Toward a SARS-CoV-2 VLP Vaccine: HBc/G as a Carrier for SARS-CoV-2 Spike RBM and Nucleocapsid Protein-Derived Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of S and N Protein Fragments of SARS-CoV-2 for Insertion into the MIR of HBc/G
2.2. CTL Epitopes of SARS-CoV-2 Used for Insertion at the C-Terminus of the HBc/G of Different Length
2.3. Construction of Vectors for the Expression of HBc/G Fusion Proteins in E. coli
2.4. Expression of HBc/G Fusion Proteins, Disintegration of Cells, and Solubility Test of Fusion Proteins
2.4.1. Purification of Fusion Proteins with Insertions of the RBM or B-Cell Epitope-Containing Peptide (Bep) from the N Protein in the HBc/G MIR
2.4.2. Purification of Fusion Proteins with the Insertion of the CTL Epitope String at the C-Terminus of Truncated HBc/G Proteins
2.5. Characterization of Purified HBc/G Fusion Proteins
2.6. Immunization of BALB/c Mice
2.7. Immunological Tests
2.7.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7.2. Flow Cytometry for the Determination of SARS-CoV-2-Epitope-Specific T-Cells
2.7.3. Staining of Surface Markers and Intracellular Cytokines
2.8. SARS-CoV-2-Pseudotyped Murine Retrovirus Neutralization Assay
2.8.1. Plasmids and Production of SARS-CoV-2 Pseudoparticles
2.8.2. Assays for Neutralization and Infection
2.9. Data Analysis
3. Results
3.1. Expression of HBc/G Fusion Proteins
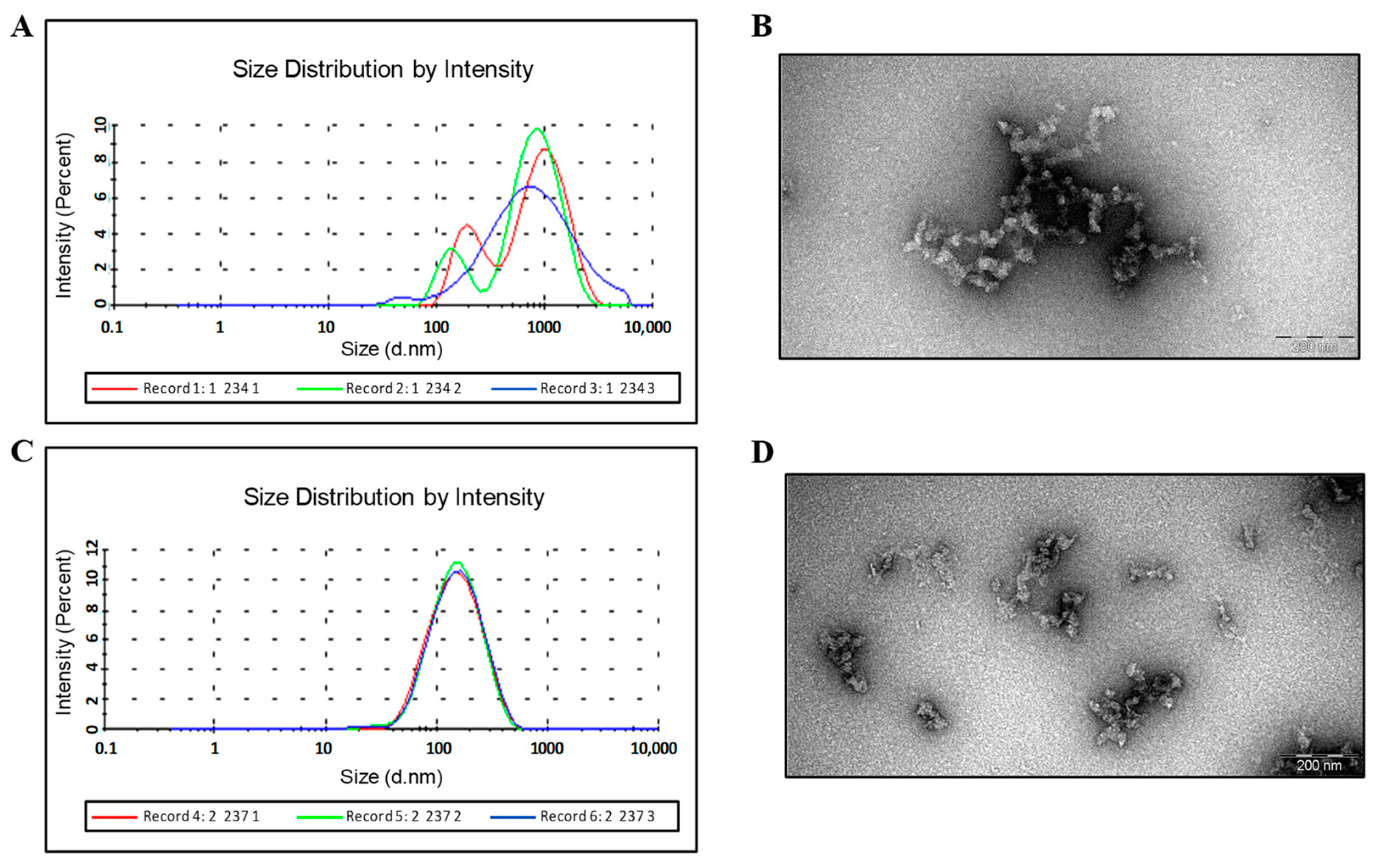
3.2. Purification of Fusion Proteins with Insertions in the MIR of HBc/G
Purification and Characterization of the HBc/G-RBM and HBc/G-Bep Fusion Proteins Obtained through the Stepwise Extraction and Refolding of Insoluble Proteins
3.3. Purification of HBc/G Fusion Proteins with the C-end Insertions of CTL Epitopes
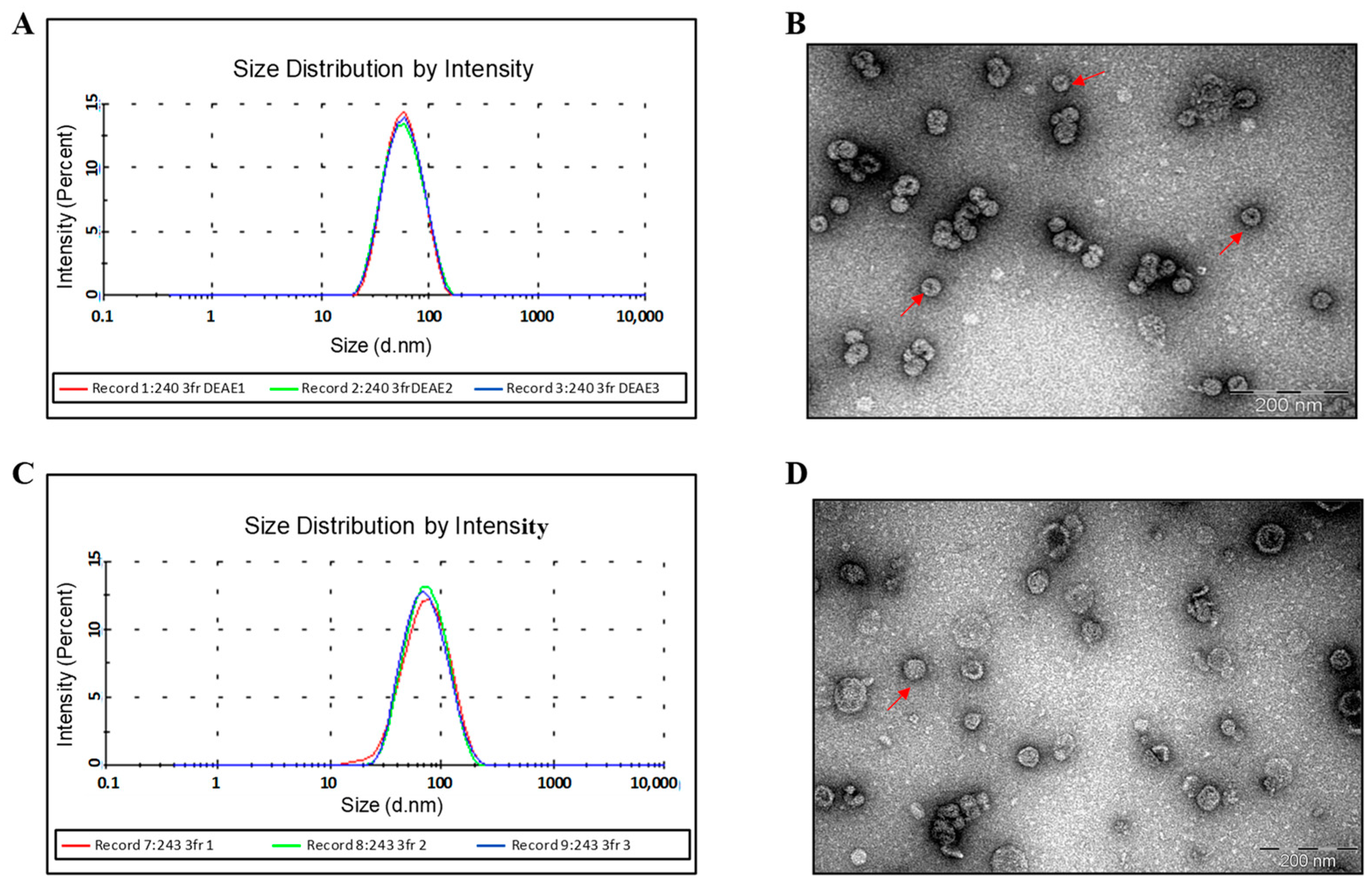
3.3.1. Use of Gel Filtration for the Purification of the HBc/G175-CTL and HBc/G161-CTL Proteins
3.3.2. IEX Chromatography of HBc/G175-CTL
3.4. Immunological Results
3.4.1. B-Cell Response
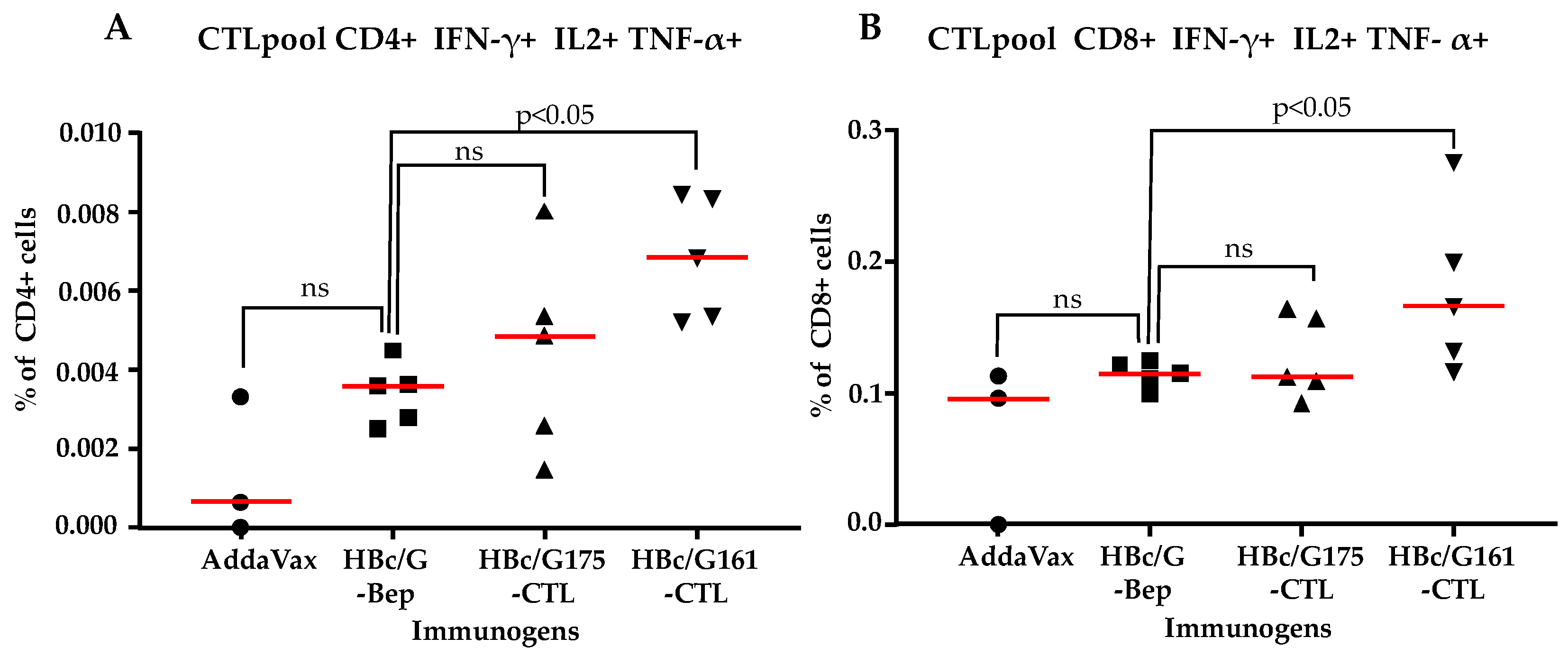
3.4.2. CTL Response
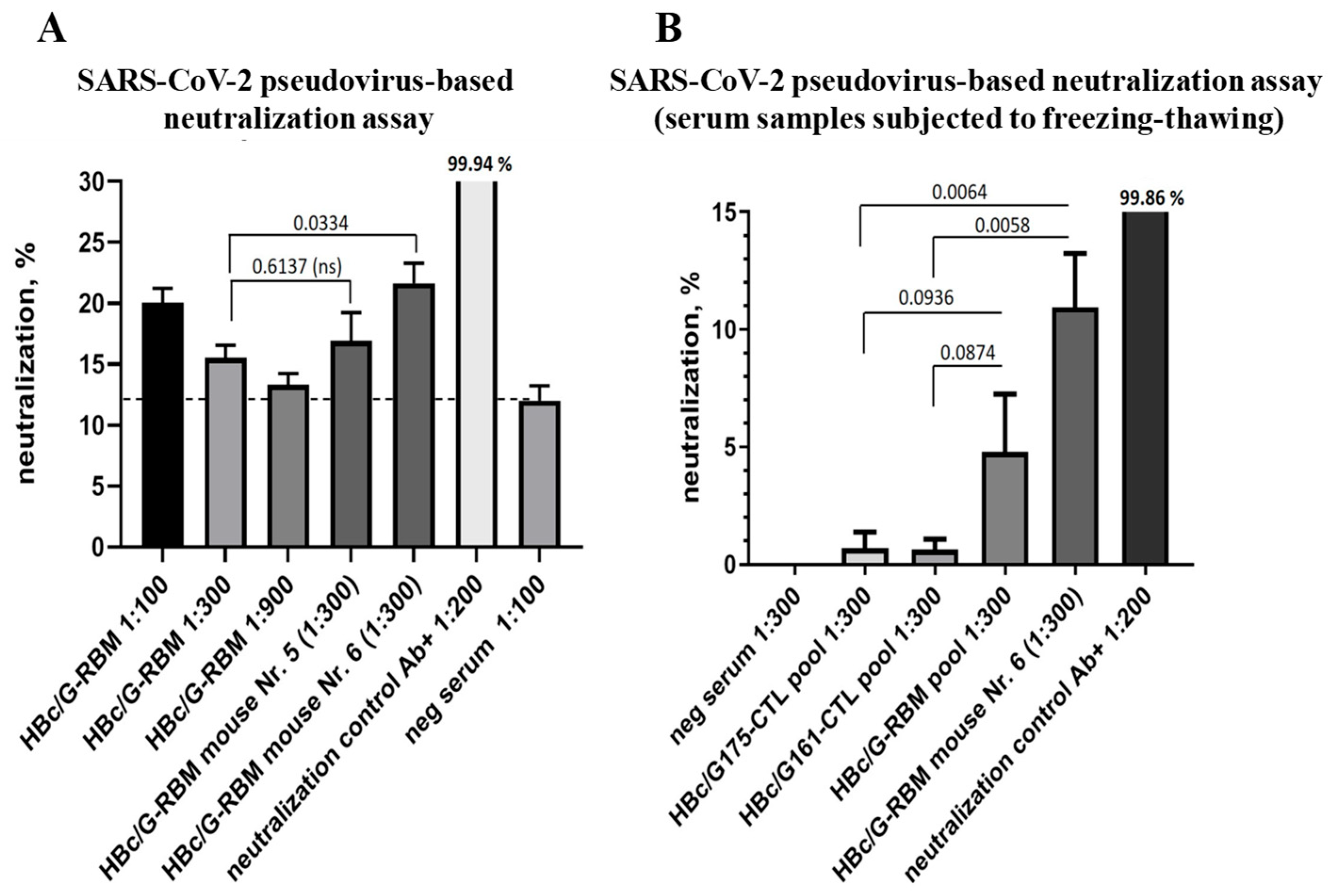
3.4.3. The Ability of Mice Sera to Neutralize SARS-CoV-2-Pseudotyped MLV Particles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 17 January 2024).
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Strych, U.; Hotez, P.J.; Bottazzi, M.E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.P.; Klasse, J.P. COVID-19 Vaccines: “Warp Speed” Needs Mind Melds, Not Warped Minds. J. Virol. 2020, 94, e01083-20. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.M.; Manchester, M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Augusto, G.; Bachmann, M.F. The 3Ds in virus-like particle based-vaccines: “Design, Delivery and Dynamics”. Immunol. Rev. 2020, 296, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- van der Sande, M.A.; Mendy, M.; Waight, P.; Doherty, C.; McConkey, S.J.; Hall, A.J.; Whittle, H.C. Similar long-term vaccine efficacy of two versus three doses of HBV vaccine in early life. Vaccine 2007, 25, 1509–1512. [Google Scholar] [CrossRef]
- Whittle, H.; Jaffar, S.; Wansbrough, M.; Mendy, M.; Dumpis, U.; Collinson, A.; Hall, A. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ 2002, 325, 569. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Haber, P.; Moro, P.L.; Ng, C.; Lewis, P.W.; Hibbs, B.; Schillie, S.F.; Nelson, N.P.; Li, R.; Stewart, B.; Cano, M.V. Safety of currently licensed hepatitis B surface antigen vaccines in the United States, Vaccine Adverse Event Reporting System (VAERS), 2005–2015. Vaccine 2018, 36, 559–564. [Google Scholar] [CrossRef]
- Macartney, K.K.; Chiu, C.; Georgousakis, M.; Brotherton, J.M. Safety of human papillomavirus vaccines: A review. Drug. Saf. 2013, 36, 393–412. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Zasada, A.A.; Darlińska, A.; Wiatrzyk, A.; Woźnica, K.; Formińska, K.; Czajka, U.; Główka, M.; Lis, K.; Górska, P. COVID-19 Vaccines over Three Years after the Outbreak of the COVID-19 Epidemic. Viruses 2023, 15, 1786. [Google Scholar] [CrossRef]
- Soheili, M.; Khateri, S.; Moradpour, F.; Mohammadzedeh, P.; Zareie, M.; Mortazavi, S.M.M.; Manifar, S.; Kohan, H.G.; Moradi, Y. The efficacy and effectiveness of COVID-19 vaccines around the world: A mini-review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 42. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 17 January 2024).
- Clarke, B.E.; Newton, S.E.; Carroll, A.R.; Francis, M.J.; Appleyard, G.; Syred, A.D.; Highfield, P.E.; Rowlands, D.J.; Brown, F. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 1987, 330, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Borisova, G.; Bundule, M.; Grinstein, E.; Dreilina, D.; Dreimane, A.; Kalis, J.; Kozlovskaya, T.; Loseva, V.; Ose, V.; Pumpen, P.; et al. Recombinant capsid structures for exposure of protein antigenic epitopes. Mol. Genet. 1987, 6, 169–174. [Google Scholar]
- Peyret, H.; Stephen, S.; Stonehouse, N.J.; Rowlands, D.J. History and potential of hepatitis B virus core as a VLP vaccine platform. In Viral Nanotechnology, 1st ed.; Khudyakov, Y., Pumpens, P., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2015; pp. 177–186. ISBN 978-042-916-674-7. [Google Scholar] [CrossRef]
- Pumpens, P.; Grens, E. Artificial genes for chimeric virus-like particles. In Artificial DNA: Methods and Applications, 1st ed.; Khudiakov, Y.E., Fields, H.A., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2002; pp. 249–327. ISBN 978-042-912-888-2. [Google Scholar] [CrossRef]
- Pumpens, P.; Ulrich, R.; Sasnauskas, K.; Kazaks, A.; Ose, V.; Grens, E. Construction of novel vaccines on the basis of the virus-like particles: Hepatitis B virus proteins as vaccine carriers. In Medicinal Protein Engineering, 1st ed.; Khudyakov, Y.E., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008; pp. 205–248. ISBN 978-042-912-869-1. [Google Scholar] [CrossRef]
- Kramvis, A. Genotypes and genetic variability of hepatitis B virus. Intervirology 2014, 57, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Petrovskis, I.; Lieknina, I.; Dislers, A.; Jansons, J.; Bogans, J.; Akopjana, I.; Zakova, J.; Sominskaya, I. Production of the HBc Protein from Different HBV Genotypes in E. coli. Use of Reassociated HBc VLPs for Packaging of ss- and dsRNA. Microorganisms 2021, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Pan, Y.; Zhao, Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020, 525, 135–140. [Google Scholar] [CrossRef]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O.; et al. AP205 VLPs Based on Dimerized Capsid Proteins Accommodate RBM Domain of SARS-CoV-2 and Serve as an Attractive Vaccine Candidate. Vaccines 2021, 9, 403. [Google Scholar] [CrossRef]
- Almanza, G.; Clark, A.E.; Kouznetsova, V.; Olmedillas, E.; Castro, A.; Tsigelny, I.F.; Wu, Y.; Gao, G.F.; Leibel, S.L.; Bray, W.; et al. Structure-selected RBM immunogens prime polyclonal memory responses that neutralize SARS-CoV-2 variants of concern. PLoS Pathog. 2022, 18, e1010686. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Parray, H.A.; Agrahari, A.K.; Rizvi, Z.A.; Kaul, R.; Raj, S.; Asthana, S.; Mani, S.; Samal, S.; Awasthi, A.; et al. Designing and characterization of a SARS-CoV-2 immunogen with receptor binding motif grafted on a protein scaffold: An epitope-focused vaccine approach. Int. J. Biol. Macromol. 2022, 209, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Balke, I.; Zinkhan, S.; Zeltina, V.; Liu, X.; Chang, X.; Krenger, P.S.; Plattner, K.; Gharailoo, Z.; Vogt, A.S.; et al. A scalable and highly immunogenic virus-like particle-based vaccine against SARS-CoV-2. Allergy 2022, 77, 243–257. [Google Scholar] [CrossRef]
- Grandi, A.; Tomasi, M.; Ullah, I.; Bertelli, C.; Vanzo, T.; Accordini, S.; Gagliardi, A.; Zanella, I.; Benedet, M.; Corbellari, R.; et al. Immunogenicity and pre-clinical efficacy of an OMV-based SARS-CoV-2 vaccine. Vaccines 2023, 11, 1546. [Google Scholar] [CrossRef]
- Oliveira, S.C.; de Magalhães, M.T.Q.; Homan, E.J. Immunoinformatic Analysis of SARS-CoV-2 Nucleocapsid Protein and Identification of COVID-19 Vaccine Targets. Front. Immunol. 2020, 11, 587615. [Google Scholar] [CrossRef]
- Ahlén, G.; Frelin, L.; Nikouyan, N.; Weber, F.; Höglund, U.; Larsson, O.; Westman, M.; Tuvesson, O.; Gidlund, E.K.; Cadossi, M.; et al. OPENCORONA Consortium. The SARS-CoV-2 N Protein Is a Good Component in a Vaccine. J. Virol. 2020, 94, e01279-20. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Mazumdar, K.; Gordy, J.T. The Nucleocapsid Protein of SARS-CoV-2: A Target for Vaccine Development. J. Virol. 2020, 94, e00647-20. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Cheng, Y.; Zhou, H.; Sun, C.; Zhang, S. The SARS-CoV-2 nucleocapsid protein: Its role in the viral life cycle, structure and functions, and use as a potential target in the development of vaccines and diagnostics. Virol. J. 2023, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Devi, Y.D.; Goswami, H.B.; Konwar, S.; Doley, C.; Dolley, A.; Devi, A.; Chongtham, C.; Dowerah, D.; Biswa, V.; Jamir, L.; et al. Immunoinformatics mapping of potential epitopes in SARS-CoV-2 structural proteins. PLoS ONE 2021, 16, e0258645. [Google Scholar] [CrossRef] [PubMed]
- Pumpens, P.; Pushko, P. Order Blubervirales; Core Protein. In Virus-Like Particles: A Comprehensive Guide; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2022; pp. 563–602. ISBN 978-100-327-971-6. [Google Scholar] [CrossRef]
- De Baets, S.; Roose, K.; Schepens, B.; Saelens, X. Presenting heterologous epitopes with hepatitis B core-based virus-like particles. In Virus-like Particles in Vaccine Development; Buonaguro, F.M., Buonaguro, L., Eds.; Future Medicine: London, UK, 2014; pp. 68–84. ISBN 978-178-084-417-6. [Google Scholar] [CrossRef]
- Mihailova, M.; Boos, M.; Petrovskis, I.; Ose, V.; Skrastina, D.; Fiedler, M.; Sominskaya, I.; Ross, S.; Pumpens, P.; Roggendorf, M.; et al. Recombinant virus-like particles as a carrier of B- and T-cell epitopes of hepatitis C virus (HCV). Vaccine 2006, 24, 4369–4377. [Google Scholar] [CrossRef] [PubMed]
- Skrastina, D.; Bulavaite, A.; Sominskaya, I.; Kovalevska, L.; Ose, V.; Priede, D.; Pumpens, P.; Sasnauskas, K. High immunogenicity of a hydrophilic component of the hepatitis B virus preS1 sequence exposed on the surface of three virus-like particle carriers. Vaccine 2008, 26, 1972–1981. [Google Scholar] [CrossRef]
- Skrastina, D.; Petrovskis, I.; Petraityte, R.; Sominskaya, I.; Ose, V.; Lieknina, I.; Bogans, J.; Sasnauskas, K.; Pumpens, P. Chimeric derivatives of hepatitis B virus core particles carrying major epitopes of the rubella virus E1 glycoprotein. Clin. Vaccine Immunol. 2013, 20, 1719–1728. [Google Scholar] [CrossRef]
- Dishlers, A.; Petrovskis, I.; Skrastina, D.; Zarina, I.; Lieknina, I.; Jansons, J.; Akopjana, I.; Zakova, J.; Ose, V.; Sominskaya, I. PreS1 Containing HBc VLPs for the Development of a Combined Therapeutic/Prophylactic Hepatitis B Vaccine. Microorganisms 2023, 11, 972. [Google Scholar] [CrossRef]
- Brune, K.D.; Liekniņa, I.; Sutov, G.; Morris, A.R.; Jovicevic, D.; Kalniņš, G.; Kazāks, A.; Kluga, R.; Kastaljana, S.; Zajakina, A.; et al. N-Terminal Modification of Gly-His-Tagged Proteins with Azidogluconolactone. Chembiochem 2021, 22, 3199–3207. [Google Scholar] [CrossRef]
- Petkov, S.; Kilpeläinen, A.; Bayurova, E.; Latanova, A.; Mezale, D.; Fridrihsone, I.; Starodubova, E.; Jansons, J.; Dudorova, A.; Gordeychuk, I.; et al. HIV-1 Protease as DNA Immunogen against Drug Resistance in HIV-1 Infection: DNA Immunization with Drug Resistant HIV-1 Protease Protects Mice from Challenge with Protease-Expressing Cells. Cancers 2022, 15, 238. [Google Scholar] [CrossRef]
- Bartosch, B.; Dubuisson, J.; Cosset, F.L. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 2003, 197, 633–642. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug. Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Russo, G.; Parasiliti Palumbo, G.A.; Pappalardo, F. In silico design of recombinant multi-epitope vaccine against influenza A virus. BMC Bioinform. 2021, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, E.A.; Kuprianov, V.V.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Ravin, N.V.; Skryabin, K.G. A molecular assembly system for presentation of antigens on the surface of HBc virus-like particles. Virology 2013, 435, 293–300. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, A.; Sun, X.; Sha, W.; Ai, K.; Pan, G.; Zhou, C.; Zhou, H.; Cong, H.; He, S. Immunogenicity of a Virus-Like-Particle Vaccine Containing Multiple Antigenic Epitopes of Toxoplasma gondii Against Acute and Chronic Toxoplasmosis in Mice. Front. Immunol. 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ku, Z.; Liu, Q.; Wang, X.; Shi, J.; Zhang, Y.; Kong, L.; Cong, Y.; Huang, Z. Chimeric virus-like particle vaccines displaying conserved enterovirus 71 epitopes elicit protective neutralizing antibodies in mice through divergent mechanisms. J. Virol. 2014, 88, 72–81. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, Y.; Chai, F.; Tan, Y.; Huo, C.; Pan, Z. Chimeric virus-like particles containing a conserved region of the G protein in combination with a single peptide of the M2 protein confer protection against respiratory syncytial virus infection. Antivir. Res. 2016, 131, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sazegari, S.; Akbarzadeh, N.M.; Afsharifar, A.; Niazi, A.; Derakhshandeh, A.; Moradi, V.M.; Hemmati, F.; Eskandari, M.H. Chimeric Hepatitis B core virus-like particles harboring SARS-CoV2 epitope elicit a humoral immune response in mice. Microb. Cell Fact. 2023, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Hassebroek, A.M.; Sooryanarain, H.; Heffron, C.L.; Hawks, S.A.; LeRoith, T.; Cecere, T.E.; Stone, W.B.; Walter, D.; Mahsoub, H.M.; Wang, B.; et al. A hepatitis B virus core antigen-based virus-like particle vaccine expressing SARS-CoV-2 B and T cell epitopes induces epitope-specific humoral and cell-mediated immune responses but confers limited protection against SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28503. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.F.; Sun, C.; Zhuang, Z.; Yuan, R.-Y.; Zheng, Q.; Li, J.-P.; Zhou, P.P.; Chen, X.-C.; Liu, Z.; Zhang, X.; et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-Binding Domain Self-Assembled Nanoparticle Vaccine Candidates. ACS Nano 2021, 15, 2738–2752. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.M.; Stephenson, R.J. Advances in Infectious Disease Vaccine Adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Choubini, E.; Habibi, M.; Khorshidi, A.; Ghasemi, A.; Asadi Karam, M.R.; Bouzari, S. A novel multi-peptide subunit vaccine admixed with AddaVax adjuvant produces significant immunogenicity and protection against Proteus mirabilis urinary tract infection in mice model. Mol. Immunol. 2018, 96, 88–97. [Google Scholar] [CrossRef]
- Gao, F.; Liu, X.; Dang, Y.; Duan, P.; Xu, W.; Zhang, X.; Wang, S.; Luo, J.; Li, X. AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines 2022, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M. Multifunctional analysis of antigen-specific T cells: Correlates of vaccine efficiency. BMC Proc. 2010, 4 (Suppl. S3), O18. [Google Scholar] [CrossRef]
- Boyd, A.; Almeida, J.R.; Darrah, P.A.; Sauce, D.; Seder, R.A.; Appay, V.; Gorochov, G.; Larsen, M. Pathogen-Specific T Cell Polyfunctionality Is a Correlate of T Cell Efficacy and Immune Protection. PLoS ONE 2015, 10, e0128714. [Google Scholar] [CrossRef]
- Han, Q.; Bagheri, N.; Bradshaw, E.M.; Hafler, D.A.; Lauffenburger, D.A.; Love, J.C. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 1607–1612. [Google Scholar] [CrossRef]
- Chen, M.; Venturi, V.; Munier, C.M.L. Dissecting the Protective Effect of CD8+ T Cells in Response to SARS-CoV-2 mRNA Vaccination and the Potential Link with Lymph Node CD8+ T Cells. Biology 2023, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Babji, S.; Parthiban, C.; Madhavan, R.; Adiga, V.; Eveline, J.C.; Kumar, N.C.; Ahmed, A.; Shivalingaiah, S.; Shashikumar, N.; et al. Polyfunctional CD4 T-cells correlating with neutralising antibody is a hallmark of COVISHIELDTM and COVAXIN® induced immunity in COVID-19 exposed Indians. NPJ Vaccines 2023, 8, 134–151. [Google Scholar] [CrossRef] [PubMed]
- Portmann, K.; Linder, A.; Eyer, K. Stimulation-induced cytokine polyfunctionality as a dynamic concept. Life 2023, 12, RP89781. [Google Scholar] [CrossRef]
- Bai, Z.; Cao, Y.; Liu, W.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef]
- Sominskaya, I.; Skrastina, D.; Dislers, A.; Vasiljev, D.; Mihailova, M.; Ose, V.; Dreilina, D.; Pumpens, P. Construction and immunological evaluation of multivalent hepatitis B virus (HBV) core virus-like particles carrying HBV and HCV epitopes. Clin. Vaccine Immunol. 2010, 17, 1027–1033. [Google Scholar] [CrossRef]
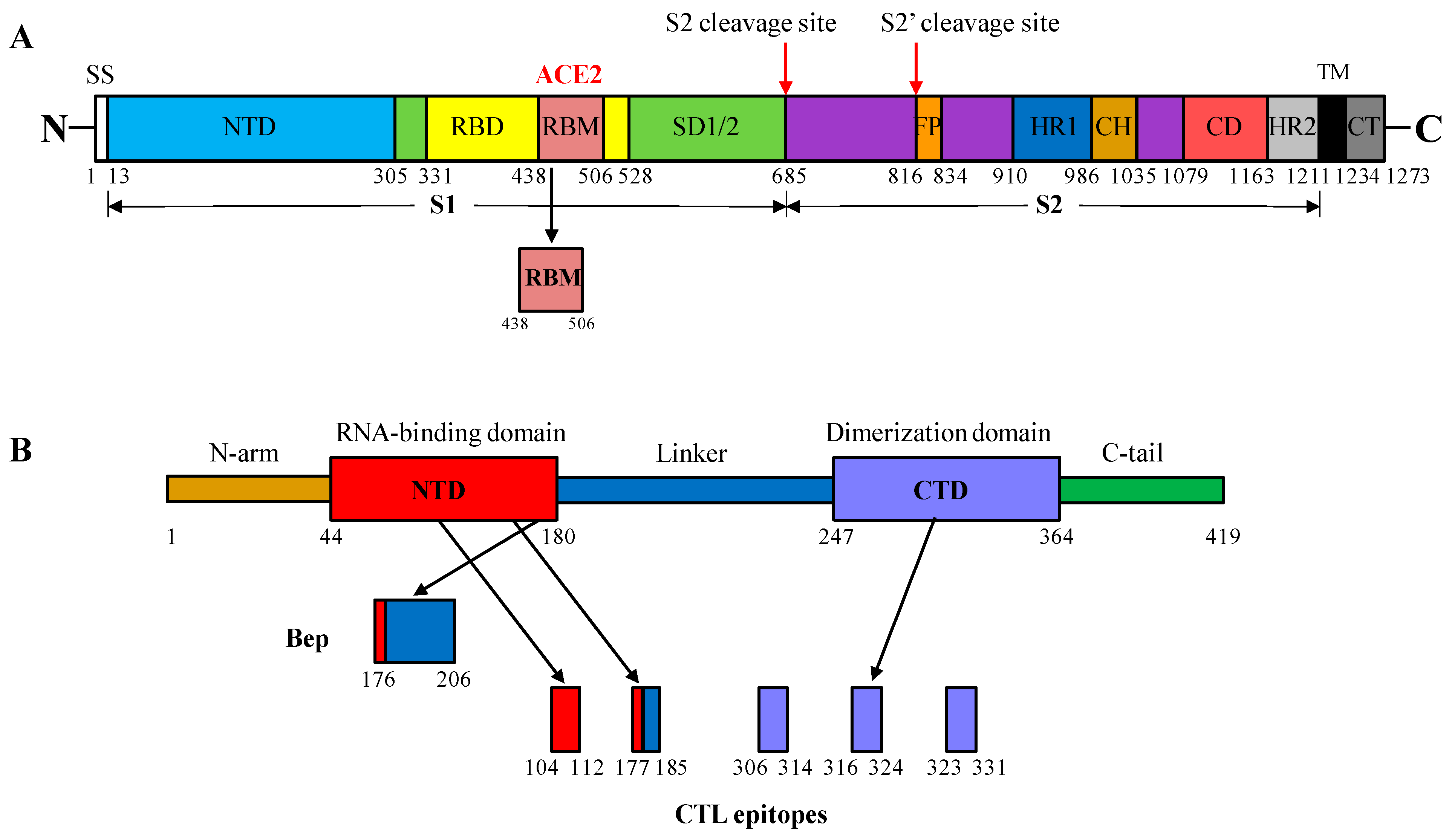



| Expression Vector | Fusion Protein (aa) | Inserted Sequence (aa) | Insertion Site in HBc/G |
|---|---|---|---|
| pET28- RBM | HBc/G-RBM (271 aa) | RBM, 438–506 aa region from the S protein (72 aa) | Between 90 and 91 aa (MIR) |
| pET28- Bep | HBc/G-Bep (226 aa) | Peptide, 176–206 aa region from the N protein (31 aa) | Between 90 and 91 aa (MIR) |
| pET28- CTL161 | HBc/G161-CTL (232 aa) | String of five CTL epitopes from the N protein (57 aa) | After 161 aa |
| pET28- CTL175 | HBc/G175-CTL (218 aa) | String of five CTL epitopes from the N protein (57 aa) | After 175 aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovskis, I.; Skrastina, D.; Jansons, J.; Dislers, A.; Bogans, J.; Spunde, K.; Neprjakhina, A.; Zakova, J.; Zajakina, A.; Sominskaya, I. Toward a SARS-CoV-2 VLP Vaccine: HBc/G as a Carrier for SARS-CoV-2 Spike RBM and Nucleocapsid Protein-Derived Peptides. Vaccines 2024, 12, 267. https://doi.org/10.3390/vaccines12030267
Petrovskis I, Skrastina D, Jansons J, Dislers A, Bogans J, Spunde K, Neprjakhina A, Zakova J, Zajakina A, Sominskaya I. Toward a SARS-CoV-2 VLP Vaccine: HBc/G as a Carrier for SARS-CoV-2 Spike RBM and Nucleocapsid Protein-Derived Peptides. Vaccines. 2024; 12(3):267. https://doi.org/10.3390/vaccines12030267
Chicago/Turabian StylePetrovskis, Ivars, Dace Skrastina, Juris Jansons, Andris Dislers, Janis Bogans, Karina Spunde, Anastasija Neprjakhina, Jelena Zakova, Anna Zajakina, and Irina Sominskaya. 2024. "Toward a SARS-CoV-2 VLP Vaccine: HBc/G as a Carrier for SARS-CoV-2 Spike RBM and Nucleocapsid Protein-Derived Peptides" Vaccines 12, no. 3: 267. https://doi.org/10.3390/vaccines12030267
APA StylePetrovskis, I., Skrastina, D., Jansons, J., Dislers, A., Bogans, J., Spunde, K., Neprjakhina, A., Zakova, J., Zajakina, A., & Sominskaya, I. (2024). Toward a SARS-CoV-2 VLP Vaccine: HBc/G as a Carrier for SARS-CoV-2 Spike RBM and Nucleocapsid Protein-Derived Peptides. Vaccines, 12(3), 267. https://doi.org/10.3390/vaccines12030267






