The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Propagation and DNA Extraction
2.2. Cloning, Protein Expression and Purification
2.3. Immunization and Challenge
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Histopathology and Immunohistochemistry Analyses
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Construction of Expression Vectors
3.2. Expression and Purification of Recombinant Proteins
3.3. Challenge Protection Test and Detection of Antibody Titers
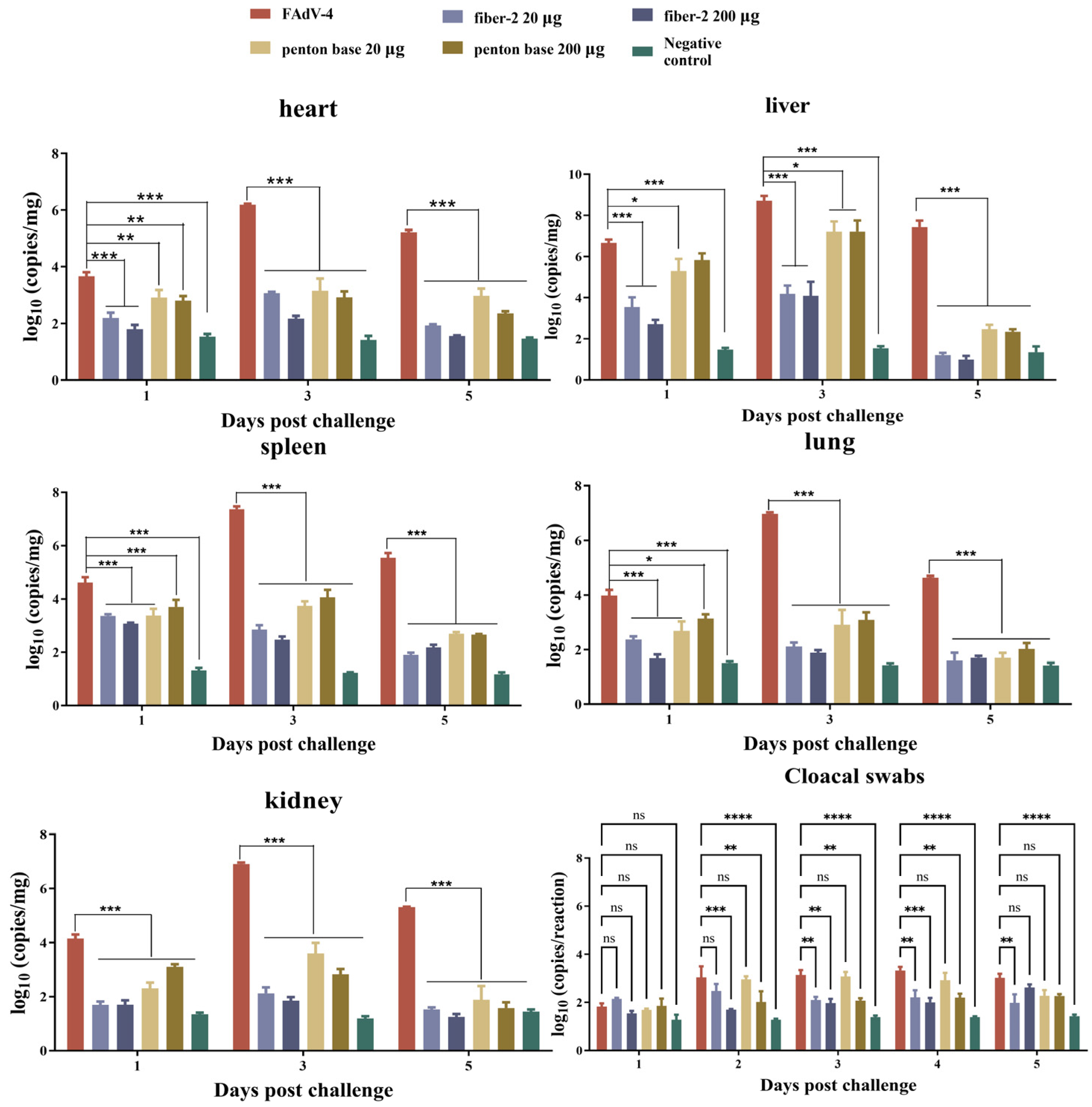
3.4. Viral Loads in Target Tissues and Cloacal Swabs
3.5. Gross Lesions, Histopathology and Immunohistochemistry
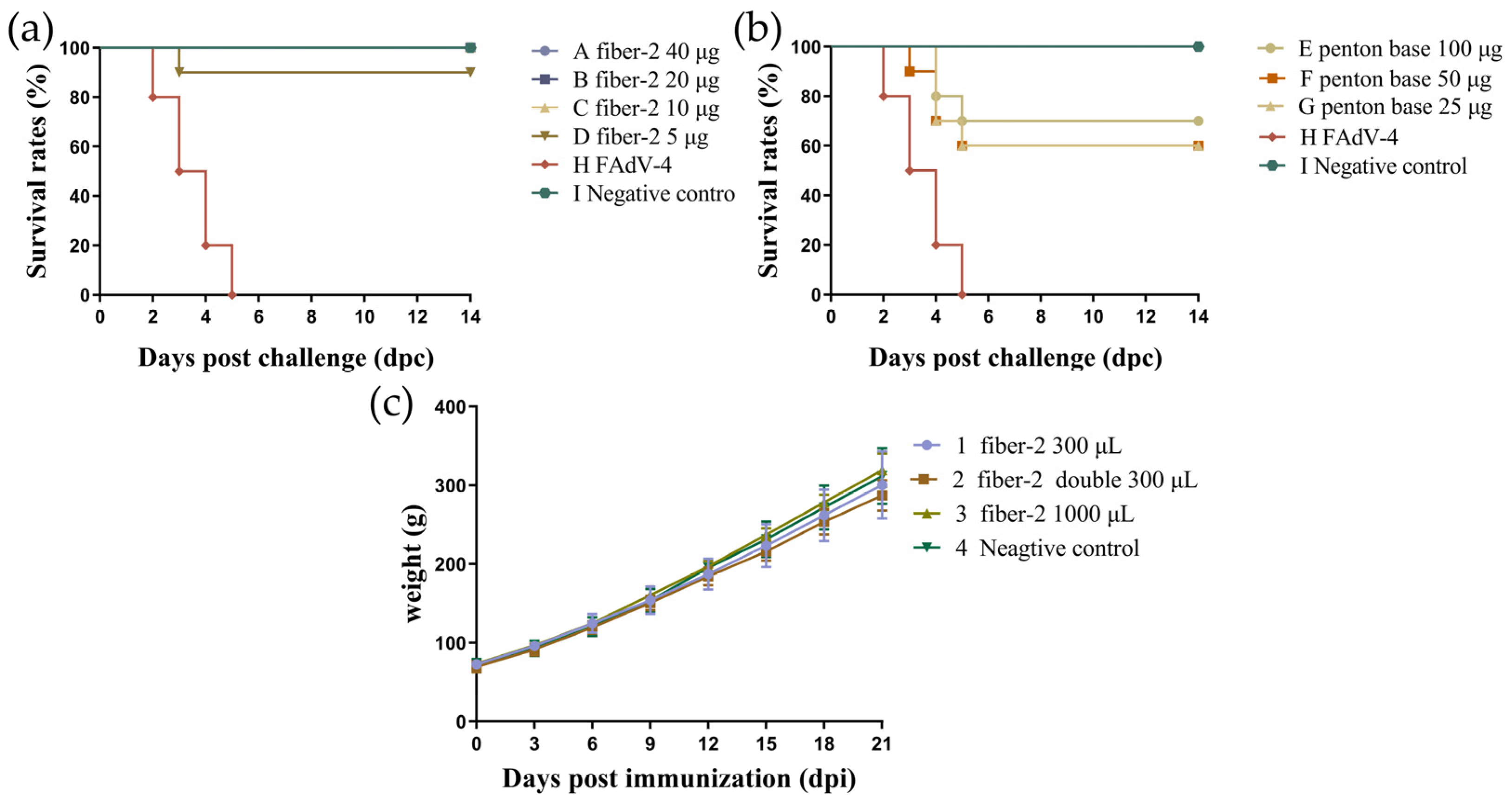
3.6. Determination of Minimum Immune Dose and Safety of Recombinant Fiber-2 Protein and Penton Base Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Wang, J.; Qiu, L.; Han, Z.; Liu, S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis-hydropericardium syndrome in chickens in China. Infect. Genet. Evol. 2016, 45, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Ashraf, A.; Khan, M.I.; Rahman, M.; Habib, M.; Chughtai, M.I.; Qureshi, J.A. Fowl adenovirus: History, emergence, biology and development of a vaccine against hydropericardium syndrome. Arch. Virol. 2017, 162, 1833–1843. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, W.; Gao, D.; Li, Y.; Yang, X.; Liu, H.; Yao, H.; Chen, L.; Wang, C.; Zhao, J. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China. Emerg. Microbes Infect. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Nolte, V.; Schachner, A.; Berger, E.; Schlötterer, C.; Hess, M. Two fiber genes of nearly equal lengths are a common and distinctive feature of Fowl adenovirus C members. Vet. Microbiol. 2012, 156, 411–417. [Google Scholar] [CrossRef]
- Reddy, V.S.; Nemerow, G.R. Structures and organization of adenovirus cement proteins provide insights into the role of capsid maturation in virus entry and infection. Proc. Natl. Acad. Sci. USA 2014, 111, 11715–11720. [Google Scholar] [CrossRef]
- Li, W.; You, G.; Haiyilati, A.; Wang, H.; Jiao, H.; Wang, Y.; Gao, L.; Cao, H.; Li, X.; Zheng, S.J. Critical Role of Viral Protein Hexon in Hypervirulent Fowl Adenovirus Serotype-4-Induced Autophagy by Interaction with BAG3 and Promotion of Viral Replication in LMH Cells. J. Virol. 2023, 97, e0028423. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Tian, K.; Wang, Z.; Yang, X.; Gao, D.; Zhang, Y.; Fu, J.; Wang, H.; Zhao, J. Fiber2 and hexon genes are closely associated with the virulence of the emerging and highly pathogenic fowl adenovirus 4. Emerg. Microbes Infect. 2018, 7, 199. [Google Scholar] [CrossRef]
- Cao, J.; Liu, S.; Liu, M.; Wang, S.; Bi, Z.; Fan, W.; Shi, Z.; Song, S.; Yan, L. Hsp70 Inhibits the Replication of Fowl Adenovirus Serotype 4 by Suppressing Viral Hexon with the Assistance of DnaJC7. J. Virol. 2022, 96, e0080722. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, M.; Duan, X.; Wang, Y.; Cao, H.; Li, X.; Zheng, S.J. Requirement of Cellular Protein CCT7 for the Replication of Fowl Adenovirus Serotype 4 (FAdV-4) in Leghorn Male Hepatocellular Cells Via Interaction with the Viral Hexon Protein. Viruses 2019, 11, 107. [Google Scholar] [CrossRef]
- Wang, B.; Song, C.; Yang, P.; Song, M.; Zhao, S.; Qiao, Q.; Wang, Z.; Zhao, J. The Role of Hexon Amino Acid 188 Varies in Fowl Adenovirus Serotype 4 Strains with Different Virulence. Microbiol. Spectr. 2022, 10, e0149322. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, W.; Li, L.; Kan, Q.; Fu, H.; Geng, T.; Li, T.; Wan, Z.; Gao, W.; Shao, H.; et al. Domain in Fiber-2 interacted with KPNA3/4 significantly affects the replication and pathogenicity of the highly pathogenic FAdV-4. Virulence 2021, 12, 754–765. [Google Scholar] [CrossRef]
- Huang, R.; He, Q.; Lu, S.; Yan, M.; Xu, L.; Wang, Q. Importin alpha 1 is required for the nucleus entry of Fowl Adenovirus serotype 4 Fiber-1 protein. Vet. Microbiol. 2022, 266, 109351. [Google Scholar] [CrossRef]
- Griffin, B.D.; Nagy, É. Coding potential and transcript analysis of fowl adenovirus 4: Insight into upstream ORFs as common sequence features in adenoviral transcripts. J. Gen. Virol. 2011, 92 Pt 6, 1260–1272. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Guo, H.; Li, N.; Wang, B.; Tian, K.; Wang, Z.; Yang, X.; Li, Y.; Wang, H.; et al. The increased virulence of hypervirulent fowl adenovirus 4 is independent of fiber-1 and penton. Res. Vet. Sci. 2020, 131, 31–37. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, L.; Gao, Y.; Liu, C.; Qi, X.; Zhang, Y.; Wang, Y.; Li, K.; Gao, L.; Wang, X.; et al. Characterization of a hypervirulent fowl adenovirus 4 with the novel genotype newly prevalent in China and establishment of reproduction infection model of hydropericardium syndrome in chickens. Poult. Sci. 2017, 96, 1581–1588. [Google Scholar] [CrossRef]
- El-Shall, N.A.; El-Hamid, H.S.A.; Elkady, M.F.; Ellakany, H.F.; Elbestawy, A.R.; Gado, A.R.; Geneedy, A.M.; Hasan, M.E.; Jaremko, M.; Selim, S.; et al. Epidemiology, pathology, prevention, and control strategies of inclusion body hepatitis and hepatitis-hydropericardium syndrome in poultry: A comprehensive review. Front. Vet. Sci. 2022, 9, 963199. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Matos, M.; Grafl, B.; Hess, M. Fowl adenovirus-induced diseases and strategies for their control—A review on the current global situation. Avian Pathol. 2018, 47, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Meng, F.; Chen, C.; Shen, Y.; Li, P.; Xu, J.; Feng, Z.; Qu, X.; Wang, F.; Li, B.; et al. Pathogenicity and epidemiological survey of fowl adenovirus in Shandong Province from 2021 to 2022. Front. Microbiol. 2023, 14, 1166078. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xie, Z.; Fan, Q.; Xie, Z.; Deng, X.; Luo, S.; Li, X.; Zhang, Y.; Zeng, T.; Huang, J.; et al. Pathogenicity and molecular characteristics of fowl adenovirus serotype 4 with moderate virulence in Guangxi Province, China. Front. Vet. Sci. 2023, 10, 1190126. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Yang, L.; Chen, F.; He, X.; Zhang, R.; Zhao, Y.; Gao, G.; Mu, W.; Chen, X.; Luo, S.; et al. Prevalence and Molecular Characteristics of FAdV-4 from Indigenous Chicken Breeds in Yunnan Province, Southwestern China. Microorganisms 2023, 11, 2631. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liang, G.; Zhang, J.; Wang, W.; Song, N.; Wang, P.; Zheng, W.; Xie, Q.; Shao, H.; Wan, Z.; et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg. Microbes Infect. 2016, 5, e50. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Feng, J.; Duan, B.; Shi, Q.; Li, Y.; Chen, Z.; Ma, L.; Liu, H.; Wang, Y. Epidemiological survey of avian adenovirus in China from 2015 to 2021 and the genetic variability of highly pathogenic Fadv-4 isolates. Infect. Genet. Evol. 2022, 101, 105277. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Yang, Q.; Wu, M.J.; Zhang, Z.; Song, J.; Wang, W.; Yang, J.; Ji, J.; Zhang, Y.; Dai, H.; et al. Genomic and Pathologic Characterization of the First FAdV-C Serotype 4 Isolate from Black-Necked Crane. Viruses 2023, 15, 1653. [Google Scholar] [CrossRef]
- Pouladi, I.; Najafi, H.; Jaydari, A. Research Note: Overview of fowl adenovirus serotype 4: Structure, pathogenicity, and progress in vaccine development. Poult. Sci. 2024, 103, 103479. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, Z.; Liu, L.; Li, Y.; Gao, W.; Song, X.; Li, X. Knob domain of Fiber 2 protein provides full protection against fowl adenovirus serotype 4. Virus Res. 2023, 330, 199113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Q.; Chu, Z.; Wang, P.; Luo, C.; Zhang, Y.; Fang, X.; Qiu, L.; Dang, R.; Yang, Z. Immune protection efficacy of FAdV-4 surface proteins fiber-1, fiber-2, hexon and penton base. Virus Res. 2018, 245, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, Y.; Jiang, H.; Xu, Z.; Guo, Y.; Cao, X.; Li, T.; Wan, Z.; Shao, H.; Qin, A.; et al. Efficient cross-protection against serotype 4/8a fowl adenoviruses (FAdVs): Recombinant FAdV-4 with FAdV-8a Fiber. Microbiol. Spectr. 2023, 11, e0246223. [Google Scholar] [CrossRef]
- Shah, M.S.; Ashraf, A.; Rahman, M.; Khan, M.I.; Qureshi, J.A. A subunit vaccine against hydropericardium syndrome using adenovirus penton capsid protein. Vaccine 2012, 30, 7153–7156. [Google Scholar] [CrossRef]
- Chen, L.; Yin, L.; Zhou, Q.; Li, Q.; Luo, Y.; Xu, Z.; Zhang, Y.; Xue, C.; Cao, Y. Immunogenicity and protective efficacy of recombinant fiber-2 protein in protecting SPF chickens against fowl adenovirus 4. Vaccine 2018, 36, 1203–1208. [Google Scholar] [CrossRef]
- Ruan, S.; Zhao, J.; Yin, X.; He, Z.; Zhang, G. A subunit vaccine based on fiber-2 protein provides full protection against fowl adenovirus serotype 4 and induces quicker and stronger immune responses than an inactivated oil-emulsion vaccine. Infect. Genet. Evol. 2018, 61, 145–150. [Google Scholar] [CrossRef]
- Hu, J.; Li, G.; Wang, X.; Cai, L.; Rong, M.; Li, H.; Xie, M.; Zhang, Z.; Rong, J. Development of a subunit vaccine based on fiber2 and hexon against fowl adenovirus serotype 4. Virus Res. 2021, 305, 198552. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; He, L.; Zhu, E.; Fang, T.; Yue, J.; Wen, M.; Wang, K.; Cheng, Z. A fowl adenovirus serotype 4 (FAdV-4) Fiber2 subunit vaccine candidate provides complete protection against challenge with virulent FAdV-4 strain in chickens. Vet. Microbiol. 2021, 263, 109250. [Google Scholar] [CrossRef] [PubMed]
- Schachner, A.; Marek, A.; Jaskulska, B.; Bilic, I.; Hess, M. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis-hydropericardium syndrome (HHS). Vaccine 2014, 32, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, E.; Jaspers, R.; Paul, G.; Hess, M. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis. 2010, 54, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Cao, S.; Zhang, W.; Wang, W.; Li, L.; Kan, Q.; Fu, H.; Geng, T.; Li, T.; Wan, Z.; et al. A novel fiber-2-edited live attenuated vaccine candidate against the highly pathogenic serotype 4 fowl adenovirus. Vet. Res. 2021, 52, 35. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Yang, Y.; Gao, Y.; Qi, X.; Liu, C.; Zhang, Y.; Cui, H.; Wang, X. An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China. Viruses 2017, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Inclusion bodies and purification of proteins in biologically active forms. Adv. Biochem. Eng. Biotechnol. 1997, 56, 61–109. [Google Scholar]
- Hashemzadeh, M.S.; Mohammadi, M.; Ghaleh, H.E.G.; Sharti, M.; Choopani, A.; Panda, A.K. Expression, Solubilization, Refolding and Final Purification of Recombinant Proteins as Expressed in the form of “Classical Inclusion Bodies” in E. coli. Protein Pept. Lett. 2021, 28, 122–130. [Google Scholar] [CrossRef]
- Fu, W.; Lin, J.; Cen, P. 5-Aminolevulinate production with recombinant Escherichia coli using a rare codon optimizer host strain. Appl. Microbiol. Biotechnol. 2007, 75, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, Y.; Huang, Z.; Liu, L.; Liao, H.; Sun, W.; Tao, M.; Li, L.; Li, X. Immunity analysis against Fowl Adenovirus serotype 4 (FAdV-4) based on Fiber-2 trimer Protein with the different virulence. Virus Res. 2022, 308, 198652. [Google Scholar] [CrossRef]
- Murakoshi, H.; Kuse, N.; Akahoshi, T.; Zhang, Y.; Chikata, T.; Borghan, M.A.; Gatanaga, H.; Oka, S.; Sakai, K.; Takiguchi, M. Broad Recognition of Circulating HIV-1 by HIV-1-Specific Cytotoxic T-Lymphocytes with Strong Ability to Suppress HIV-1 Replication. J. Virol. 2019, 93, e01480-18. [Google Scholar] [CrossRef]
- Qin, Y.; Tu, K.; Teng, Q.; Feng, D.; Zhao, Y.; Zhang, G. Identification of Novel T-Cell Epitopes on Infectious Bronchitis Virus N Protein and Development of a Multi-epitope Vaccine. J. Virol. 2021, 95, e0066721. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Schachner, A.; Heidl, S.; Hess, M.; Liebhart, D.; Mitra, T. Local cellular immune response plays a key role in protecting chickens against hepatitis-hydropericardium syndrome (HHS) by vaccination with a recombinant fowl adenovirus (FAdV) chimeric fiber protein. Front. Immunol. 2022, 13, 1026233. [Google Scholar] [CrossRef] [PubMed]



| Primers Name | Sequence (5′-3′) b | Produces Sizes (bp) |
|---|---|---|
| fiber 2-F | ggtcgcgatccgaattcATGCTCCGGGCCCCTAAAAGA | 1440 |
| fiber 2-R | gtggtggtggtgctcgagTTACGGGAGGGAGGCCGCTGG | |
| penton base-F | ggtcgcgatccgaattcATGTGGGGGTTGCAGCCGCCGA | 1578 |
| penton base-R | gtggtggtggtgctcgagTACTGCAAGGTCGCGGAACTC | |
| qORF14-F a | AGTGTGTATGTGCGTTGGGTAG | 136 |
| qORF14-R a | CATTGTCATAACGATGGTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, M.; Wang, S.; Tang, Z.; Liu, J.; Song, S.; Yan, L. The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants. Vaccines 2024, 12, 263. https://doi.org/10.3390/vaccines12030263
Liu W, Liu M, Wang S, Tang Z, Liu J, Song S, Yan L. The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants. Vaccines. 2024; 12(3):263. https://doi.org/10.3390/vaccines12030263
Chicago/Turabian StyleLiu, Wenjian, Meng Liu, Shuaiwen Wang, Zhihui Tang, Jiwen Liu, Suquan Song, and Liping Yan. 2024. "The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants" Vaccines 12, no. 3: 263. https://doi.org/10.3390/vaccines12030263
APA StyleLiu, W., Liu, M., Wang, S., Tang, Z., Liu, J., Song, S., & Yan, L. (2024). The Development of a Novel Fiber-2 Subunit Vaccine against Fowl Adenovirus Serotype 4 Formulated with Oil Adjuvants. Vaccines, 12(3), 263. https://doi.org/10.3390/vaccines12030263





