Herpes Zoster and Cardiovascular Disease: Exploring Associations and Preventive Measures through Vaccination
Abstract
1. Introduction
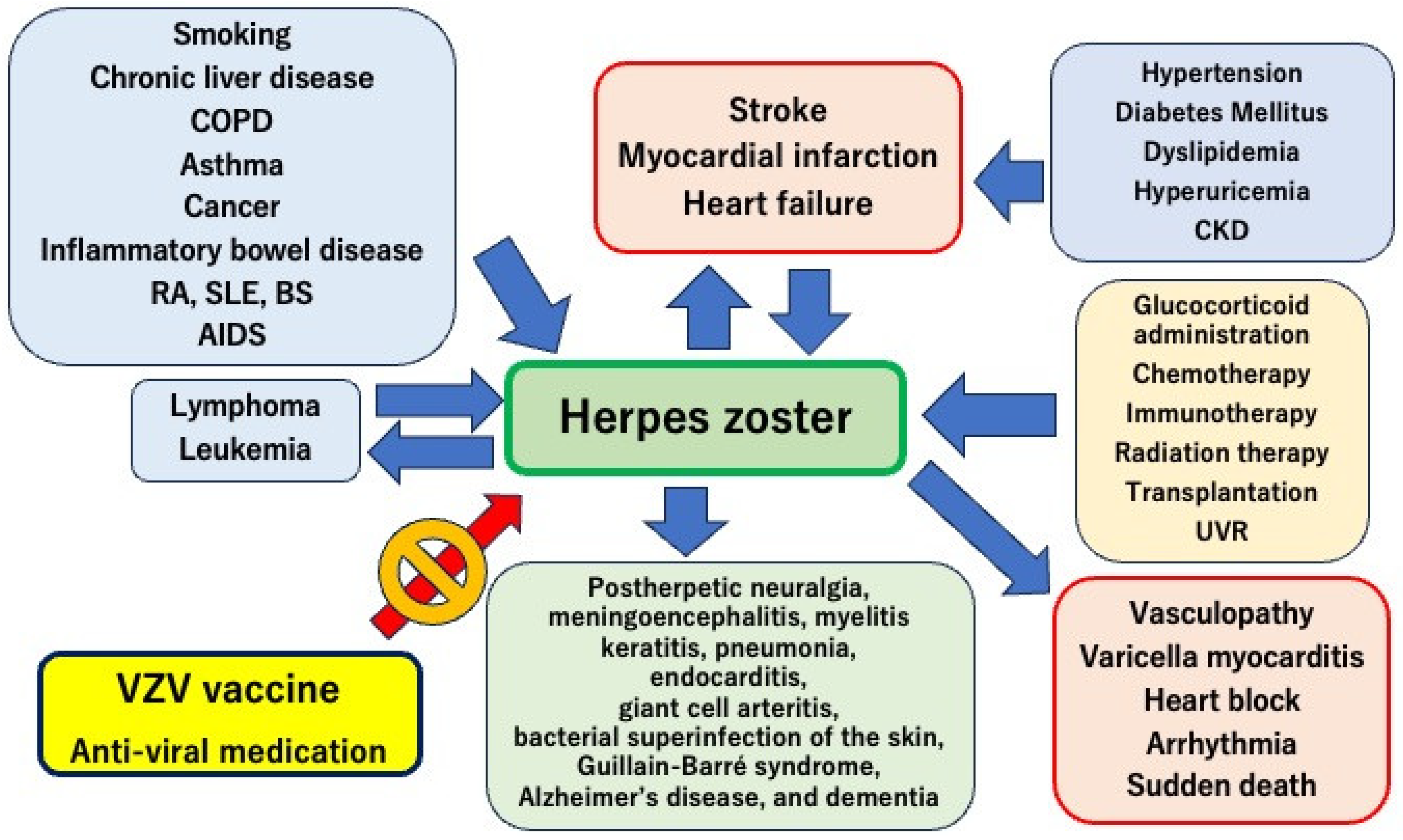
2. Risk Factors for Herpes Zoster
2.1. Literature Search Results for Cardiovascular Disease and Herpes Zoster
2.2. Cardiovascular Diseases as Risk Factors for Herpes Zoster
2.3. Diabetes
| Risks [Reference] | Results (Country; Herpes Zoster Cases) | Subgroups | HR (95% CI) |
|---|---|---|---|
| Cardiovascular diseases [22] | Cardiovascular diseases increase the risk of herpes zoster hospitalization (Korea; 20,311) | Myocardial infarction | 1.625 (1.144–2.308) |
| Stroke | 1.518 (1.177–1.957) | ||
| Heart failure | 1.485 (1.041–2.117) | ||
| Rheumatoid arthritis [27] | RA increases the risk of herpes zoster (USA and UK; 1611) | USA | 1.91 (1.80–2.03) |
| UK | 1.65 (1.57–1.75) | ||
| Rheumatic diseases [20] | Compared with RA, SLE and BS are stronger risk factors for herpes zoster (Korea; 1869) | SLE | 4.29 (3.49–5.27) |
| Behçet’s syndrome | 4.54 (3.66–5.64) | ||
| Infliximab | 2.92 (1.72–4.89) | ||
| Glucocorticoids | 2.91 (1.72–4.89) | ||
| Heart failure [21] | Heart failure (Taiwan; 211) | All | 2.07 (1.54–2.78) |
| Men | 2.30 (1.51–3.50) | ||
| Respiratory diseases [28] | COPD, asthma, and lung cancer are related to a high risk of contracting herpes zoster (Spain; 31,765) | COPD | 1.16 (1.13–1.19) |
| Asthma | 1.67 (1.63–1.71) | ||
| Lung cancer | 1.68 (1.60–1.76) | ||
| UVR [29] | There is a higher risk of herpes zoster in men, especially those with a history of severe sunburn (US; 24,201) | Men | 1.14 (1.02–1.29) |
| Higher lifetime number of severe sunburn | 1.13 (1.00–1.28) |
2.4. Respiratory Diseases
2.5. Malignancy
2.6. Immunocompromised Conditions
2.7. Rheumatoid Arthritis
2.8. Inflammatory Bowel Disease
2.9. Iatrogenic Risk Factors
2.10. Ultraviolet Radiation (UVR)
3. Postherpetic Complications
3.1. Neuralgia
3.2. Meningoencephalitis
3.3. Myelitis
3.4. Cranial Nerve Damage
3.5. Keratitis
3.6. Respiratory Complications
3.7. Subacute Endocarditis
3.8. Giant Cell Arteritis
3.9. Bacterial Superinfection of the Skin and Soft Tissue
3.10. Guillain–Barré Syndrome
3.11. Alzheimer’s Disease and Dementia
3.12. Subsequent Cancer Risk
4. Antiviral Treatment for Herpes Zoster
5. Prevention of Herpes Zoster and Its Complications
6. Recurrence Rate of Herpes Zoster
7. Herpes Virus-Induced Vasculopathy
7.1. Vasculopathy-Related Diseases
7.2. Vasculopathy-Related Signaling
7.3. Pathological Changes
8. Mechanisms of Herpes Zoster-Induced Cardiovascular Diseases
8.1. Inflammation
8.2. Autonomic Nervous System Dysfunction
8.3. Vasculopathy
9. Herpes Zoster-Related Cardiovascular Diseases
9.1. All Major Adverse Cardiac and Cerebrovascular Events (MACCEs)
9.2. Stroke
9.3. Myocardial Infarction
9.4. Coronary Heart Disease
9.5. Myocarditis
9.6. Heart Failure
9.7. Arrhythmia
10. Conclusions
Funding
Conflicts of Interest
References
- Gnann, J.W., Jr.; Whitley, R.J. Clinical practice. Herpes zoster. N. Engl. J. Med. 2002, 347, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Itzler, R.F.; Wollan, P.C.; Pellissier, J.M.; Sy, L.S.; Saddier, P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin. Proc. 2009, 84, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Huang, L.M.; Chang, I.S.; Tsai, F.Y.; Lu, C.Y.; Shao, P.L.; Chang, L.Y.; Varicella-Zoster Working Group, Advisory Committee on Immunization Practices, Taiwan. Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine 2010, 28, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Saddier, P.; Wollan, P.C.; St Sauver, J.L.; Kurland, M.J.; Sy, L.S. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin. Proc. 2007, 82, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef]
- Rimland, D.; Moanna, A. Increasing incidence of herpes zoster among Veterans. Clin. Infect. Dis. 2010, 50, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Yawn, B.P.; Wollan, P.; Harpaz, R. Increasing Incidence of Herpes Zoster Over a 60-year Period From a Population-based Study. Clin. Infect. Dis. 2016, 63, 221–226. [Google Scholar] [CrossRef]
- Schmader, K. Herpes zoster in older adults. Clin. Infect. Dis. 2001, 32, 1481–1486. [Google Scholar] [CrossRef]
- Weinberg, A.; Lazar, A.A.; Zerbe, G.O.; Hayward, A.R.; Chan, I.S.; Vessey, R.; Silber, J.L.; MacGregor, R.R.; Chan, K.; Gershon, A.A.; et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J. Infect. Dis. 2010, 201, 1024–1030. [Google Scholar] [CrossRef]
- Burke, B.L.; Steele, R.W.; Beard, O.W.; Wood, J.S.; Cain, T.D.; Marmer, D.J. Immune responses to varicella-zoster in the aged. Arch. Intern. Med. 1982, 142, 291–293. [Google Scholar] [CrossRef]
- Bahouth, M.N.; Venkatesan, A. Acute Viral Illnesses and Ischemic Stroke: Pathophysiological Considerations in the Era of the COVID-19 Pandemic. Stroke 2021, 52, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.H.; Kleinschmidt-DeMasters, B.K.; Wellish, M.; Hedley-Whyte, E.T.; Rentier, B.; Mahalingam, R. Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5-1995 revisited. Neurology 1996, 47, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Takao, Y.; Miyazaki, Y.; Okeda, M.; Onishi, F.; Yano, S.; Gomi, Y.; Ishikawa, T.; Okuno, Y.; Mori, Y.; Asada, H.; et al. Incidences of Herpes Zoster and Postherpetic Neuralgia in Japanese Adults Aged 50 Years and Older From a Community-based Prospective Cohort Study: The SHEZ Study. J. Epidemiol. 2015, 25, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E. The Spectrum of Neurological Manifestations of Varicella-Zoster Virus Reactivation. Viruses 2023, 15, 1663. [Google Scholar] [CrossRef]
- Wareham, D.W.; Breuer, J. Herpes zoster. BMJ 2007, 334, 1211–1215. [Google Scholar] [CrossRef]
- Tung, Y.C.; Tu, H.P.; Wu, M.K.; Kuo, K.L.; Su, Y.F.; Lu, Y.Y.; Lin, C.L.; Wu, C.H. Higher risk of herpes zoster in stroke patients. PLoS ONE 2020, 15, e0228409. [Google Scholar] [CrossRef]
- Thomas, S.L.; Hall, A.J. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect. Dis. 2004, 4, 26–33. [Google Scholar] [CrossRef]
- Horev, A.; Horev, A.; Gordon-Irshai, A.; Gordon, M.; Andre, N.; Ifergane, G. Herpes zoster and long-term vascular risk: A retrospective cohort study. Sci. Rep. 2023, 13, 2364. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nakano, K.; Tokutsu, K.; Nakayamada, S.; Matsuda, S.; Fushimi, K.; Tanaka, Y. Short-Term Prognostic Factors in Hospitalized Herpes Zoster Patients and Its Associated Cerebro-Cardiovascular Events: A Nationwide Retrospective Cohort in Japan. Front. Med. 2022, 9, 843809. [Google Scholar] [CrossRef]
- Ryu, H.J.; Han, J.O.; Lee, S.A.; Seo, M.R.; Choi, H.J.; Ko, K.P.; Baek, H.J. Risk factors for herpes zoster in patients with rheumatic diseases: A nationwide cohort study in Korea. Rheumatology 2021, 60, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Lin, Y.T.; Lin, C.Y.; Huang, M.Y.; Chang, W.C.; Chang, W.P. A nationwide population-based cohort study to identify the correlation between heart failure and the subsequent risk of herpes zoster. BMC Infect. Dis. 2015, 15, 17. [Google Scholar] [CrossRef]
- Seo, H.M.; Cha, M.J.; Han, J.H.; Han, K.; Park, S.H.; Bang, C.H.; Lee, J.H.; Lee, J.Y.; Choi, E.K.; Park, Y.M. Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in Korea. J. Dermatol. 2018, 45, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Heymann, A.D.; Chodick, G.; Karpati, T.; Kamer, L.; Kremer, E.; Green, M.S.; Kokia, E.; Shalev, V. Diabetes as a risk factor for herpes zoster infection: Results of a population-based study in Israel. Infection 2008, 36, 226–230. [Google Scholar] [CrossRef]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Ampudia-Blasco, F.J.; Diez-Domingo, J. Risk and impact of herpes zoster on patients with diabetes: A population-based study, 2009–2014. Hum. Vaccin. Immunother. 2017, 13, 2606–2611. [Google Scholar] [CrossRef]
- Lai, S.W.; Liu, C.S.; Kuo, Y.H.; Lin, C.L.; Hwang, B.F.; Liao, K.F. The incidence of herpes zoster in patients with diabetes mellitus: A meta-analysis of cohort studies. Medicine 2021, 100, e25292. [Google Scholar] [CrossRef] [PubMed]
- Poirrier, J.E.; Meyers, J.L.; Nagar, S.P.; Patterson, B.J.; Glasser, L.I.; Jabbour, S.A. Herpes Zoster Incidence and Burden in Adults With Type 2 Diabetes in the U.S.: A Retrospective Database Analysis. Diabetes Care 2022, 45, 2585–2593. [Google Scholar] [CrossRef]
- Smitten, A.L.; Choi, H.K.; Hochberg, M.C.; Suissa, S.; Simon, T.A.; Testa, M.A.; Chan, K.A. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007, 57, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Morena, D.; Lumbreras, S.; Rodriguez, J.M.; Campos, C.; Castillo, M.; Benavent, M.; Izquierdo, J.L. Chronic Respiratory Diseases as a Risk Factor for Herpes Zoster Infection. Arch. Bronconeumol. 2023, 59, 797–804. [Google Scholar] [CrossRef]
- Kawai, K.; VoPham, T.; Drucker, A.; Curhan, S.G.; Curhan, G.C. Ultraviolet Radiation Exposure and the Risk of Herpes Zoster in Three Prospective Cohort Studies. Mayo Clin. Proc. 2020, 95, 283–292. [Google Scholar] [CrossRef]
- Batram, M.; Witte, J.; Schwarz, M.; Hain, J.; Ultsch, B.; Steinmann, M.; Bhavsar, A.; Wutzler, P.; Criee, C.P.; Hermann, C.; et al. Burden of Herpes Zoster in Adult Patients with Underlying Conditions: Analysis of German Claims Data, 2007–2018. Dermatol. Ther. 2021, 11, 1009–1026. [Google Scholar] [CrossRef]
- Lasagna, A.; Mele, D.; Bergami, F.; Alaimo, D.; Dauccia, C.; Alessio, N.; Comolli, G.; Pasi, F.; Muzzi, A.; Novelli, V.; et al. The immunogenicity and the safety of the adjuvanted glycoprotein E (gE)-based recombinant vaccine against herpes zoster (RZV) in cancer patients during immunotherapy. Hum. Vaccin. Immunother. 2023, 19, 2288282. [Google Scholar] [CrossRef]
- Marra, F.; Parhar, K.; Huang, B.; Vadlamudi, N. Risk Factors for Herpes Zoster Infection: A Meta-Analysis. Open Forum Infect. Dis. 2020, 7, ofaa005. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Yawn, B.P. Risk of Herpes Zoster in Cancer Patients and the Promise of New Vaccines. J. Infect. Dis. 2019, 220, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Tayyar, R.; Ho, D. Herpes Simplex Virus and Varicella Zoster Virus Infections in Cancer Patients. Viruses 2023, 15, 439. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Heywood, A.E.; Karki, S.; Banks, E.; Macartney, K.; Chantrill, L.; Liu, B. Risk of Herpes Zoster Prior to and Following Cancer Diagnosis and Treatment: A Population-Based Prospective Cohort Study. J. Infect. Dis. 2019, 220, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Serra, F.; Cassaniti, I.; Lilleri, D.; Pedrazzoli, P.; Baldanti, F.; Lasagna, A. Herpes zoster in patients with solid tumors treated with immune checkpoint inhibitors. Immunotherapy 2022, 14, 389–393. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, Y.M.; Chen, T.J.; Lan, J.L.; Lin, C.H.; Chen, D.Y. Risk of herpes zoster in patients with systemic lupus erythematosus: A three-year follow-up study using a nationwide population-based cohort. Clinics 2011, 66, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Michaud, K.; Chakravarty, E.F. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology 2006, 45, 1370–1375. [Google Scholar] [CrossRef]
- Imafuku, S.; Dormal, G.; Goto, Y.; Jegou, C.; Rosillon, D.; Matsuki, T. Risk of herpes zoster in the Japanese population with immunocompromising and chronic disease conditions: Results from a claims database cohort study, from 2005 to 2014. J. Dermatol. 2020, 47, 236–244. [Google Scholar] [CrossRef]
- Gilden, D.H.; Cohrs, R.J.; Mahalingam, R. Clinical and molecular pathogenesis of varicella virus infection. Viral Immunol. 2003, 16, 243–258. [Google Scholar] [CrossRef]
- Mueller, N.H.; Gilden, D.H.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Varicella zoster virus infection: Clinical features, molecular pathogenesis of disease, and latency. Neurol. Clin. 2008, 26, 675–697. [Google Scholar] [CrossRef] [PubMed]
- Wung, P.K.; Holbrook, J.T.; Hoffman, G.S.; Tibbs, A.K.; Specks, U.; Min, Y.I.; Merkel, P.A.; Spiera, R.; Davis, J.C.; St Clair, E.W.; et al. Herpes zoster in immunocompromised patients: Incidence, timing, and risk factors. Am. J. Med. 2005, 118, 1416. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.H.; Kleinschmidt-DeMasters, B.K.; LaGuardia, J.J.; Mahalingam, R.; Cohrs, R.J. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 2000, 342, 635–645. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.R.; Zeringue, A.L.; Caplan, L.; Ranganathan, P.; Xian, H.; Burroughs, T.E.; Fraser, V.J.; Cunningham, F.; Eisen, S.A. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin. Infect. Dis. 2009, 48, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Chun, J.; Han, K.; Park, S.; Choi, G.; Kim, J.; Lee, J.; Im, J.P.; Kim, J.S. Increased Risk of Herpes Zoster in Young and Metabolically Healthy Patients with Inflammatory Bowel Disease: A Nationwide Population-Based Study. Gut Liver 2019, 13, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt-DeMasters, B.K.; Gilden, D.H. Varicella-Zoster virus infections of the nervous system: Clinical and pathologic correlates. Arch. Pathol. Lab. Med. 2001, 125, 770–780. [Google Scholar] [CrossRef]
- Yang, Y.W.; Chen, Y.H.; Wang, K.H.; Wang, C.Y.; Lin, H.W. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: A population-based study. CMAJ 2011, 183, E275–E280. [Google Scholar] [CrossRef]
- Lasagna, A.; Cassaniti, I.; Sacchi, P.; Baldanti, F.; Bruno, R.; Pedrazzoli, P. Infectious complications and immunotherapy: Old pitfalls and new horizons. Future Oncol. 2022, 18, 2377–2381. [Google Scholar] [CrossRef]
- Xu, Q.; He, L.; Yin, Y. Risk of herpes zoster associated with JAK inhibitors in immune-mediated inflammatory diseases: A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1241954. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef]
- Schmader, K. Postherpetic neuralgia in immunocompetent elderly people. Vaccine 1998, 16, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Quiles, C.; Lopez-Lacort, M.; Orrico-Sanchez, A.; Diez-Domingo, J. Impact of postherpetic neuralgia: A six year population-based analysis on people aged 50 years or older. J. Infect. 2018, 77, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lizzi, J.; Hill, T.; Jakubowski, J. Varicella Zoster Virus Encephalitis. Clin. Pract. Cases Emerg. Med. 2019, 3, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.J.; Jeng, B.H. Herpes Zoster: A Brief Definitive Review. Cornea 2021, 40, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, C.Q.; Ge, Q.M.; Tang, L.Y.; Su, T.; Li, Q.Y.; Pan, Y.C.; Shu, H.Y.; Zhang, L.J.; Shao, Y. Investigation of Altered Spontaneous Brain Activity Patterns in Herpes Zoster Keratitis Using the Percent Amplitude of Fluctuation Method: A Resting-State Functional Magnetic Resonance Imaging Study. Neuropsychiatr. Dis. Treat. 2023, 19, 1781–1789. [Google Scholar] [CrossRef]
- Gallon, P.; Parekh, M.; Ferrari, S.; Fasolo, A.; Ponzin, D.; Borroni, D. Metagenomics in ophthalmology: Hypothesis or real prospective? Biotechnol. Rep. 2019, 23, e00355. [Google Scholar] [CrossRef]
- Yadav, P.S.; Adhikari, P.; Jha, N.M.; Khadka, S.; Kafle, S.; Singh, S.; Subedi, S.; Bhatta, M. Varicella pneumonia in an immunocompetent child: A case report. IDCases 2023, 33, e01870. [Google Scholar] [CrossRef]
- Agur Cohen, D.; Braun, E. Unusual subacute endocarditis following herpes zoster infection. a case report. Fam. Pract. 2023, cmad113. [Google Scholar] [CrossRef]
- Borchers, A.T.; Gershwin, M.E. Giant cell arteritis: A review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun. Rev. 2012, 11, A544–A554. [Google Scholar] [CrossRef]
- Lee, D.H.; Iovieno, A.; Sheldon, C.A. Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study. J. Clin. Med. 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Drerup, C.; Eveslage, M.; Sunderkotter, C.; Ehrchen, J. Diagnostic Value of Laboratory Parameters for Distinguishing Between Herpes Zoster and Bacterial Superficial Skin and Soft Tissue Infections. Acta Derm. Venereol. 2020, 100, adv00009. [Google Scholar] [CrossRef]
- Bae, S.; Yun, S.C.; Kim, M.C.; Yoon, W.; Lim, J.S.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.Y.; et al. Association of herpes zoster with dementia and effect of antiviral therapy on dementia: A population-based cohort study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Lopatko Lindman, K.; Hemmingsson, E.S.; Weidung, B.; Brannstrom, J.; Josefsson, M.; Olsson, J.; Elgh, F.; Nordstrom, P.; Lovheim, H. Herpesvirus infections, antiviral treatment, and the risk of dementia-a registry-based cohort study in Sweden. Alzheimers Dement. 2021, 7, e12119. [Google Scholar] [CrossRef] [PubMed]
- Elhalag, R.H.; Motawea, K.R.; Talat, N.E.; Rouzan, S.S.; Reyad, S.M.; Elsayed, S.M.; Chebl, P.; Abowafia, M.; Shah, J. Herpes Zoster virus infection and the risk of developing dementia: A systematic review and meta-analysis. Medicine 2023, 102, e34503. [Google Scholar] [CrossRef] [PubMed]
- Warren-Gash, C.; Breuer, J. Herpes zoster in people who are immunocompromised: What are the options for prevention? Lancet Infect. Dis. 2019, 19, 922–924. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, K.; Yoo, S.A.; Lee, J.H. Herpes Zoster and Subsequent Cancer Risk: A Nationwide Population-Based Cohort Study in Korea. Dermatology 2021, 237, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Cho, H.S.; Kim, Y.D.; Mun, J.; Kim, S.B.; Lee, J.H.; Leem, J.G. The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study. Curr. Oncol. 2021, 28, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, N.; Basit, S.; Wohlfahrt, J.; Pasternak, B.; Munch, T.N.; Nielsen, L.P.; Melbye, M. The short- and long-term risk of stroke after herpes zoster—A nationwide population-based cohort study. PLoS ONE 2013, 8, e69156. [Google Scholar] [CrossRef]
- Buturak, A.; Norgaz, T.; Gorgulu, S. Brivudine induced coronary vasospasm as a manifestation of Kounis syndrome: First report. Int. J. Cardiol. 2015, 185, 4–6. [Google Scholar] [CrossRef]
- Wu, P.H.; Chuang, Y.S.; Lin, Y.T. Does Herpes Zoster Increase the Risk of Stroke and Myocardial Infarction? A Comprehensive Review. J. Clin. Med. 2019, 8, 547. [Google Scholar] [CrossRef]
- Langan, S.M.; Minassian, C.; Smeeth, L.; Thomas, S.L. Risk of stroke following herpes zoster: A self-controlled case-series study. Clin. Infect. Dis. 2014, 58, 1497–1503. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.; Lee, H.S.; Lee, K.Y. Association Between the Risk for Cardiovascular Events and Antiviral Treatment for Herpes Zoster. Clin. Infect. Dis. 2021, 73, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.J.; Liu, K.; Danesh-Meyer, H.V.; Niederer, R.L. Prompt Antiviral Therapy Is Associated With Lower Risk of Cerebrovascular Accident Following Herpes Zoster Ophthalmicus. Am. J. Ophthalmol. 2022, 242, 215–220. [Google Scholar] [CrossRef]
- Parameswaran, G.I.; Drye, A.F.; Wattengel, B.A.; Carter, M.T.; Doyle, K.M.; Mergenhagen, K.A. Increased Myocardial Infarction Risk Following Herpes Zoster Infection. Open Forum Infect. Dis. 2023, 10, ofad137. [Google Scholar] [CrossRef]
- Strezova, A.; Diez-Domingo, J.; Al Shawafi, K.; Tinoco, J.C.; Shi, M.; Pirrotta, P.; Mwakingwe-Omari, A.; Zoster-049 Study Group. Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination. Open Forum Infect. Dis. 2022, 9, ofac485. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Thomas, S.L.; Smeeth, L.; Margolis, D.J.; Nitsch, D. Zoster vaccination is associated with a reduction of zoster in elderly patients with chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 2095–2098. [Google Scholar] [CrossRef]
- Pedrazzoli, P.; Lasagna, A.; Cassaniti, I.; Ferrari, A.; Bergami, F.; Silvestris, N.; Sapuppo, E.; Di Maio, M.; Cinieri, S.; Baldanti, F. Vaccination for herpes zoster in patients with solid tumors: A position paper on the behalf of the Associazione Italiana di Oncologia Medica (AIOM). ESMO Open 2022, 7, 100548. [Google Scholar] [CrossRef]
- Vink, P.; Delgado Mingorance, I.; Maximiano Alonso, C.; Rubio-Viqueira, B.; Jung, K.H.; Rodriguez Moreno, J.F.; Grande, E.; Marrupe Gonzalez, D.; Lowndes, S.; Puente, J.; et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: A randomized trial. Cancer 2019, 125, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Farraye, F.A.; Winthrop, K.L.; Willer, D.O.; Vink, P.; Tavares-Da-Silva, F. Safety and efficacy of recombinant and live herpes zoster vaccines for prevention in at-risk adults with chronic diseases and immunocompromising conditions. Vaccine 2023, 41, 36–48. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, C.N.; Lee, M.S.; Lee, J.H.; Lee, J.Y.; Han, K.; Park, Y.M. Recurrence Rate of Herpes Zoster and Its Risk Factors: A Population-based Cohort Study. J. Korean Med. Sci. 2019, 34, e1. [Google Scholar] [CrossRef]
- Nagel, M.A.; Jones, D.; Wyborny, A. Varicella zoster virus vasculopathy: The expanding clinical spectrum and pathogenesis. J. Neuroimmunol. 2017, 308, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Bubak, A.N. Varicella Zoster Virus Vasculopathy. J. Infect. Dis. 2018, 218, S107–S112. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Wollan, P.C.; Nagel, M.A.; Gilden, D. Risk of Stroke and Myocardial Infarction After Herpes Zoster in Older Adults in a US Community Population. Mayo Clin. Proc. 2016, 91, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Yun, S.C.; Lee, H.B.; Lee, P.H.; Lee, S.W.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H.; Kwon, S.U. Herpes Zoster Increases the Risk of Stroke and Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 70, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Lindsay, A.C.; Yousefi, M.; Wang, C. Risk of, and risk factors for, vasculopathy associated with acute herpes zoster. J. Stroke Cerebrovasc. Dis. 2023, 32, 106891. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Forghani, B.; Mahalingam, R.; Wellish, M.C.; Cohrs, R.J.; Russman, A.N.; Katzan, I.; Lin, R.; Gardner, C.J.; Gilden, D.H. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology 2007, 68, 1069–1073. [Google Scholar] [CrossRef]
- Nagel, M.A.; Traktinskiy, I.; Stenmark, K.R.; Frid, M.G.; Choe, A.; Gilden, D. Varicella-zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology 2013, 80, 62–68. [Google Scholar] [CrossRef]
- Oaklander, A.L. The pathology of shingles: Head and Campbell’s 1900 monograph. Arch. Neurol. 1999, 56, 1292–1294. [Google Scholar] [CrossRef]
- Nagashima, K.; Nakazawa, M.; Endo, H. Pathology of the human spinal ganglia in varicella-zoster virus infection. Acta Neuropathol. 1975, 33, 105–117. [Google Scholar] [CrossRef]
- Ghatak, N.R.; Zimmerman, H.M. Spinal ganglion in herpes zoster. A light and electron microscopic study. Arch. Pathol. 1973, 95, 411–415. [Google Scholar]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef]
- Jones, D.; Alvarez, E.; Selva, S.; Gilden, D.; Nagel, M.A. Proinflammatory cytokines and matrix metalloproteinases in CSF of patients with VZV vasculopathy. Neurol. Neuroimmunol. Neuroinflamm 2016, 3, e246. [Google Scholar] [CrossRef]
- Sakakibara, R.; Sawai, S.; Ogata, T. Varicella-zoster virus infection and autonomic dysfunction. Auton. Neurosci. 2022, 242, 103018. [Google Scholar] [CrossRef]
- Gilden, D.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Varicella zoster virus vasculopathies: Diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009, 8, 731–740. [Google Scholar] [CrossRef]
- Warren-Gash, C. Herpes Zoster: Epidemiological Links With Stroke and Myocardial Infarction. J. Infect. Dis. 2018, 218, S102–S106. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Huang, Y.; Yu, Q.; Wang, L.; Li, K. Risk of Stroke/Transient Ischemic Attack or Myocardial Infarction with Herpes Zoster: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Erskine, N.; Tran, H.; Levin, L.; Ulbricht, C.; Fingeroth, J.; Kiefe, C.; Goldberg, R.J.; Singh, S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS ONE 2017, 12, e0181565. [Google Scholar] [CrossRef] [PubMed]
- Minassian, C.; Thomas, S.L.; Smeeth, L.; Douglas, I.; Brauer, R.; Langan, S.M. Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States. PLoS Med. 2015, 12, e1001919. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Shingles is linked to increased risk of cardiovascular events. BMJ 2015, 351, h6757. [Google Scholar] [CrossRef]
- Curhan, S.G.; Kawai, K.; Yawn, B.; Rexrode, K.M.; Rimm, E.B.; Curhan, G.C. Herpes Zoster and Long-Term Risk of Cardiovascular Disease. J. Am. Heart Assoc. 2022, 11, e027451. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Ruckenstein, J.; Richardson, K. A meta-analysis of stroke risk following herpes zoster infection. BMC Infect. Dis. 2017, 17, 198. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Zhu, Y.; Tang, F.; Yang, B.; Duan, R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0171182. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, G.I.; Wattengel, B.A.; Chua, H.C.; Swiderek, J.; Fuchs, T.; Carter, M.T.; Goode, L.; Doyle, K.; Mergenhagen, K.A. Increased Stroke Risk Following Herpes Zoster Infection and Protection With Zoster Vaccine. Clin. Infect. Dis. 2023, 76, e1335–e1340. [Google Scholar] [CrossRef] [PubMed]
- Schink, T.; Behr, S.; Thone, K.; Bricout, H.; Garbe, E. Risk of Stroke after Herpes Zoster—Evidence from a German Self-Controlled Case-Series Study. PLoS ONE 2016, 11, e0166554. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chien, C.W.; Ho, J.D. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Pacou, M.; Gauthier, A.; Brown, M.M. Herpes zoster as a risk factor for stroke and TIA: A retrospective cohort study in the UK. Neurology 2014, 82, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, P. Stroke and myocardial infarction after herpes zoster. MMW Fortschr. Med. 2017, 159, 66–68. [Google Scholar] [CrossRef]
- Morales, A.R.; Adelman, S.; Fine, G. Varicella myocarditis. A case of sudden death. Arch. Pathol. 1971, 91, 29–31. [Google Scholar]
- Elikowski, W.; Marszalek, A.; Malek-Elikowska, M.; Ganowicz-Kaatz, T.; Mozer-Lisewska, I. Myopericarditis in a 23-year-old male with herpes zoster. Pol. Merkur. Lekarski 2016, 40, 97–101. [Google Scholar]
- Kelly, E.; Cullen, G.; McGurk, C. When an MI is not an MI: A case of varicella zoster myocarditis. Cardiology 2008, 109, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.S.; Collins, T.C.; Habib, G.; Bredikis, A.; Carabello, B.A. Herpes zoster and its cardiovascular complications in the elderly--another look at a dormant virus. Cardiology 2007, 107, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kalender, E.; Sensoy, B.; Cay, S. Heart block after herpes zoster reactivation. Int. J. Cardiol. 2016, 203, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Jin, P.; Nassereddin, A.T.; Lopez, J.E.; Keeley, E.C. Varicella Zoster Virus-Induced Complete Heart Block. Am. J. Med. 2023, 136, e63–e64. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, V.; Fernandez, M.C.; Zide, K. Herpes Zoster-induced Progressive Heart Block: A Case Report. Cureus 2018, 10, e3674. [Google Scholar] [CrossRef]
| Disease [Reference] | Results (Country, Incident) | Years Since Herpes Zoster | HR (95% CI) |
|---|---|---|---|
| Stroke [100] | Higher long-term risk of stroke (US, 3603) | 1–4 years | 1.05 (0.88–1.25) |
| 5–8 years | 1.38 (1.10–1.74) | ||
| 9–12 years | 1.28 (1.03–1.59) | ||
| ≥13 years | 1.19 (0.90–1.56) | ||
| Coronary heart disease [100] | Higher long-term risk of coronary heart disease (US, 8620) | 1–4 years | 1.13 (1.01–1.27) |
| 5–8 years | 1.16 (1.02–1.32) | ||
| 9–12 years | 1.25 (1.07–1.46) | ||
| ≥13 years | 1.00 (0.83–1.21) | ||
| Cardiovascular disease [22] | Severe herpes zoster is associated with cardiovascular disease (Korea, 20311) | Myocardial infarction | 1.83 (1.35–2.48) |
| Ischemic stroke | 1.52 (1.21–1.92) | ||
| Heart failure | 2.03 (1.62–2.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaoka-Tojo, M.; Tojo, T. Herpes Zoster and Cardiovascular Disease: Exploring Associations and Preventive Measures through Vaccination. Vaccines 2024, 12, 252. https://doi.org/10.3390/vaccines12030252
Yamaoka-Tojo M, Tojo T. Herpes Zoster and Cardiovascular Disease: Exploring Associations and Preventive Measures through Vaccination. Vaccines. 2024; 12(3):252. https://doi.org/10.3390/vaccines12030252
Chicago/Turabian StyleYamaoka-Tojo, Minako, and Taiki Tojo. 2024. "Herpes Zoster and Cardiovascular Disease: Exploring Associations and Preventive Measures through Vaccination" Vaccines 12, no. 3: 252. https://doi.org/10.3390/vaccines12030252
APA StyleYamaoka-Tojo, M., & Tojo, T. (2024). Herpes Zoster and Cardiovascular Disease: Exploring Associations and Preventive Measures through Vaccination. Vaccines, 12(3), 252. https://doi.org/10.3390/vaccines12030252







