Mechanism of Action of Oral Salmonella-Based Vaccine to Prevent and Reverse Type 1 Diabetes in NOD Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Salmonella Vaccine
2.2. Animal Vaccination
2.3. Flow Cytometry
2.4. In Vitro Antigen-Specific Suppression Assay
2.5. Adoptive Transfer of Diabetes
2.6. ELISA and Cytokine Measurement
2.7. Gene Expression
2.8. Statistical Analyses
3. Results
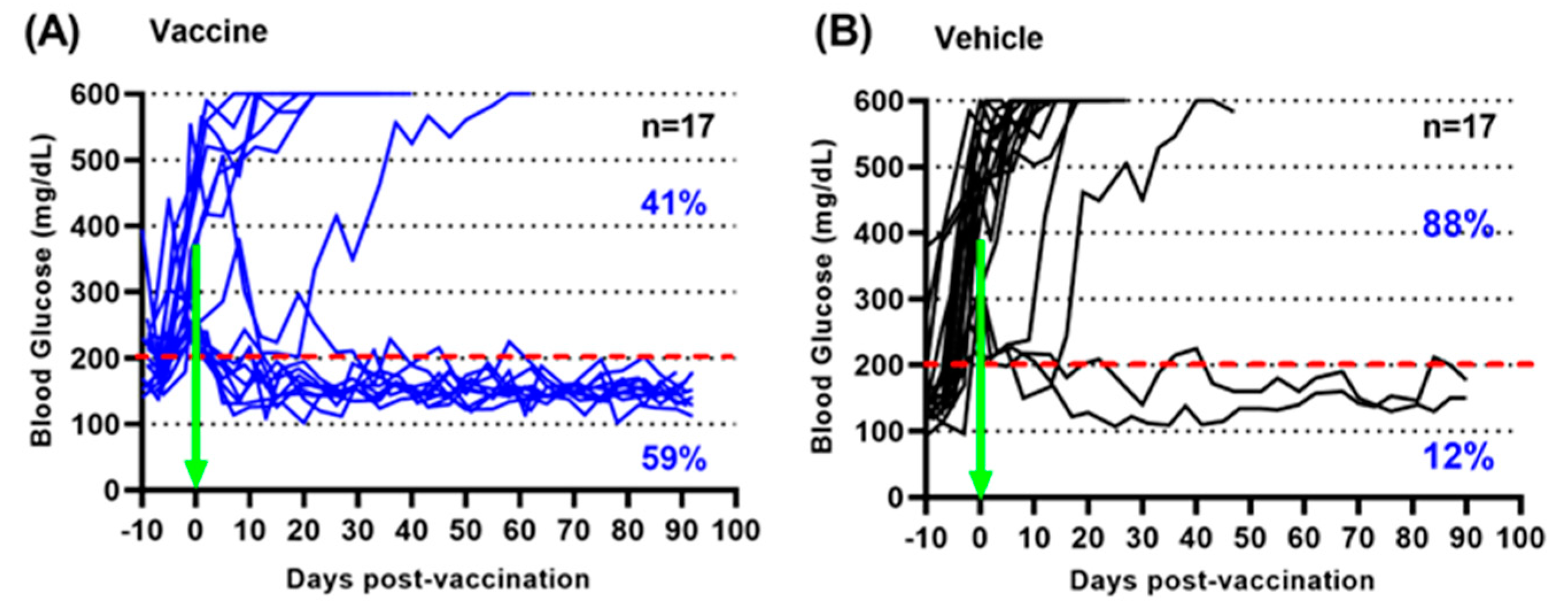
3.1. A Salmonella-Based Vaccine Reverses Diabetes in Diabetic Mice
3.2. A Salmonella-Based Vaccine Increases Tolerogenic Cytokines and Decreases Pro-Inflammatory Cytokines without Altering the Immune Response to Salmonella
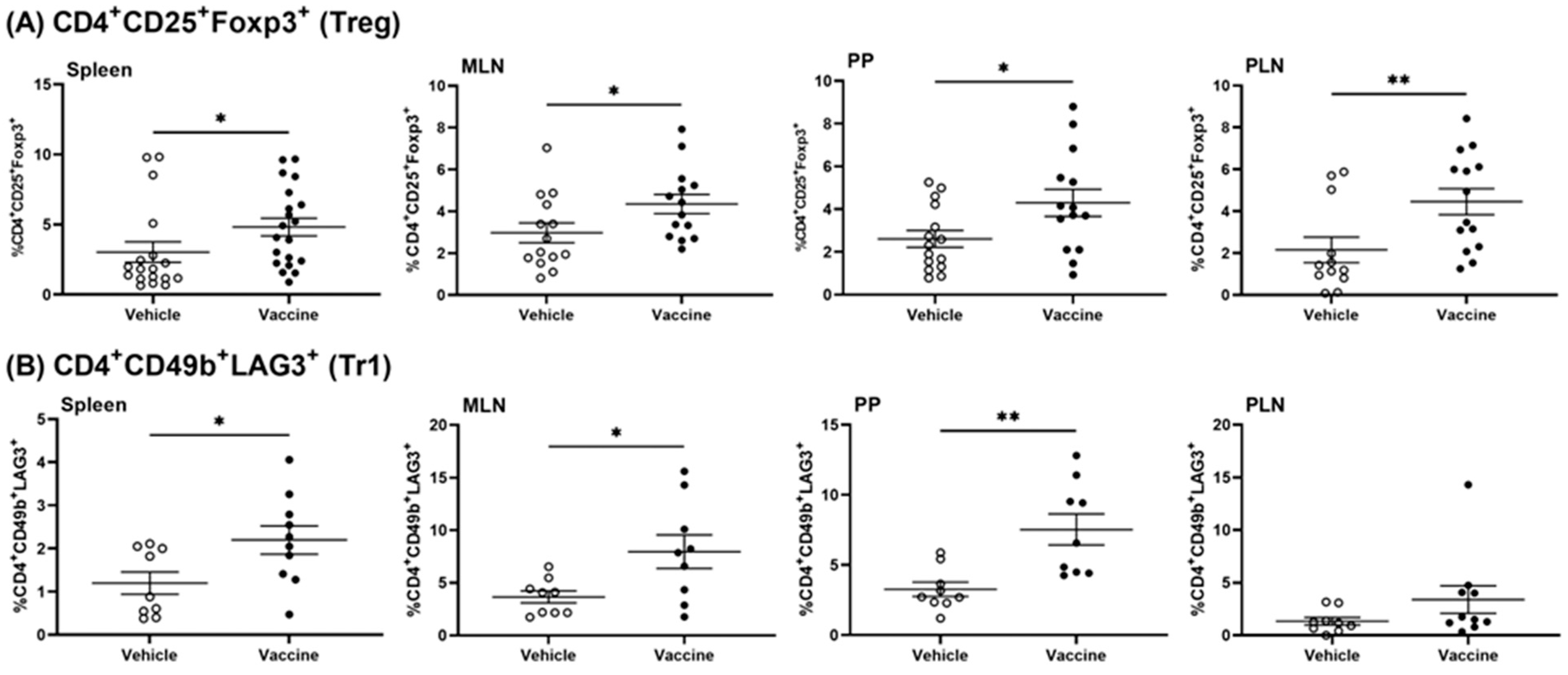
3.3. A Salmonella-Based Vaccine Induces Regulatory T-Cells
3.4. A Salmonella-Based Vaccine Induces Functional Tregs
3.5. A Salmonella-Based Vaccine Induces Antigen-Specific Suppressor Tregs
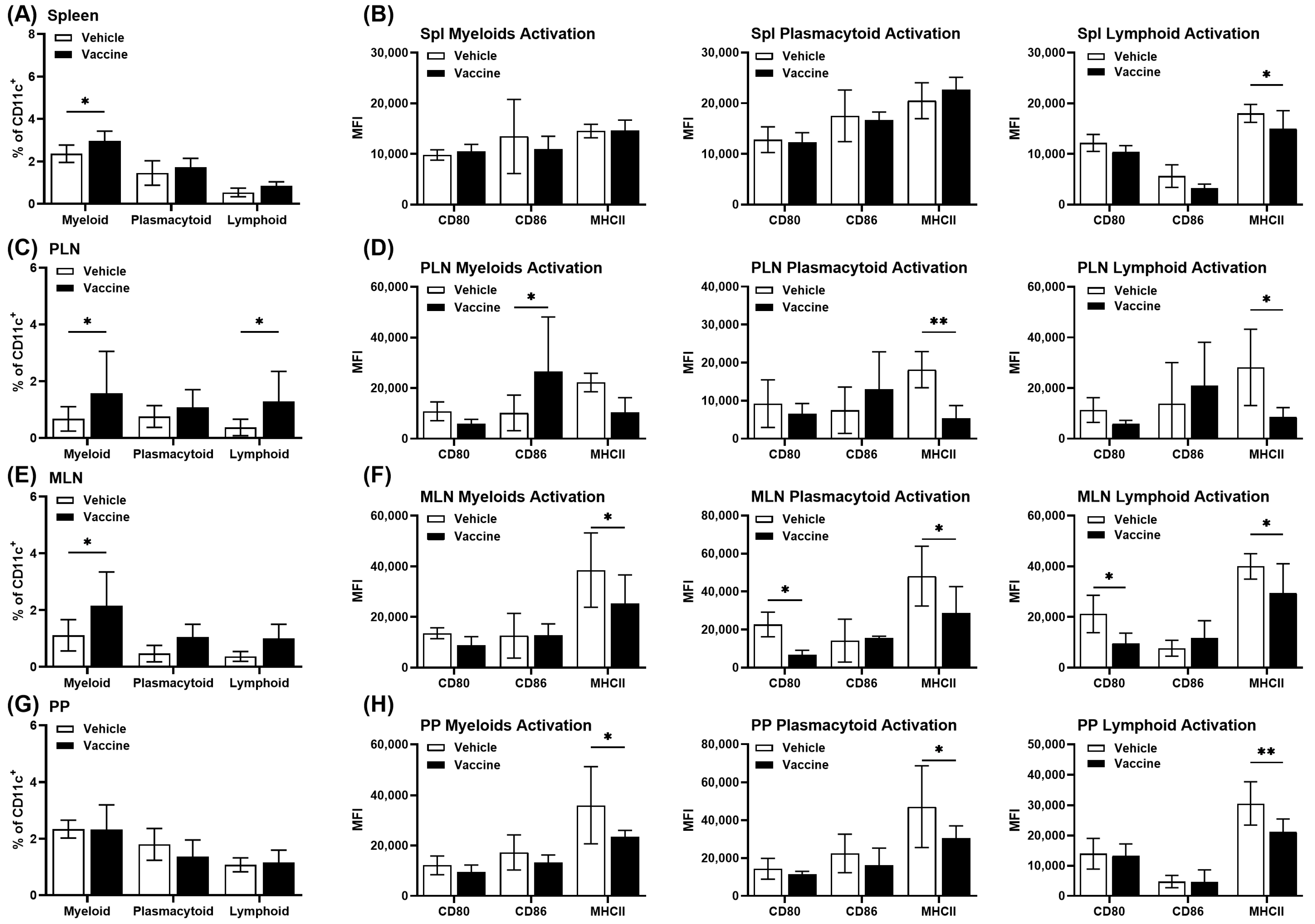
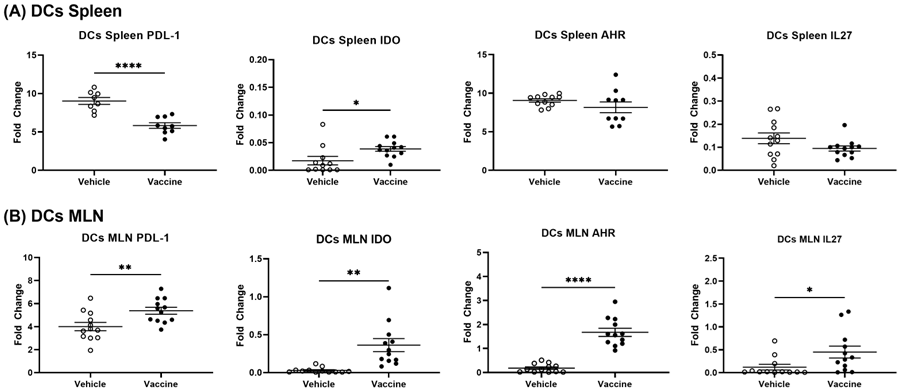
3.6. A Salmonella-Based Vaccine Induces Tolerogenic Dendritic Cells (tol-DCs)
3.7. Tolerogenic DCs Contribute to Diabetes Prevention by a Salmonella-Based Vaccine
3.8. A Salmonella-Based Vaccine Increases the Expression of the Autoimmune Inhibitory Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.-X.; Qiao, Y.-C.; Li, W.; Zou, X.; Chen, Y.-L.; Shen, J.; Liao, Q.-Y.; Zhang, Q.-J.; He, L.; Zhao, H.-L. Human amylin induces CD4+Foxp3+ regulatory T cells in the protection from autoimmune diabetes. Immunol. Res. 2018, 66, 179–186. [Google Scholar] [CrossRef]
- Cordero, O.J.; Reche, P.A.; Rodríguez, M.; Sueiro, A.M.; Viñuela, J.E.; Calviño, R.V.; Calviño-Sampedro, C.; Gomez-Tourino, I.; Gomez-Perosanz, M.; Sánchez-Trincado, J.L. Naturally presented HLA class l–restricted epitopes from the neurotrophic factor S100-β are targets of the autoimmune response in type 1 diabetes. FASEB J. 2019, 33, 6390–6401. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Araña, M.; Radichev, I.; Smith, P.; Huarte, E.; Barajas, M. New insights into immunotherapy strategies for treating autoimmune diabetes. Int. J. Mol. Sci. 2019, 20, 4789. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Beilke, J.N.; Jasinski, J.M.; Kobayashi, M.; Miao, D.; Li, M.; Coulombe, M.G.; Liu, E.; Elliott, J.F.; Gill, R.G.; et al. Priming and effector dependence on insulin B:9–23 peptide in NOD islet autoimmunity. J. Clin. Investig. 2007, 117, 1835–1843. [Google Scholar] [CrossRef]
- Achenbach, P.; Koczwara, K.; Knopff, A.; Naserke, H.; Ziegler, A.-G.; Bonifacio, E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J. Clin. Investig. 2004, 114, 589–597. [Google Scholar] [CrossRef]
- Kronenberg, D.; Knight, R.R.; Estorninho, M.; Ellis, R.J.; Kester, M.G.; de Ru, A.; Eichmann, M.; Huang, G.C.; Powrie, J.; Dayan, C.M.; et al. Circulating preproinsulin signal peptide–specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells. Diabetes 2012, 61, 1752–1759. [Google Scholar] [CrossRef]
- Skowera, A.; Ellis, R.J.; Varela-Calviño, R.; Arif, S.; Huang, G.C.; Van-Krinks, C.; Zaremba, A.; Rackham, C.; Allen, J.S.; Tree, T.I.; et al. CTLs are targeted to kill β cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Investig. 2008, 118, 3390–3402. [Google Scholar] [CrossRef]
- Xu, D.; Prasad, S.; Miller, S.D. Inducing immune tolerance: A focus on type 1 diabetes mellitus. Diabetes Manag. 2013, 3, 415–426. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Rawson, J.; Kaye, A.; Nair, I.; Todorov, I.; Hensel, M.; Kandeel, F.; Ferreri, K. An oral vaccine for type 1 diabetes based on live attenuated Salmonella. Vaccine 2014, 32, 2300–2307. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Du, W.; Mbongue, J.; Lenz, A.; Rawson, J.; Kandeel, F.; Ferreri, K. Factors affecting Salmonella-based combination immunotherapy for prevention of type 1 diabetes in non-obese diabetic mice. Vaccine 2018, 36, 8008–8018. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Rawson, J.; Garcia, P.A.; Gonzalez, N.; Cobb, J.; Kandeel, F.; Ferreri, K.; Husseiny, M.I. Reversal of new onset type 1 diabetes by oral Salmonella-based combination therapy and mediated by regulatory T-cells in NOD mice. Front. Immunol. 2019, 10, 320. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Alhoshani, A.; Rawson, J.; Garcia, P.A.; Gonzalez, N.; Ferreri, K.; Kandeel, F.; Husseiny, M.I. Tracking of an oral Salmonella-based vaccine for type 1 diabetes in non-obese diabetic mice. Front. Immunol. 2020, 11, 712. [Google Scholar] [CrossRef]
- Cobb, J.; Rawson, J.; Gonzalez, N.; Hensel, M.; Kandeel, F.; Husseiny, M.I. Oral Salmonella msbB mutant as a carrier for a Salmonella-based vaccine for prevention and reversal of type 1 diabetes. Front. Immunol. 2021, 12, 667897. [Google Scholar] [CrossRef]
- Cobb, J.; Soliman, S.S.M.; Retuerto, M.; Quijano, J.C.; Orr, C.; Ghannoum, M.; Kandeel, F.; Husseiny, M.I. Changes in the gut microbiota of NOD mice in response to an oral Salmonella-based vaccine against type 1 diabetes. PLoS ONE 2023, 18, e0285905. [Google Scholar] [CrossRef]
- Xu, X.; Husseiny, M.I.; Goldwich, A.; Hensel, M. Efficacy of intracellular activated promoters for generation of Salmonella-based vaccines. Infect. Immun. 2010, 78, 4828–4838. [Google Scholar] [CrossRef]
- Xiong, G.; Husseiny, M.I.; Song, L.; Erdreich-Epstein, A.; Shackleford, G.M.; Seeger, R.C.; Jäckel, D.; Hensel, M.; Metelitsa, L.S. Novel cancer vaccine based on genes of Salmonella pathogenicity island 2. Int. J. Cancer 2010, 126, 2622–2634. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Hensel, M. Evaluation of Salmonella live vaccines with chromosomal expression cassettes for translocated fusion proteins. Vaccine 2009, 27, 3780–3787. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Hensel, M. Construction of highly attenuated Salmonella enterica serovar Typhimurium live vectors for delivering heterologous antigens by chromosomal integration. Microbiol. Res. 2008, 163, 605–615. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Hensel, M. Evaluation of an intracellular-activated promoter for the generation of live Salmonella recombinant vaccines. Vaccine 2005, 23, 2580–2590. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Hensel, M. Rapid method for the construction of Salmonella enterica serovar Typhimurium vaccine carrier strains. Infect. Immun. 2005, 73, 1598–1605. [Google Scholar] [CrossRef]
- Husseiny, M.I.; Wartha, F.; Hensel, M. Recombinant vaccines based on translocated effector proteins of Salmonella pathogenicity island 2. Vaccine 2007, 25, 185–193. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sato, E.; Briones, G.; Chen, L.-M.; Matsuo, M.; Nagata, Y.; Ritter, G.; Jäger, E.; Nomura, H.; Kondo, S.; et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Investig. 2006, 116, 1946–1954. [Google Scholar] [CrossRef]
- Evans, D.T.; Chen, L.-M.; Gillis, J.; Lin, K.-C.; Harty, B.; Mazzara, G.P.; Donis, R.O.; Mansfield, K.G.; Lifson, J.D.; Desrosiers, R.C.; et al. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 2003, 77, 2400–2409. [Google Scholar] [CrossRef]
- Jeker, L.T.; Bour-Jordan, H.; Bluestone, J.A. Breakdown in peripheral tolerance in type 1 diabetes in mice and humans. Cold Spring Harb. Perspect. Med. 2012, 2, a007807. [Google Scholar] [CrossRef]
- Parent, A.V.; Faleo, G.; Chavez, J.; Saxton, M.; Berrios, D.I.; Kerper, N.R.; Tang, Q.; Hebrok, M. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 2021, 36, 109538. [Google Scholar] [CrossRef]
- Gojanovich, G.S.; Hess, P.R. Making the most of major histocompatibility complex molecule multimers: Applications in type 1 diabetes. J. Immunol. Res. 2012, 2012, 380289. [Google Scholar] [CrossRef]
- Schweiger, D.S. Recent advances in immune-based therapies for type 1 diabetes. Horm. Res. Paediatr. 2022, 96, 631–645. [Google Scholar] [CrossRef]
- Bluestone, J.A.; Tang, Q. Solving the puzzle of immune tolerance for β-cell replacement therapy for type 1 diabetes. Cell Stem Cell 2020, 27, 505–507. [Google Scholar] [CrossRef]
- Gitelman, S.E.; Bluestone, J.A. Regulatory T cell therapy for type 1 diabetes: May the force be with you. J. Autoimmun. 2016, 71, 78–87. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1564. [Google Scholar] [CrossRef]
- Rigby, M.R.; Harris, K.M.; Pinckney, A.; DiMeglio, L.A.; Rendell, M.S.; Felner, E.I.; Dostou, J.M.; Gitelman, S.E.; Griffin, K.J.; Tsalikian, E.; et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Investig. 2015, 125, 3285–3296. [Google Scholar] [CrossRef]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of Treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef]
- Lu, J.; Liu, J.; Li, L.; Lan, Y.; Liang, Y. Cytokines in type 1 diabetes: Mechanisms of action and immunotherapeutic targets. Clin. Transl. Immunol. 2020, 9, e1122. [Google Scholar] [CrossRef]
- Velaga, S.; Ukena, S.N.; Dringenberg, U.; Alter, C.; Pardo, J.; Kershaw, O.; Franzke, A. Granzyme A is required for regulatory T-cell mediated prevention of gastrointestinal graft-versus-host disease. PLoS ONE 2015, 10, e0124927. [Google Scholar] [CrossRef]
- Zóka, A.; Barna, G.; Somogyi, A.; Műzes, G.; Oláh, A.; Al-Aissa, Z.; Hadarits, O.; Kiss, K.; Firneisz, G. Extension of the CD4+Foxp3+CD25−/low regulatory T-cell subpopulation in type 1 diabetes mellitus. Autoimmunity 2015, 48, 289–297. [Google Scholar] [CrossRef]
- Ryba-Stanisławowska, M.; Rybarczyk-Kapturska, K.; Myśliwiec, M.; Myśliwska, J. Elevated levels of serum IL-12 and IL-18 are associated with lower frequencies of CD4+CD25highFOXP3+ regulatory T cells in young patients with type 1 diabetes. Inflammation 2014, 37, 1513–1520. [Google Scholar] [CrossRef]
- Aghili, B.; Amirzargar, A.A.; Rajab, A.; Rabbani, A.; Sotoudeh, A.; Assadiasl, S.; Larijani, B.; Massoud, A. Altered suppressor function of regulatory T cells in type 1 diabetes. Iran J. Immunol. 2015, 12, 240–251. [Google Scholar]
- Funda, D.P.; Goliáš, J.; Hudcovic, T.; Kozáková, H.; Špíšek, R.; Palová-Jelínková, L. Antigen loading (e.g., Glutamic Acid Decarboxylase 65) of tolerogenic DCs (tolDCs) reduces their capacity to prevent diabetes in the non-obese diabetes (NOD)-severe combined immunodeficiency model of adoptive co-transfer of diabetes as well as in NOD mice. Front. Immunol. 2018, 9, 290. [Google Scholar] [CrossRef]
- Yu, H.; Paiva, R.; Flavell, R.A. Harnessing the power of regulatory T-cells to control autoimmune diabetes: Overview and perspective. Immunology 2017, 153, 161–170. [Google Scholar] [CrossRef]
- Badami, E.; Sorini, C.; Coccia, M.; Usuelli, V.; Molteni, L.; Bolla, A.M.; Scavini, M.; Mariani, A.; King, C.; Bosi, E.; et al. Defective differentiation of regulatory Foxp3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes 2011, 60, 2120–2124. [Google Scholar] [CrossRef]
- Bellemore, S.M.; Nikoopour, E.; A Schwartz, J.; Krougly, O.; Lee-Chan, E.; Singh, B. Preventative role of interleukin-17 producing regulatory T helper type 17 (Treg17) cells in type 1 diabetes in non-obese diabetic mice. Clin. Exp. Immunol. 2015, 182, 261–269. [Google Scholar] [CrossRef]
- Gregori, S.; Tomasoni, D.; Pacciani, V.; Scirpoli, M.; Battaglia, M.; Magnani, C.F.; Hauben, E.; Roncarolo, M.-G. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10–dependent ILT4/HLA-G pathway. Blood 2010, 116, 935–944. [Google Scholar] [CrossRef]
- Lohr, J.; Knoechel, B.; Abbas, A.K. Regulatory T cells in the periphery. Immunol. Rev. 2006, 212, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Raker, V.K.; Domogalla, M.P.; Steinbrink, K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front. Immunol. 2015, 6, 569. [Google Scholar] [CrossRef] [PubMed]
- Wakkach, A.; Fournier, N.; Brun, V.; Breittmayer, J.-P.; Cottrez, F.; Groux, H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003, 18, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Vasco, C.; Gruarin, P.; Bonnal, R.; Rossetti, G.; Silvestri, Y.; Carelli, E.; Pulvirenti, N.; Scantamburlo, M.; Moschetti, G.; et al. Eomesodermin-expressing type 1 regulatory (EOMES+Tr1)-like T cells: Basic biology and role in immune-mediated diseases. Eur. J. Immunol. 2023, 53, e2149775. [Google Scholar] [CrossRef]
- Zhu, D.; Mengyue, M.; Qimuge, A.; Bilige, B.; Baiyin, T.; Temuqile, T.; Chen, S.; Borjigen, S.; Baigude, H.; Yang, D. Oral delivery of SARS-CoV-2 DNA vaccines using attenuated Salmonella typhimurium as a carrier in rat. Mol. Genet. Microbiol. Virol. 2022, 37, 159–166. [Google Scholar] [CrossRef]
- Takiishi, T.; Korf, H.; Van Belle, T.L.; Robert, S.; Grieco, F.A.; Caluwaerts, S.; Galleri, L.; Spagnuolo, I.; Steidler, L.; Van Huynegem, K.; et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J. Clin. Investig. 2012, 122, 1717–1725. [Google Scholar] [CrossRef]
- Harrison, L.C.; Wentworth, J.M.; Zhang, Y.; Bandala-Sanchez, E.; Böhmer, R.M.; Neale, A.M.; Stone, N.L.; Naselli, G.; Bosco, J.J.; Auyeung, P.; et al. Antigen-based vaccination and prevention of type 1 diabetes. Curr. Diabetes Rep. 2013, 13, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Peakman, M.; von Herrath, M. Antigen-specific immunotherapy for type 1 diabetes: Maximizing the potential. Diabetes 2010, 59, 2087–2093. [Google Scholar] [CrossRef]
- Schneider, D.A.; Kretowicz, A.M.; von Herrath, M.G. Emerging immune therapies in type 1 diabetes and pancreatic islet transplantation. Diabetes Obes. Metab. 2013, 15, 581–592. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hawiger, D.; Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003, 21, 685–711. [Google Scholar] [CrossRef]
- Alemohammad, H.; Najafzadeh, B.; Asadzadeh, Z.; Baghbanzadeh, A.; Ghorbaninezhad, F.; Najafzadeh, A.; Safarpour, H.; Bernardini, R.; Brunetti, O.; Sonnessa, M.; et al. The importance of immune checkpoints in immune monitoring: A future paradigm shift in the treatment of cancer. Biomed. Pharmacother. 2022, 146, 112516. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, J.; Mi, Y.; Jin, T. Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis. J. Neuroinflamm. 2022, 19, 298. [Google Scholar] [CrossRef]
- Mao, R.-F.; Chen, Y.-Y.; Zhang, J.; Chang, X.; Wang, Y.-F. Type 1 diabetes mellitus and its oral tolerance therapy. World J. Diabetes 2020, 11, 400–415. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaqq, S.A.; Weiner, D.B. DNA vaccines: Developing new strategies to enhance immune responses. Immunol. Res. 2008, 42, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.J.; Wahren, B.; Liu, M.A. DNA vaccines: Progress and challenges. J. Immunol. 2005, 175, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, S.; Grimes-Serrano, J.M. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines 2008, 7, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Solvason, N.; Lou, Y.-P.; Peters, W.; Evans, E.; Martinez, J.; Ramirez, U.; Ocampo, A.; Yun, R.; Ahmad, S.; Liu, E.; et al. Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J. Immunol. 2008, 181, 8298–8307. [Google Scholar] [CrossRef] [PubMed]
- Coon, B.; An, L.-L.; Whitton, J.L.; von Herrath, M.G. DNA immunization to prevent autoimmune diabetes. J. Clin. Investig. 1999, 104, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Draghici, E.; Migliavacca, B.; Gregori, S.; Bonifacio, E.; Roncarolo, M.-G. Induction of tolerance in type 1 diabetes via both CD4+CD25+ T regulatory cells and T regulatory type 1 cells. Diabetes 2006, 55, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Montanucci, P.; Pescara, T.; Greco, A.; Basta, G.; Calafiore, R. Human induced pluripotent stem cells (hiPSC), enveloped in elastin-like recombinamers for cell therapy of type 1 diabetes mellitus (T1D): Preliminary data. Front. Bioeng. Biotechnol. 2023, 11, 1046206. [Google Scholar] [CrossRef]
- Linsley, P.S.; Long, S.A. Enforcing the checkpoints: Harnessing T-cell exhaustion for therapy of T1D. Curr. Opin. Endocrinol. Diabetes 2019, 26, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Herold, K.C.; Miller, S.D. Immunotherapy of type 1 diabetes: Where are we and where should we be going? Immunity 2010, 32, 488–499. [Google Scholar] [CrossRef]
- Bone, R.N.; Evans-Molina, C. Combination immunotherapy for type 1 diabetes. Curr. Diabetes Rep. 2017, 17, 50. [Google Scholar] [CrossRef]
- Griffin, K.J.; Thompson, P.A.; Gottschalk, M.; Kyllo, J.H.; Rabinovitch, A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2014, 2, 710–718. [Google Scholar] [CrossRef]
- Preisser, T.M.; da Cunha, V.P.; Santana, M.P.; Pereira, V.B.; Cara, D.C.; Souza, B.M.; Miyoshi, A. Recombinant Lactococcus lactis carrying IL-4 and IL-10 coding vectors protects against type 1 diabetes in NOD mice and attenuates insulitis in the STZ-induced model. J. Diabetes Res. 2021, 2021, 6697319. [Google Scholar] [CrossRef]
- Bender, C.; Rajendran, S.; von Herrath, M.G. New insights into the role of autoreactive CD8 T cells and cytokines in human type 1 diabetes. Front. Endocrinol. 2021, 11, 606434. [Google Scholar] [CrossRef] [PubMed]
- Frørup, C.; Gerwig, R.; Svane, C.A.S.; de Melo, J.M.L.; Henriksen, K.; Fløyel, T.; Pociot, F.; Kaur, S.; Størling, J. Characterization of the functional and transcriptomic effects of pro-inflammatory cytokines on human EndoC-βH5 beta cells. Front. Endocrinol. 2023, 14, 1128523. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G.; Gregori, S.; Bacchetta, R.; Battaglia, M. Tr1 Cells and the counter-regulation of immunity: Natural mechanisms and therapeutic applications. Curr. Top. Microbiol. Immunol. 2014, 380, 39–68. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ohayon-Steckel, L.; Coppin, E.; Johny, E.; Dasari, A.; Florentin, J.; Vasamsetti, S.; Dutta, P. Epidermal growth factor receptor in hepatic endothelial cells suppresses MCP-1–dependent monocyte recruitment in diabetes. J. Immunol. 2023, 210, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Garrigan, E.; Belkin, N.S.; Alexander, J.J.; Han, Z.; Seydel, F.; Carter, J.; Atkinson, M.; Wasserfall, C.; Clare-Salzler, M.J.; Amick, M.A.; et al. Persistent STAT5 phosphorylation and epigenetic dysregulation of GM-CSF and PGS2/COX2 expression in type 1 diabetic human monocytes. PLoS ONE 2013, 8, e76919. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Ramirez, N.; Woytschak, J.; Boyman, O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015, 36, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Malek, T.R.; Bayer, A.L. Tolerance, not immunity, crucially depends on IL-2. Nat. Rev. Immunol. 2004, 4, 665–674. [Google Scholar] [CrossRef]
- Hulme, M.A.; Wasserfall, C.H.; Atkinson, M.A.; Brusko, T.M. Central role for interleukin-2 in type 1 diabetes. Diabetes 2012, 61, 14–22. [Google Scholar] [CrossRef]
- Jhala, G.; Selck, C.; Chee, J.; Kwong, C.-T.J.; Pappas, E.G.; Thomas, H.E.; Kay, T.W.; Krishnamurthy, B. Tolerance to proinsulin-1 reduces autoimmune diabetes in NOD mice. Front. Immunol. 2021, 12, 645817. [Google Scholar] [CrossRef]
- Eggenhuizen, P.J.; Ng, B.H.; Ooi, J.D. Treg enhancing therapies to treat autoimmune diseases. Int. J. Mol. Sci. 2020, 21, 7015. [Google Scholar] [CrossRef]
- Ahmed, S.; Bae, Y.-S. Dendritic cell-based immunotherapy for rheumatoid arthritis: From bench to bedside. Immune Netw. 2016, 16, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, B. Tolerogenic dendritic cells and their applications in transplantation. Cell. Mol. Immunol. 2015, 12, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kulhankova, K.; Rouse, T.; Nasr, M.E.; Field, E.H. Dendritic cells control CD4+CD25+ Treg cell suppressor function in vitro through juxtacrine delivery of IL-2. PLoS ONE 2012, 7, e43609. [Google Scholar] [CrossRef]
- Zanoni, I.; Granucci, F. The regulatory role of dendritic cells in the induction and maintenance of T-cell tolerance. Autoimmunity 2011, 44, 23–32. [Google Scholar] [CrossRef]
- Wright, G.P.; Notley, C.A.; Xue, S.-A.; Bendle, G.M.; Holler, A.; Schumacher, T.N.; Ehrenstein, M.R.; Stauss, H.J. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc. Natl. Acad. Sci. USA 2009, 106, 19078–19083. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Yang, X.; Hu, H.; Liu, P.; Liu, H. Crosstalk between dendritic cells and regulatory T cells: Protective effect and therapeutic potential in multiple sclerosis. Front. Immunol. 2022, 13, 970508. [Google Scholar] [CrossRef]
- Serr, I.; Fürst, R.W.; Achenbach, P.; Scherm, M.G.; Gökmen, F.; Haupt, F.; Sedlmeier, E.-M.; Knopff, A.; Shultz, L.; Willis, R.A.; et al. Type 1 diabetes vaccine candidates promote human Foxp3+Treg induction in humanized mice. Nat. Commun. 2016, 7, 10991. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Dong, Y.; Tsurushita, N.; Tso, J.Y.; Fu, W. CD122 blockade restores immunological tolerance in autoimmune type 1 diabetes via multiple mechanisms. J. Clin. Investig. 2018, 3, e96600. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, S.; Rigby, M.; Mirmira, R. Targeting regulatory T cells in the treatment of type 1 diabetes mellitus. Curr. Mol. Med. 2012, 12, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chen, H.; Lan, J.; Lan, C.; Liang, L.; Yu, J.; Zhong, H.; Zhou, X.; Lu, J.; Tan, X.; et al. Th1 or Th2 cytokines are correlated with Tregs and T cell subsets and pregnancy outcomes in patients with autoimmune thyroid disease during early, middle, late pregnancy, and postpartum period. Hum. Immunol. 2023, 84, 525–533. [Google Scholar] [CrossRef]
- Dowling, M.R.; Kan, A.; Heinzel, S.; Marchingo, J.M.; Hodgkin, P.D.; Hawkins, E.D. Regulatory T cells suppress effector T cell proliferation by limiting division destiny. Front. Immunol. 2018, 9, 2461. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, C.; Ortiz, A.Z.; Presa, M.; Malo, S.; Montoya, A.; Garabatos, N.; Mora, C.; Verdaguer, J.; Stratmann, T. Treatment of T1D via optimized expansion of antigen-specific Tregs induced by IL-2/anti-IL-2 monoclonal antibody complexes and peptide/MHC tetramers. Sci. Rep. 2018, 8, 8106. [Google Scholar] [CrossRef]
- Yu, H.; Gagliani, N.; Ishigame, H.; Huber, S.; Zhu, S.; Esplugues, E.; Herold, K.C.; Wen, L.; Flavell, R.A. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc. Natl. Acad. Sci. USA 2017, 114, 10443–10448. [Google Scholar] [CrossRef] [PubMed]
- Spanier, J.A.; Fung, V.; Wardell, C.M.; Alkhatib, M.H.; Chen, Y.; Swanson, L.A.; Dwyer, A.J.; Weno, M.E.; Silva, N.; Mitchell, J.S.; et al. Insulin B peptide-MHC class II-specific chimeric antigen receptor-Tregs prevent autoimmune diabetes in mice. J. Clin. Investig. 2023, 133, e168601. [Google Scholar] [CrossRef] [PubMed]
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef]
- Jia, L.; Lu, J.; Zhou, Y.; Tao, Y.; Xu, H.; Zheng, W.; Zhao, J.; Liang, G.; Xu, L. Tolerogenic dendritic cells induced the enrichment of CD4+Foxp3+ regulatory T cells via TGF-β in mesenteric lymph nodes of murine LPS-induced tolerance model. Clin. Immunol. 2018, 197, 118–129. [Google Scholar] [CrossRef]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.E.; Garciafigueroa, Y.; Trucco, M.; Giannoukakis, N. Clinical tolerogenic dendritic cells: Exploring therapeutic impact on human autoimmune disease. Front. Immunol. 2017, 8, 1279. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Quintana, F.J. Tolerogenic dendritic cells. Semin. Immunopathol. 2017, 39, 113–120. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Wang, J.; Zhang, W.; Xu, B.; Xu, X.; Zong, L. Targeted delivery of antigen to intestinal dendritic cells induces oral tolerance and prevents autoimmune diabetes in NOD mice. Diabetologia 2018, 61, 1384–1396. [Google Scholar] [CrossRef]
- Brown, C.C.; Gudjonson, H.; Pritykin, Y.; Deep, D.; Lavallée, V.-P.; Mendoza, A.; Fromme, R.; Mazutis, L.; Ariyan, C.; Leslie, C.; et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell 2019, 179, 846–863.e24. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front. Immunol. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Pina, A.; de Araujo, E.F.; Felonato, M.; Loures, F.V.; Feriotti, C.; Bernardino, S.; Barbuto, J.A.M.; Calich, V.L.G. Myeloid dendritic cells (DCs) of mice susceptible to paracoccidioidomycosis suppress T cell responses whereas myeloid and plasmacytoid DCs from resistant mice induce effector and regulatory T cells. Infect. Immun. 2013, 81, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.F.; Tussiwand, R. Novel concepts in plasmacytoid dendritic cell (pDC) development and differentiation. Mol. Immunol. 2020, 126, 25–30. [Google Scholar] [CrossRef]
- Castenmiller, C.; Keumatio-Doungtsop, B.-C.; van Ree, R.; de Jong, E.C.; van Kooyk, Y. Tolerogenic immunotherapy: Targeting DC surface receptors to induce antigen-specific tolerance. Front. Immunol. 2021, 12, 643240. [Google Scholar] [CrossRef] [PubMed]
- Morel, P.A. Dendritic cell subsets in type 1 diabetes: Friend or foe? Front. Immunol. 2013, 4, 415. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Hachimura, S.; Ametani, A.; Sato, T.; Kojima, H.; Kumagai, Y.; Habu, S.; Kobata, T.; Kaminogawa, S. Naïve CD4+ T cells of Peyer’s patches produce more IL-6 than those of spleen in response to antigenic stimulation. Immunol. Lett. 2011, 141, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Peron, J.P.S.; de Oliveira, A.P.L.; Rizzo, L.V. It takes guts for tolerance: The phenomenon of oral tolerance and the regulation of autoimmune response. Autoimmun. Rev. 2009, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Kelsall, B.L. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999, 190, 229–240. [Google Scholar] [CrossRef]
- Tarbell, K.V.; Yamazaki, S.; Olson, K.; Toy, P.; Steinman, R.M. CD25+ CD4+ T Cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004, 199, 1467–1477. [Google Scholar] [CrossRef]
- Giannoukakis, N. Tolerogenic dendritic cells in type 1 diabetes: No longer a concept. Front. Immunol. 2023, 14, 1212641. [Google Scholar] [CrossRef]
- Vives-Pi, M.; Rodríguez-Fernández, S.; Pujol-Autonell, I. How apoptotic β-cells direct immune response to tolerance or to autoimmune diabetes: A review. Apoptosis 2015, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, M.J.; Hilkens, C.M.U.; Martínez-Cáceres, E.M. Challenges in tolerogenic dendritic cell therapy for autoimmune diseases: The route of administration. Immunother. Adv. 2023, 3, ltad012. [Google Scholar] [CrossRef]
- Nikolic, T.; Suwandi, J.S.; Wesselius, J.; Laban, S.; Joosten, A.M.; Sonneveld, P.; Mul, D.; Aanstoot, H.-J.; Kaddis, J.S.; Zwaginga, J.J.; et al. Tolerogenic dendritic cells pulsed with islet antigen induce long-term reduction in T-cell autoreactivity in type 1 diabetes patients. Front. Immunol. 2022, 13, 1054968. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Sun, F.; Yang, C.; Wang, F.; Luo, J.; Yang, P.; Xiong, F.; Zhang, S.; Yu, Q.; Wang, C.-Y. The AHR signaling attenuates autoimmune responses during the development of type 1 diabetes. Front. Immunol. 2020, 11, 1510. [Google Scholar] [CrossRef]
- Abram, D.M.; Fernandes, L.G.R.; Filho, A.C.S.R.; Simioni, P.U. The modulation of enzyme indoleamine 2,3-dioxygenase from dendritic cells for the treatment of type 1 diabetes mellitus. Drug Des. Dev. Ther. 2017, 11, 2171–2178. [Google Scholar] [CrossRef]
- Anquetil, F.; Mondanelli, G.; Gonzalez, N.; Calvo, T.R.; Gonzalo, J.Z.; Krogvold, L.; Dahl-Jørgensen, K.; Eynde, B.V.D.; Orabona, C.; Grohmann, U.; et al. Loss of IDO1 expression from human pancreatic β-cells precedes their destruction during the development of type 1 diabetes. Diabetes 2018, 67, 1858–1866. [Google Scholar] [CrossRef]
- Orabona, C.; Mondanelli, G.; Pallotta, M.T.; Carvalho, A.; Albini, E.; Fallarino, F.; Vacca, C.; Volpi, C.; Belladonna, M.L.; Berioli, M.G.; et al. Deficiency of immunoregulatory indoleamine 2,3-dioxygenase 1 in juvenile diabetes. J. Clin. Investig. 2018, 3, e96244. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef]
- Morita, Y.; Masters, E.A.; Schwarz, E.M.; Muthukrishnan, G. Interleukin-27 and its diverse effects on bacterial infections. Front. Immunol. 2021, 12, 678515. [Google Scholar] [CrossRef]
- Meka, R.R.; Venkatesha, S.H.; Dudics, S.; Acharya, B.; Moudgil, K.D. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun. Rev. 2015, 14, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Wojno, E.D.T.; Hosken, N.; Stumhofer, J.S.; O’hara, A.C.; Mauldin, E.; Fang, Q.; Turka, L.A.; Levin, S.D.; Hunter, C.A. A role for IL-27 in limiting T regulatory cell populations. J. Immunol. 2011, 187, 266–273. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobb, J.; Rawson, J.; Gonzalez, N.; Singer, M.; Kandeel, F.; Husseiny, M.I. Mechanism of Action of Oral Salmonella-Based Vaccine to Prevent and Reverse Type 1 Diabetes in NOD Mice. Vaccines 2024, 12, 276. https://doi.org/10.3390/vaccines12030276
Cobb J, Rawson J, Gonzalez N, Singer M, Kandeel F, Husseiny MI. Mechanism of Action of Oral Salmonella-Based Vaccine to Prevent and Reverse Type 1 Diabetes in NOD Mice. Vaccines. 2024; 12(3):276. https://doi.org/10.3390/vaccines12030276
Chicago/Turabian StyleCobb, Jacob, Jeffrey Rawson, Nelson Gonzalez, Mahmoud Singer, Fouad Kandeel, and Mohamed I. Husseiny. 2024. "Mechanism of Action of Oral Salmonella-Based Vaccine to Prevent and Reverse Type 1 Diabetes in NOD Mice" Vaccines 12, no. 3: 276. https://doi.org/10.3390/vaccines12030276




