing, Y.; Tang, X.; Yang, G.; Wang, P.; Harrison, A.G.; Li, H.; Lv, X.; Yu, X.; et al. Intrinsic Cardiac Adrenergic Cells Contribute to Septic Cardiomyopathy. bioRxiv 2021, bioRxiv:2021.03.02.433552. [Google Scholar] [CrossRef]
- Le Vu, S.; Bertrand, M.; Jabagi, M.-J.; Botton, J.; Drouin, J.; Baricault, B.; Weill, A.; Dray-Spira, R.; Zureik, M. Age and Sex-Specific Risks of Myocarditis and Pericarditis Following Covid-19 Messenger RNA Vaccines. Nat. Commun. 2022, 13, 3633. [Google Scholar] [CrossRef]
- Lane, S.; Yeomans, A.; Shakir, S. Reports of Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: A Systematic Review of Spontaneously Reported Data from the UK, Europe and the USA and of the Scientific Literature. BMJ Open 2022, 12, e059223. [Google Scholar] [CrossRef]
- Hulscher, N.; Hodkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Wong, H.-L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beachler, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of Myocarditis and Pericarditis after the COVID-19 mRNA Vaccination in the USA: A Cohort Study in Claims Databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef]
- SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents|Vaccination|JAMA Cardiology|JAMA Network. Available online: https://jamanetwork.com/journals/jamacardiology/fullarticle/2791253 (accessed on 18 September 2023).
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; Martinez de la Escalera, G.; Clapp, C. The Spike Protein of SARS-CoV-2 Induces Endothelial Inflammation through Integrin A5β1 and NF-κB Signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The Emerging Role of Toll-like Receptor 4 in Myocardial Inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Giannotta, G.; Giannotta, N. Post-Vaccination Inflammatory Syndrome: A New Syndrome. Clin. Case Rep. Rev. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Terpos, E.; Rosati, M.; Angel, M.; Bear, J.; Stellas, D.; Karaliota, S.; Apostolakou, F.; Bagratuni, T.; Patseas, D.; et al. Systemic IL-15, IFN-γ, and IP-10/CXCL10 Signature Associated with Effective Immune Response to SARS-CoV-2 in BNT162b2 mRNA Vaccine Recipients. Cell Rep. 2021, 36, 109504. [Google Scholar] [CrossRef]
- Patel, P.; DeCuir, J.; Abrams, J.; Campbell, A.P.; Godfred-Cato, S.; Belay, E.D. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults. JAMA Netw. Open 2021, 4, e2126456. [Google Scholar] [CrossRef]
- Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online: https://www.who.int/news-room/commentaries/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 23 September 2023).
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210–1212. [Google Scholar] [CrossRef]
- Wassif, M.; Lo, P.; Satouris, P.; Swan, L.; Tardo, D.; Kovacic, J.C.; Muller, D.; Muthiah, K.; Kotlyar, E.; Bart, N.K. Acute Myocarditis and Pericarditis After mRNA COVID-19 Vaccinations—A Single-Centre Retrospective Analysis. Heart Lung Circ. 2023, 32, 467–479. [Google Scholar] [CrossRef]

| Day 7 | Day 17 | Day 21 | Day 31 | |
|---|---|---|---|---|
| Hemoglobin (g/dL) | - | 9.24 | 9.5 | 9.87 |
| Hematocrit (%) | - | 28.1 | 26.7 | 28.3 |
| Leukocytes (cells/μL) | 170,000 | 14,600 | 15,000 | 28,900 |
| Segmented (%) | 76.0 | - | - | - |
| Neutrophils (%) | 76.9 | 59.2 | 59.6 | 64.1 |
| Lymphocytes (%) | - | 29 | 32.9 | 2.6 |
| Platelets (mm3) | - | 453,000 | 467,000 | 445,000 |
| OSA (U/mL) * | - | 4.1 | 1425.2 | |
| C-reactive protein (mg/dL) | - | - | 8.38 | 13.21 |
| PT/INR ** | - | - | 10.9/1.00 | |
| aTTP *** | - | - | 29.9 | |
| Urea (mg/dL) | - | - | 24.69 | 31.72 |
| Creatinine (mg/dL) | - | - | 0.74 | 0.41 |
| Sodiu (mmol/L) | - | - | 138 | - |
| Potassium (mol/L) | - | - | 4.7 | - |
| CPK (U/L) **** | - | - | - | 49.97 |
| Brain | Heart | Lungs | Liver | Rins | |
|---|---|---|---|---|---|
| Weight (g) | 1362 | 298 | Right: 404 Left: 304 | 1368 | Right: 116 Left: 134 |
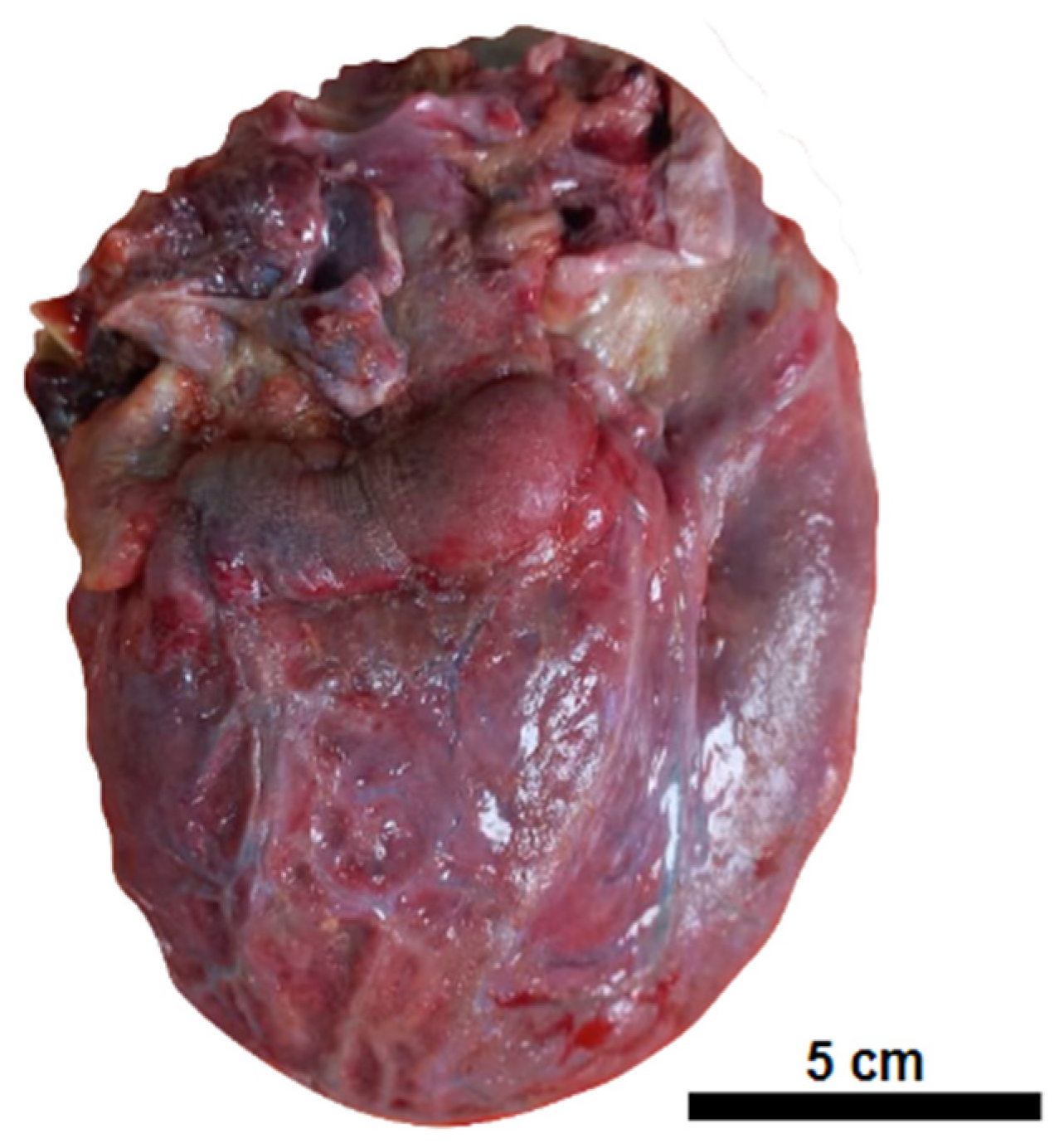
| Macroscopic findings | Edema and tonsil herniation. | Fibrinous perimyocarditis with hemorrhage. Valves unchanged | Edema and congestion. | Moderate steatosis hemorrhagic appearance | Signs of shock. |
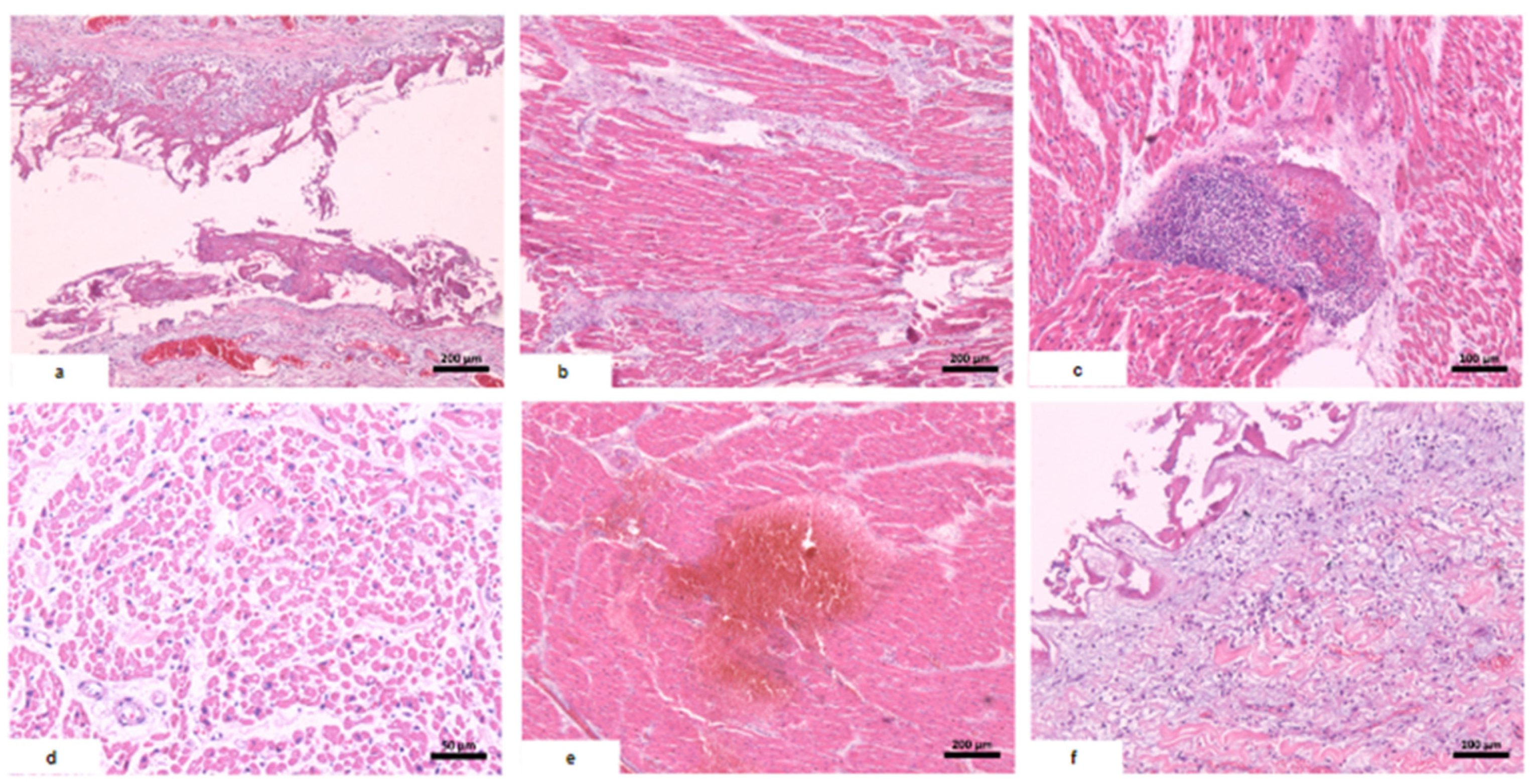
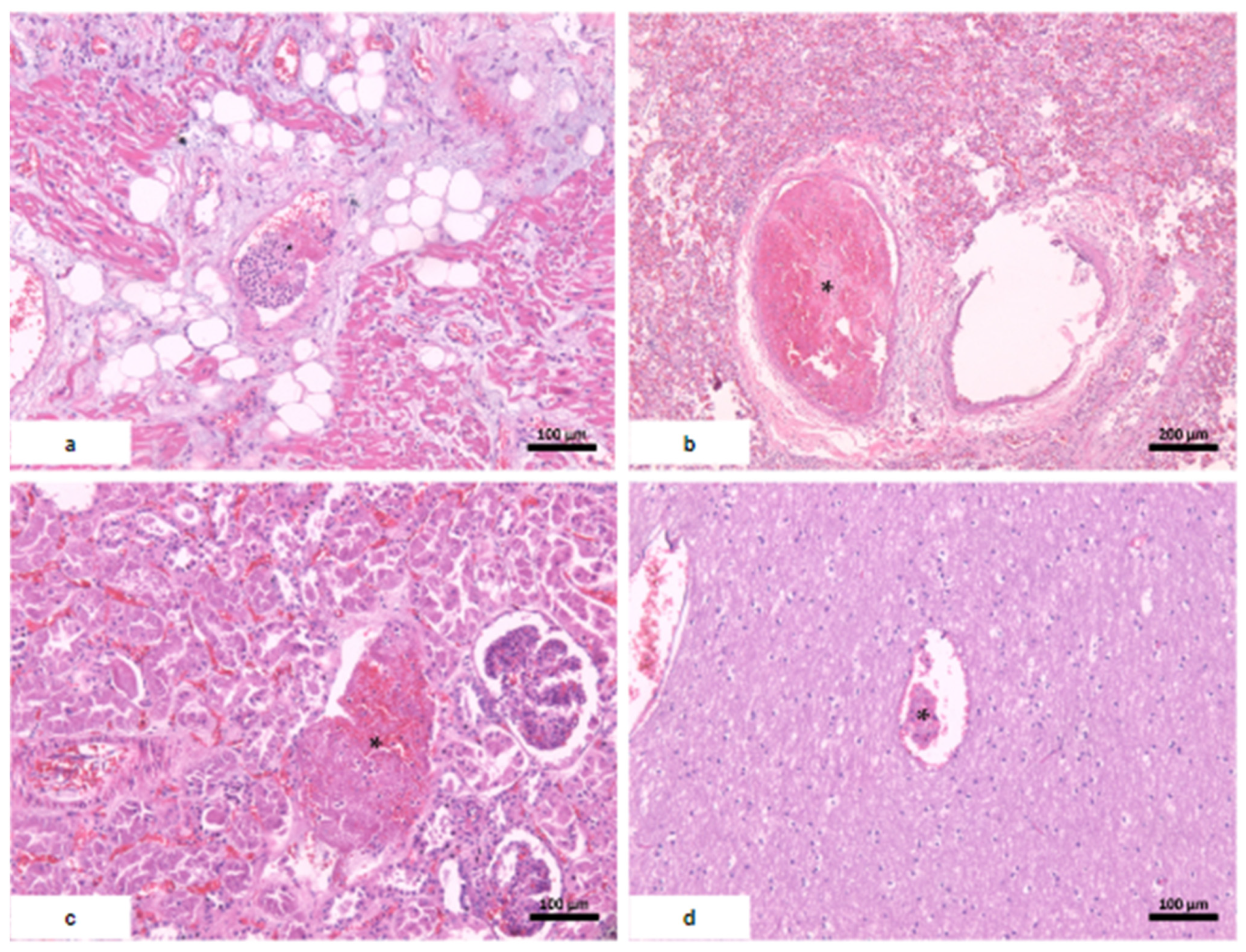
| Microscopic findings | Tissue edema. Reactive gliosis. Vascular thromboembolism. | Pericardium: intense deposition of fibrin, red blood cells, and inflammatory cells. Myocardium: interfascicular edema, coagulative myocyte necrosis. Hemorrhage and inflammatory infiltrate rich in macrophages and t-cells. Aschoff’s nodules and/or Anitschkow’s cells: not observed. Endocardium: slight loose fibrosis and inflammatory infiltrate. | Parenchymal hemorrhage. Vascular thromboembolism. Inflammatory infiltrate of lymphocytes, neutrophils, and xanthomatous macrophages. | Hemorrhagic necrosis. Vascular thromboembolism. Microvesicular steatosis. | Acute tubular necrosis. |
| Rheumatic Fever | COVID-19 | Multisystem Inflammatory Syndrome (MIS) | Post COVID-19 mRNAV | |
|---|---|---|---|---|
| Diagnostic criteria | Revised Jones criteria * | Presence of cardiac involvement (pericarditis, myocarditis, or valvulitis) during proven COVID-19 infection. | World Health Organization ** | Temporal relationship + exclusion of other causes. |
| Presentation | Valvulitis in 90% of cases. Myocardium and pericardium are occasionally affected. | Cardiomegaly, Variable clinic, cardiac dysfunction when associated with MIS. | Fever. Presence of multisystem involvement (gastrointestinal, central nervous system). Carditis in >80% of cases. | Favorable evolution when not associated with MIS. Predilection for young men. |
| Mechanism | Cross-inflammatory reaction centered on connective tissue. | Spike protein, endothelial activation, macrophage activation. | Lymphocytes and macrophages, neutrophils, and eosinophils possible. | Lymphocytes and macrophages, neutrophils, and eosinophils possible |
| Inflammatory infiltrate | Inflammatory infiltrate in scattered collagenous bands, with lymphocytes and macrophages. | Variable. Can be discrete and unorganized or in a pattern of myocarditis. | Not described | Lymphocytes and macrophages, neutrophils, and eosinophils possible. |
| Myocardial necrosis | Discrete, related to local inflammatory infiltrate. | Frequent. | -- | Present, focal. |
| Specific findings | Aschoff’s nodules in up to 67% of cases. | Absent | Absent. | Absent. |
| Vascular effects | Aortic involvement in 20% of cases of mitral valve disease. | Vasculitis and thromboembolism in the microvasculature. | Not reported. | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, P.M.B.d.; Silva, E.A.; Campos, M.A.G.; Lages, J.S.; Corrêa, R.d.G.C.F.; Silva, G.E.B. Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review. Vaccines 2024, 12, 194. https://doi.org/10.3390/vaccines12020194
Sousa PMBd, Silva EA, Campos MAG, Lages JS, Corrêa RdGCF, Silva GEB. Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review. Vaccines. 2024; 12(2):194. https://doi.org/10.3390/vaccines12020194
Chicago/Turabian StyleSousa, Pedro Manuel Barros de, Elon Almeida Silva, Marcos Adriano Garcia Campos, Joyce Santos Lages, Rita da Graça Carvalhal Frazão Corrêa, and Gyl Eanes Barros Silva. 2024. "Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review" Vaccines 12, no. 2: 194. https://doi.org/10.3390/vaccines12020194
APA StyleSousa, P. M. B. d., Silva, E. A., Campos, M. A. G., Lages, J. S., Corrêa, R. d. G. C. F., & Silva, G. E. B. (2024). Fatal Myocarditis following COVID-19 mRNA Immunization: A Case Report and Differential Diagnosis Review. Vaccines, 12(2), 194. https://doi.org/10.3390/vaccines12020194





