Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design
Abstract
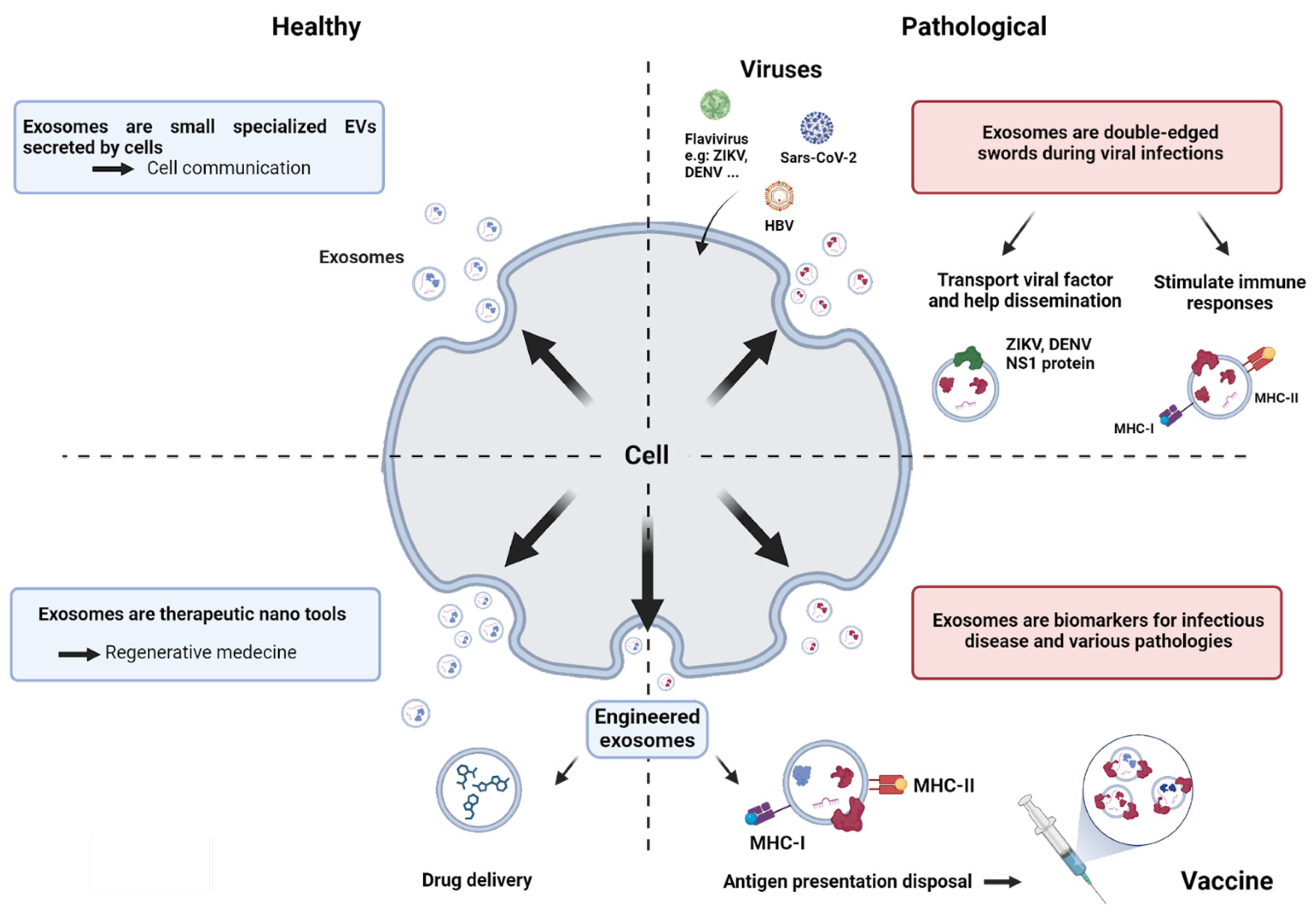
:1. Introduction
2. Exosomes Are Small, Specialized Extracellular Vesicles (EVs)
2.1. Exosome Biogenesis
2.2. Exosomes Composition

2.3. Exosome Biological Activities according to Their Mode of Interaction with Recipient Cells
3. Exosomes and Biology of Infection
3.1. Exosomes May Contribute to Viral Dissemination
3.2. Exosomes Contribute to Infection Resolution
4. Exosomes as Biomarkers of Pathologies in Human Medicine
5. Exosomes as Therapeutic Tools
5.1. Exosomes from Mesenchymal Stromal Cells in Regenerative Medicine
5.2. Engineered Exosomes for Drug Delivery
6. Exosomes and Antigen Presentation: Perspectives in Vaccinology
6.1. Exosomes and Antigen Presentation
6.2. Exosomes in Vaccinology
6.3. Exploring Strategies for Loading Exosomes with Antigens
6.4. Ongoing Exosome-Based Vaccine Strategies against Viruses
7. Challenges and Issues
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALIX | ALG-2-interacting protein X |
| APCs | antigen-presenting cells |
| APOBEC3G | apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3G |
| BMDCs | bone marrow-derived dendritic cells |
| CCL2 | C-C motif chemokine ligand 2 |
| cGAS-STING | cyclic GMP-AMP synthase stimulator of interferon genes |
| CHIKV | Chikungunya virus |
| CHMP4 | charged multivesicular body protein 4a |
| circRNAs | circular RNAs |
| CMV | cytomegalovirus |
| CTL | cytotoxic T lymphocyte |
| DC | dendritic cell |
| DENV | Dengue virus |
| DUBs | de-ubiquitylating enzymes |
| EBV | Epstein–Barr virus |
| EMT | epithelial–mesenchymal transition |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| EV | extracellular vesicles |
| HBsAg | hepatitis B antigen |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HGF | hepatocyte growth factor |
| HIV | human immunodeficiency virus |
| HLA-DR | Human Leukocyte Antigen DR isotype |
| HNRNPs | heterogeneous nuclear ribonucleoproteins |
| HNSCC | Head and Neck Squamous-Cell Carcinoma |
| HSP70 | heat shock protein 70 |
| ICAM-1 | intercellular adhesion molecule 1 |
| IC50 | half maximal inhibitory concentration |
| IFITM3 | interferon-induced transmembrane protein 3 |
| IFNα | interferon alpha |
| IFNγ | interferon gamma |
| IgG | immunoglobulin G |
| IL-1β | interleukin-1β |
| ILVs | intraluminal vesicles |
| IP-10 | interferon gamma-induced protein |
| ISGs | interferon-stimulated genes |
| JEV | Japanese encephalitis virus |
| L1 | non-LTR retrotransposons LINE-1 |
| LPS | lipopolysaccharides |
| MCP-1 | monocyte chemoattractant protein-1 |
| MEVs | multi-epitope vaccines |
| MHC | major histocompatibility complex |
| MSCs | mesenchymal stromal cells |
| mo-DCs | monocyte-derived dendritic cells |
| MVBs | multivesicular bodies |
| NS1 | non-structural protein-1 |
| PRRs | pattern recognition receptors |
| RANTES | regulated upon activation normal T cell expressed and secreted |
| RBD | receptor-binding domain |
| RLRs | RIG-I like receptors |
| RSV | respiratory syncytial virus |
| SIgA | secretory IgA |
| SNARE | soluble NSF attachment protein receptor |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| STING | stimulator of interferon genes |
| TAR | trans-activation response element |
| TBEV | tick-borne encephalitis virus |
| TGFβ | Tumor Growth Factor β |
| TIM4 | T-cell immunoglobulin- and mucin-domain-containing molecule 4 |
| TNFα | Tumor Necrosis Factor α |
| TSG101 | tumor susceptibility gene 101 |
| VLPs | virus-like particles |
| VSV-G | vesicular stomatitis virus glycoprotein |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Poetsch, A. Proteomic Research of Extracellular Vesicles in Clinical Biofluid. Proteomes 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, F.; Li, C.; Mao, J.; Wang, Y.; Zhou, X.; Xie, H.; Wen, C. Application of Single Extracellular Vesicle Analysis Techniques. Int. J. Nanomed. 2023, 18, 5365–5376. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Théry, C.; Welsh, J.A.; Blenkiron, C.; Buzás, E.I.; Di Vizio, D.; Erdbrügger, U.; Falcón-Pérez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef] [PubMed]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Hendrix, A.; Lippens, L.; Pinheiro, C.; Théry, C.; Martin-Jaular, L.; Lötvall, J.; Lässer, C.; Hill, A.F.; Witwer, K.W. Extracellular vesicle analysis. Nat. Rev. Methods Primer 2023, 3, 56. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Cappe, B.; Vandenabeele, P.; Riquet, F.B. A guide to the expanding field of extracellular vesicles and their release in regulated cell death programs. FEBS J. 2023; early view. [Google Scholar] [CrossRef] [PubMed]
- Deatherage, B.L.; Cookson, B.T. Membrane vesicle release in bacteria, eukaryotes, and archaea: A conserved yet underappreciated aspect of microbial life. Infect. Immun. 2012, 80, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Gomez, C.; Goreham, R.V.; Serra, J.J.B.; Nann, T.; Kussmann, M. “Exosomics”—A Review of Biophysics, Biology and Biochemistry of Exosomes with a Focus on Human Breast Milk. Front. Genet. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef]
- Izadpanah, M.; Seddigh, A.; Barough, S.E.; Fazeli, S.A.S.; Ai, J. Potential of Extracellular Vesicles in Neurodegenerative Diseases: Diagnostic and Therapeutic Indications. J. Mol. Neurosci. MN 2018, 66, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Petroni, D.; Fabbri, C.; Babboni, S.; Menichetti, L.; Basta, G.; Del Turco, S. Extracellular Vesicles and Intercellular Communication: Challenges for In Vivo Molecular Imaging and Tracking. Pharmaceutics 2023, 15, 1639. [Google Scholar] [CrossRef]
- Théry, C. Exosomes: Secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011, 3, 15. [Google Scholar] [CrossRef]
- Meldolesi, J. Unconventional Protein Secretion Dependent on Two Extracellular Vesicles: Exosomes and Ectosomes. Front. Cell Dev. Biol. 2022, 10, 877344. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Mori, T.; Giovannelli, L.; Bilia, A.R.; Margheri, F. Exosomes: Potential Next-Generation Nanocarriers for the Therapy of Inflammatory Diseases. Pharmaceutics 2023, 15, 2276. [Google Scholar] [CrossRef] [PubMed]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J. Life in the lumen: The multivesicular endosome. Traffic 2020, 21, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Naslavsky, N.; Caplan, S. The enigmatic endosome—Sorting the ins and outs of endocytic trafficking. J. Cell Sci. 2018, 131, jcs216499. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.; Grant, B.D. Endosomal microdomains: Formation and function. Curr. Opin. Cell Biol. 2020, 65, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Solinger, J.A.; Spang, A. Sorting of cargo in the tubular endosomal network. BioEssays 2022, 44, 2200158. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Lanzetti, L.; Scita, G.; Di Fiore, P.P. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat. Rev. Mol. Cell Biol. 2021, 22, 625–643. [Google Scholar] [CrossRef]
- Pfitzner, A.-K.; Zivkovic, H.; Bernat-Silvestre, C.; West, M.; Peltier, T.; Humbert, F.; Odorizzi, G.; Roux, A. Vps60 initiates alternative ESCRT-III filaments. J. Cell Biol. 2023, 222, e202206028. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Taguchi, T. Lysosomal microautophagy: An emerging dimension in mammalian autophagy. Trends Cell Biol. 2023, S0962892423002386. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation: Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.G.; Baum, B. Roles of ESCRT-III polymers in cell division across the tree of life. Curr. Opin. Cell Biol. 2023, 85, 102274. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, J.; Lee, I.-H.; Iwasa, J.H.; Hurley, J.H. Reverse-topology membrane scission by the ESCRT proteins. Nat. Rev. Mol. Cell Biol. 2017, 18, 5–17. [Google Scholar] [CrossRef]
- Rivera-Cuevas, Y.; Carruthers, V.B. The multifaceted interactions between pathogens and host ESCRT machinery. PLoS Pathog. 2023, 19, e1011344. [Google Scholar] [CrossRef]
- Jin, X.; Xia, T.; Luo, S.; Zhang, Y.; Xia, Y.; Yin, H. Exosomal lipid PI4P regulates small extracellular vesicle secretion by modulating intraluminal vesicle formation. J. Extracell. Vesicles 2023, 12, 12319. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, S.M.; Giovagnoni, C.; Zhu, Z.; Tripathi, P.; Elsherbini, A.; Quadri, Z.; Pu, J.; Zhang, L.; Ferko, B.; Berkes, D.; et al. Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12233. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef] [PubMed]
- Tancini, B.; Buratta, S.; Delo, F.; Sagini, K.; Chiaradia, E.; Pellegrino, R.M.; Emiliani, C.; Urbanelli, L. Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle. Membranes 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The Intracellular Interactome of Tetraspanin-enriched Microdomains Reveals Their Function as Sorting Machineries toward Exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Xu, S.; Xu, J. A Review of the Roles of Specialized Extracellular Vesicles, Migrasomes, and Exosomes in Normal Cell Physiology and Disease. Med. Sci. Monit. 2023, 29, e940118. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.-M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective Enrichment of Tetraspan Proteins on the Internal Vesicles of Multivesicular Endosomes and on Exosomes Secreted by Human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef] [PubMed]
- Suwakulsiri, W.; Xu, R.; Rai, A.; Shafiq, A.; Chen, M.; Greening, D.W.; Simpson, R.J. Comparative proteomic analysis of three major extracellular vesicle classes secreted from human primary and metastatic colorectal cancer cells: Exosomes, microparticles, and shed midbody remnants. Proteomics 2023, 2300057. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Karim, M.A.; Samyn, D.R.; Mattie, S.; Brett, C.L. Distinct features of multivesicular body-lysosome fusion revealed by a new cell-free content-mixing assay. Traffic Cph. Denver 2018, 19, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ji, J.; Jin, D.; Wu, Y.; Wu, T.; Lin, R.; Zhu, S.; Jiang, F.; Ji, Y.; Bao, B.; et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): Intercellular shuttles and implications in human diseases. Genes Dis. 2023, 10, 1894–1907. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Casari, I.; Howard, J.A.; Robless, E.E.; Falasca, M. Exosomal integrins and their influence on pancreatic cancer progression and metastasis. Cancer Lett. 2021, 507, 124–134. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Segura, E.; Nicco, C.; Lombard, B.; Véron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Théry, C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef]
- Braun, J.E.A. Extracellular chaperone networks and the export of J-domain proteins. J. Biol. Chem. 2023, 299, 102840. [Google Scholar] [CrossRef]
- Linder, M.; von Strandmann, E.P. The Role of Extracellular HSP70 in the Function of Tumor-Associated Immune Cells. Cancers 2021, 13, 4721. [Google Scholar] [CrossRef]
- Kreimer, S.; Belov, A.M.; Ghiran, I.; Murthy, S.K.; Frank, D.A.; Ivanov, A.R. Mass-Spectrometry-Based Molecular Characterization of Extracellular Vesicles: Lipidomics and Proteomics. J. Proteome Res. 2015, 14, 2367–2384. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. BioScience 2015, 65, 783–797. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, X.; Miller-Little, W.; Wang, H.; Willard, B.; Bulek, K.; Zhao, J.; Li, X. The Ras GTPase -activating-like protein IQGAP1 bridges Gasdermin D to the ESCRT system to promote IL-1β release via exosomes. EMBO J. 2023, 42, e110780. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Sturm, R.; Henrich, D.; Lupu, L.; Rottluff, K.; Marzi, I.; Leppik, L. Diagnostic and Prognostic Potential of Exosomal Cytokines IL-6 and IL-10 in Polytrauma Patients. Int. J. Mol. Sci. 2023, 24, 11830. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Bun, P.; Huveneers, S.; van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-time imaging of multivesicular body–plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020, 15, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.C.; Katzmann, D.J. Biogenesis and Function of Multivesicular Bodies. Annu. Rev. Cell Dev. Biol. 2007, 23, 519–547. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Geis-Asteggiante, L.; Belew, A.T.; Clements, V.K.; Edwards, N.J.; Ostrand-Rosenberg, S.; El-Sayed, N.M.; Fenselau, C. Differential Content of Proteins, mRNAs, and miRNAs Suggests that MDSC and Their Exosomes May Mediate Distinct Immune Suppressive Functions. J. Proteome Res. 2018, 17, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Zichittella, C.; Alessandro, R.; Conigliaro, A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells 2021, 10, 3355. [Google Scholar] [CrossRef] [PubMed]
- Croft, P.K.-D.; Sharma, S.; Sobrevia, L.; Salomon, C. Extracellular vesicle interactions with the external and internal exposome in mediating carcinogenesis. Mol. Asp. Med. 2022, 87, 101039. [Google Scholar] [CrossRef] [PubMed]
- Omrani, M.; Beyrampour-Basmenj, H.; Jahanban-Esfahlan, R.; Talebi, M.; Raeisi, M.; Serej, Z.A.; Akbar-Gharalari, N.; Khodakarimi, S.; Wu, J.; Ebrahimi-Kalan, A. Global trend in exosome isolation and application: An update concept in management of diseases. Mol. Cell. Biochem. 2023. [Google Scholar] [CrossRef]
- Heydari, R.; Koohi, F.; Rasouli, M.; Rezaei, K.; Abbasgholinejad, E.; Bekeschus, S.; Doroudian, M. Exosomes as Rheumatoid Arthritis Diagnostic Biomarkers and Therapeutic Agents. Vaccines 2023, 11, 687. [Google Scholar] [CrossRef]
- Tavasolian, F.; Moghaddam, A.S.; Rohani, F.; Abdollahi, E.; Janzamin, E.; Momtazi-Borojeni, A.A.; Moallem, S.A.; Jamialahmadi, T.; Sahebkar, A. Exosomes: Effectual players in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102511. [Google Scholar] [CrossRef]
- Silva, J.; Garcia, V.; Zaballos, Á.; Provencio, M.; Lombardia, L.; Almonacid, L.; Garcia, J.M.; Dominguez, G.; Pena, C.; Diaz, R.; et al. Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur. Respir. J. 2011, 37, 617–623. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood–brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- García-Romero, N.; Carrión-Navarro, J.; Esteban-Rubio, S.; Lázaro-Ibáñez, E.; Peris-Celda, M.; Alonso, M.M.; Guzmán-De-Villoria, J.; Fernández-Carballal, C.; de Mendivil, A.O.; García-Duque, S.; et al. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 2017, 8, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L.; et al. Induction of Lymphocyte Apoptosis by Tumor Cell Secretion of FasL-bearing Microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Somiya, M.; Kuroda, S. Reporter gene assay for membrane fusion of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12171. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sane, S.; Kim, J.-E.; Yun, S.; Kim, H.-J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and delivery of extracellular vesicles: Harnessing the power of EVs for diagnostics and therapeutics. Front. Mol. Biosci. 2024, 10, 1330400. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Levy, D.; Do, M.A.; Zhang, J.; Brown, A.; Lu, B. Orchestrating Extracellular Vesicle with Dual Reporters for Imaging and Capturing in Mammalian Cell Culture. Front. Mol. Biosci. 2021, 8, 680580. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Gonda, A.; Kabagwira, J.; Senthil, G.N.; Wall, N.R. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol. Cancer Res. 2019, 17, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Christianson, H.C.; Svensson, K.J.; van Kuppevelt, T.H.; Li, J.-P.; Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA 2013, 110, 17380–17385. [Google Scholar] [CrossRef]
- Ochieng, J.; Nangami, G.; Sakwe, A.; Rana, T.; Ingram, S.; Goodwin, J.S.; Moye, C.; Lammers, P.; Adunyah, S.E. Extracellular histones are the ligands for the uptake of exosomes and hydroxyapatite-nanoparticles by tumor cells via syndecan-4. FEBS Lett. 2018, 592, 3274–3285. [Google Scholar] [CrossRef]
- Bonsergent, E.; Grisard, E.; Buchrieser, J.; Schwartz, O.; Théry, C.; Lavieu, G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 2021, 12, 1864. [Google Scholar] [CrossRef]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef]
- De Lope, L.R.; Sánchez-Herrero, E.; Serna-Blasco, R.; Provencio, M.; Romero, A. Cancer as an infective disease: The role of EVs in tumorigenesis. Mol. Oncol. 2023, 17, 390–406. [Google Scholar] [CrossRef]
- Wandrey, M.; Jablonska, J.; Stauber, R.H.; Gül, D. Exosomes in Cancer Progression and Therapy Resistance: Molecular Insights and Therapeutic Opportunities. Life 2023, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Lima, L.G.; Ham, S.; Shin, H.; Chai, E.P.Z.; Lek, E.S.H.; Lobb, R.J.; Müller, A.F.; Mathivanan, S.; Yeo, B.; Choi, Y.; et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat. Commun. 2021, 12, 3543. [Google Scholar] [CrossRef]
- You, X.; Wang, Y.; Meng, J.; Han, S.; Liu, L.; Sun, Y.; Zhang, J.; Sun, S.; Li, X.; Sun, W.; et al. Exosomal miR-663b exposed to TGF-β1 promotes cervical cancer metastasis and epithelial-mesenchymal transition by targeting MGAT3. Oncol. Rep. 2021, 45, 12. [Google Scholar] [CrossRef]
- Vignard, V.; Labbé, M.; Marec, N.; André-Grégoire, G.; Jouand, N.; Fonteneau, J.-F.; Labarrière, N.; Fradin, D. MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol. Res. 2020, 8, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Ginini, L.; Gil, Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist. Updat. 2019, 45, 1–12. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Schiffelers, R.M.; Zarovni, N.; Vago, R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol. Res. 2016, 111, 487–500. [Google Scholar] [CrossRef]
- Qian, K.; Fu, W.; Li, T.; Zhao, J.; Lei, C.; Hu, S. The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy. J. Exp. Clin. Cancer Res. 2022, 41, 286. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Wang, Y.; Iaria, J.; Dijke, P.T.; Zhu, H.-J. Simultaneously targeting extracellular vesicle trafficking and TGF-β receptor kinase activity blocks signaling hyperactivation and metastasis. Signal Transduct. Target. Ther. 2023, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Briolay, T.; Petithomme, T.; Fouet, M.; Nguyen-Pham, N.; Blanquart, C.; Boisgerault, N. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol. Cancer 2021, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.; Tousif, S.; Wang, Y.; Hough, K.; Khan, S.; Strenkowski, J.; Chacko, B.K.; Darley-Usmar, V.M.; Deshane, J.S. Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization. Cells 2020, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shang, J.; Yang, Q.; Dai, Z.; Liang, Y.; Lai, C.; Feng, T.; Zhong, D.; Zou, H.; Sun, L.; et al. Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism. J. Nanobiotechnol. 2023, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, J.; Zhou, X.; Zhao, X.; Huang, B.; Qin, Y. Exosomes Regulate the Epithelial–Mesenchymal Transition in Cancer. Front. Oncol. 2022, 12, 864980. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther. 2019, 10, 225. [Google Scholar] [CrossRef]
- Lang, Z.; Li, Y.; Lin, L.; Li, X.; Tao, Q.; Hu, Y.; Bao, M.; Zheng, L.; Yu, Z.; Zheng, J. Hepatocyte-derived exosomal miR-146a-5p inhibits hepatic stellate cell EMT process: A crosstalk between hepatocytes and hepatic stellate cells. Cell Death Discov. 2023, 9, 304. [Google Scholar] [CrossRef]
- Chahar, H.S.; Corsello, T.; Kudlicki, A.S.; Komaravelli, N.; Casola, A. Respiratory Syncytial Virus Infection Changes Cargo Composition of Exosome Released from Airway Epithelial Cells. Sci. Rep. 2018, 8, 387. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Exosome and virus infection. Front. Immunol. 2023, 14, 1154217. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mu, S.; Tian, Y.; Shi, J.; Lan, Y.; Guan, J.; Zhao, K.; Gao, F.; He, W. Porcine Hemagglutinating Encephalomyelitis Virus Co-Opts Multivesicular-Derived Exosomes for Transmission. mBio 2023, 14, e03054-22. [Google Scholar] [CrossRef]
- Sato, Y.; Yaguchi, M.; Okuno, Y.; Ishimaru, H.; Sagou, K.; Ozaki, S.; Suzuki, T.; Inagaki, T.; Umeda, M.; Watanabe, T.; et al. Epstein–Barr virus tegument protein BGLF2 in exosomes released from virus-producing cells facilitates de novo infection. Cell Commun. Signal. 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tan, L.; Sun, Y.; Qiu, X.; Meng, C.; Liao, Y.; Song, C.; Liu, W.; Nair, V.; Ding, C. Exosomes Carry microRNAs into Neighboring Cells to Promote Diffusive Infection of Newcastle Disease Virus. Viruses 2019, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128. Viruses 2023, 15, 622. [Google Scholar] [CrossRef]
- Safadi, D.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef]
- Ju, Y.; Bai, H.; Ren, L.; Zhang, L. The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection. Int. J. Mol. Sci. 2021, 22, 9060. [Google Scholar] [CrossRef]
- Martin, C.; Ligat, G.; Malnou, C.E. The Yin and the Yang of extracellular vesicles during viral infections. Biomed. J. 2023, 100659. [Google Scholar] [CrossRef]
- Thepparit, C.; Khongwichit, S.; Ketsuwan, K.; Libsittikul, S.; Auewarakul, P.; Smith, D.R. Dengue virus requires apoptosis linked gene-2-interacting protein X (ALIX) for viral propagation. Virus Res. 2019, 261, 65–71. [Google Scholar] [CrossRef]
- Tran, P.-T.-H.; Chiramel, A.I.; Johansson, M.; Melik, W. Roles of ESCRT Proteins ALIX and CHMP4A and Their Interplay with Interferon-Stimulated Gene 15 during Tick-Borne Flavivirus Infection. J. Virol. 2022, 96, e01624-21. [Google Scholar] [CrossRef]
- Carpp, L.N.; Galler, R.; Bonaldo, M.C. Interaction between the yellow fever virus nonstructural protein NS3 and the host protein Alix contributes to the release of infectious particles. Microbes Infect. 2011, 13, 85–95. [Google Scholar] [CrossRef]
- Wu, Q.; Glitscher, M.; Tonnemacher, S.; Schollmeier, A.; Raupach, J.; Zahn, T.; Eberle, R.; Krijnse-Locker, J.; Basic, M.; Hildt, E. Presence of Intact Hepatitis B Virions in Exosomes. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Rey-Cadilhac, F.; Rachenne, F.; Missé, D.; Pompon, J. Viral Components Trafficking with(in) Extracellular Vesicles. Viruses 2023, 15, 2333. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [PubMed]
- Nour, A.M.; Li, Y.; Wolenski, J.; Modis, Y. Viral Membrane Fusion and Nucleocapsid Delivery into the Cytoplasm are Distinct Events in Some Flaviviruses. PLoS Pathog. 2013, 9, e1003585. [Google Scholar] [CrossRef] [PubMed]
- Longatti, A.; Boyd, B.; Chisari, F.V. Virion-Independent Transfer of Replication-Competent Hepatitis C Virus RNA between Permissive Cells. J. Virol. 2015, 89, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.A.; Raj, V.S.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Latanova, A.; Karpov, V.; Starodubova, E. Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. Int. J. Mol. Sci. 2024, 25, 2144. [Google Scholar] [CrossRef]
- Le, B.C.T.; Burassakarn, A.; Tongchai, P.; Ekalaksananan, T.; Aromseree, S.; Phanthanawiboon, S.; Polsan, Y.; Alexander, N.; Overgaard, H.J.; Pientong, C. Characterization and Involvement of Exosomes Originating from Chikungunya Virus-Infected Epithelial Cells in the Transmission of Infectious Viral Elements. Int. J. Mol. Sci. 2022, 23, 12117. [Google Scholar] [CrossRef]
- Cortes-Galvez, D.; Dangerfield, J.A.; Metzner, C. Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles. Membranes 2023, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Iordanskiy, S.; Das, R.; Van Duyne, R.; Santos, S.; Jaworski, E.; Guendel, I.; Sampey, G.; Dalby, E.; Iglesias-Ussel, M.; et al. Exosomes Derived from HIV-1-infected Cells Contain Trans-activation Response Element RNA. J. Biol. Chem. 2013, 288, 20014–20033. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, Z.; Yue, H.; Bazdar, D.; Mbonye, U.; Zender, C.; Harding, C.V.; Bruggeman, L.; Karn, J.; Sieg, S.F.; et al. Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nat. Commun. 2018, 9, 4585. [Google Scholar] [CrossRef]
- Fan, C.; Tang, Y.; Wang, J.; Xiong, F.; Guo, C.; Wang, Y.; Xiang, B.; Zhou, M.; Li, X.; Wu, X.; et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer 2018, 9, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Akbar, I.; Kumari, B.; Vrati, S.; Basu, A.; Banerjee, A. Japanese Encephalitis Virus-induced let-7a/b interacted with the NOTCH–TLR 7 pathway in microglia and facilitated neuronal death via caspase activation. J. Neurochem. 2019, 149, 518–534. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, N.; Gan, X.; Yan, W.; Morrell, J.C.; Gould, S.J. Higher-Order Oligomerization Targets Plasma Membrane Proteins and HIV Gag to Exosomes. PLoS Biol. 2007, 5, e158. [Google Scholar] [CrossRef]
- Campbell, T.D.; Khan, M.; Huang, M.-B.; Bond, V.C.; Powell, M.D. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn. Dis. 2008, 18 (Suppl. S2), S2-14-9. [Google Scholar]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is Secreted in Exosomes and Triggers Apoptosis in Bystander CD4+ T Cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Naqvi, A.R.; Bano, A.S.; Jameel, S. The HIV-1 Nef Protein Binds Argonaute-2 and Functions as a Viral Suppressor of RNA Interference. PLoS ONE 2013, 8, e74472. [Google Scholar] [CrossRef]
- Plazolles, N.; Humbert, J.-M.; Vachot, L.; Verrier, B.; Hocke, C.; Halary, F. Pivotal Advance: The promotion of soluble DC-SIGN release by inflammatory signals and its enhancement of cytomegalovirus-mediated cis-infection of myeloid dendritic cells. J. Leukoc. Biol. 2010, 89, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Lata, S.; Ali, A.; Banerjea, A.C. Dengue haemorrhagic fever: A job done via exosomes? Emerg. Microbes Infect. 2019, 8, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Harris, C.L.; Court, J.; Mason, M.D.; Morgan, B.P. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur. J. Immunol. 2003, 33, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Karasu, E.; Eisenhardt, S.U.; Harant, J.; Huber-Lang, M. Extracellular Vesicles: Packages Sent with Complement. Front. Immunol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Hussain, W.A.; Jahangir, S.; Ghosh, B.; Yesmin, F.; Anis, A.; Satil, S.N.; Anwar, F.; Rashid, M.H. Exosomes for Regulation of Immune Responses and Immunotherapy. J. Nanotheranostics 2022, 3, 55–85. [Google Scholar] [CrossRef]
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 14787. [Google Scholar] [CrossRef] [PubMed]
- Klibi, J.; Niki, T.; Adhikary, D.; Mautner, J.; Busson, P.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Le Moulec, S.; et al. Blood diffusion and Th1-suppressive effects of galectin-9–containing exosomes released by Epstein-Barr virus–infected nasopharyngeal carcinoma cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, D.-J.; Baloche, V.; Zhang, L.; Xu, J.-X.; Li, B.-W.; Zhao, X.-R.; He, J.; Mai, H.-Q.; Chen, Q.-Y.; et al. Galectin-9 promotes a suppressive microenvironment in human cancer by enhancing STING degradation. Oncogenesis 2020, 9, 65. [Google Scholar] [CrossRef]
- Temme, S.; Eis-Hübinger, A.M.; McLellan, A.D.; Koch, N. The Herpes Simplex Virus-1 Encoded Glycoprotein B Diverts HLA-DR into the Exosome Pathway. J. Immunol. 2010, 184, 236–243. [Google Scholar] [CrossRef]
- Saari, H.; Turunen, T.; Lõhmus, A.; Turunen, M.; Jalasvuori, M.; Butcher, S.J.; Ylä-Herttuala, S.; Viitala, T.; Cerullo, V.; Siljander, P.R.M.; et al. Extracellular vesicles provide a capsid-free vector for oncolytic adenoviral DNA delivery. J. Extracell. Vesicles 2020, 9, 1747206. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Kuroda, S.; Kanaya, N.; Kumon, K.; Tsumura, T.; Hashimoto, M.; Yagi, C.; Sugimoto, R.; Hamada, Y.; Kikuchi, S.; et al. Local oncolytic adenovirotherapy produces an abscopal effect via tumor-derived extracellular vesicles. Mol. Ther. 2021, 29, 2920–2930. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, X.; Bao, J.; Wang, Y.; Liu, H.; Tang, L. Exosomes in Pathogen Infections: A Bridge to Deliver Molecules and Link Functions. Front. Immunol. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Kitai, Y.; Kawasaki, T.; Sueyoshi, T.; Kobiyama, K.; Ishii, K.J.; Zou, J.; Akira, S.; Matsuda, T.; Kawai, T. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity. J. Immunol. 2017, 198, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Hur, S. Regulation of cGAS- and RLR-mediated immunity to nucleic acids. Nat. Immunol. 2020, 21, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Longatti, A. The Dual Role of Exosomes in Hepatitis A and C Virus Transmission and Viral Immune Activation. Viruses 2015, 7, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Somerville, C.C. Modulating Cytokine Production via Select Packaging and Secretion from Extracellular Vesicles. Front. Immunol. 2020, 11, 1040. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.G.; Colli, L.M. Extracellular Vesicles and Interleukins: Novel Frontiers in Diagnostic and Therapeutic for Cancer. Front. Immunol. 2022, 13, 836922. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.A.; Lucs, A.V.; DeVoti, J.; Blanc, L.; Papoin, J.; Wu, R.; Papayannakos, C.J.; Abramson, A.; Bonagura, V.R.; Steinberg, B.M. Poly(I:C) induces controlled release of IL-36γ from keratinocytes in the absence of cell death. Immunol. Res. 2015, 63, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Khatua, A.K.; Taylor, H.E.; Hildreth, J.E.K.; Popik, W. Exosomes Packaging APOBEC3G Confer Human Immunodeficiency Virus Resistance to Recipient Cells. J. Virol. 2009, 83, 512–521. [Google Scholar] [CrossRef]
- Zhu, X.; He, Z.; Yuan, J.; Wen, W.; Huang, X.; Hu, Y.; Lin, C.; Pan, J.; Li, R.; Deng, H.; et al. IFITM3-containing exosome as a novel mediator for anti-viral response in dengue virus infection: Antiviral effect of IFITM3 mediated by exosome. Cell. Microbiol. 2015, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Zhou, Y.; Zhou, R.-H.; Ho, W.-Z.; Li, J.-L. Exosomes contribute to the transmission of anti-HIV activity from TLR3-activated brain microvascular endothelial cells to macrophages. Antiviral Res. 2016, 134, 167–171. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderón-Peláez, M.A.; Balbás-Tepedino, A.; Márquez-Ortiz, R.A.; Madroñero, L.J.; Prieto, A.B.; Castellanos, J.E. Extracellular vesicles of U937 macrophage cell line infected with DENV-2 induce activation in endothelial cells EA.hy926. PLoS ONE 2020, 15, e0227030. [Google Scholar] [CrossRef]
- Edgar, J.R.; Manna, P.T.; Nishimura, S.; Banting, G.; Robinson, M.S. Tetherin is an exosomal tether. eLife 2016, 5, e17180. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Mittelbrunn, M.; Fernandez-Delgado, I.; Torralba, D.; Moreno-Gonzalo, O.; Baldanta, S.; Enrich, C.; Guerra, S.; Sánchez-Madrid, F. ISGylation controls exosome secretion by promoting lysosomal degradation of MVB proteins. Nat. Commun. 2016, 7, 13588. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.-F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Maemura, T.; Fukuyama, S.; Kawaoka, Y. High Levels of miR-483-3p Are Present in Serum Exosomes Upon Infection of Mice with Highly Pathogenic Avian Influenza Virus. Front. Microbiol. 2020, 11, 144. [Google Scholar] [CrossRef]
- Rana, R.; Kant, R.; Kaul, D.; Sachdev, A.; Ganguly, N.K. Integrated view of molecular diagnosis and prognosis of dengue viral infection: Future prospect of exosomes biomarkers. Mol. Cell. Biochem. 2022, 477, 815–832. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.-M.; Xu, Y.-M.; Huang, L.-F.; Wang, X.-Z. Exosomes: Novel Biomarkers for Clinical Diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.-K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Douglas, S.R.; Yeung, K.T.; Yang, J.; Blair, S.L.; Cohen, O.; Eliceiri, B.P. Identification of CD105+ Extracellular Vesicles as a Candidate Biomarker for Metastatic Breast Cancer. J. Surg. Res. 2021, 268, 168–173. [Google Scholar] [CrossRef]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 25, 266–276. [Google Scholar] [CrossRef]
- Sabbagh, Q.; André-Grégoire, G.; Alves-Nicolau, C.; Dupont, A.; Bidère, N.; Jouglar, E.; Guével, L.; Frénel, J.-S.; Gavard, J. The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients. Sci. Rep. 2021, 11, 22792. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, C.; Wen, J.; Liu, G.; Liu, H.; Li, L. Recent advances in nanomaterial-based biosensors for the detection of exosomes. Anal. Bioanal. Chem. 2021, 413, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Dezhakam, E.; Khalilzadeh, B.; Mahdipour, M.; Isildak, I.; Yousefi, H.; Ahmadi, M.; Naseri, A.; Rahbarghazi, R. Electrochemical biosensors in exosome analysis; a short journey to the present and future trends in early-stage evaluation of cancers. Biosens. Bioelectron. 2023, 222, 114980. [Google Scholar] [CrossRef]
- Xu, L.; Shoaie, N.; Jahanpeyma, F.; Zhao, J.; Azimzadeh, M.; Al, K.T. Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens. Bioelectron. 2020, 161, 112222. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.F.; Best, T.; Wüstenhagen, E.; Adrian, K.; Rammo, O.; Saul, M.J. Novel insights into the isolation of extracellular vesicles by anion exchange chromatography. Front. Bioeng. Biotechnol. 2024, 11, 1298892. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal miR-17-5p and miR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. miR-21 in the Extracellular Vesicles (EVs) of Cerebrospinal Fluid (CSF): A Platform for Glioblastoma Biomarker Development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Jiang, P.; Peng, M.; Zhang, X.; Chen, K.; Liu, H.; Bi, H.; Liu, X.; Li, X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 177. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, D.; Wang, Y.; Liu, C.; Wang, L.; Yuan, Y.; Xu, X.; Jiang, Y. Urinary exosomes: A promising biomarker of drug-induced nephrotoxicity. Front. Med. 2023, 10, 1251839. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Makler, A.; Asghar, W. Exosomal biomarkers for cancer diagnosis and patient monitoring. Expert Rev. Mol. Diagn. 2020, 20, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Tutrone, R.; Donovan, M.J.; Torkler, P.; Tadigotla, V.; McLain, T.; Noerholm, M.; Skog, J.; McKiernan, J. Clinical utility of the exosome based ExoDx Prostate(IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.-H.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, Y.; Lebeau, G.; Guillot, X.; Dargai, F.; Guiraud, P.; Neal, J.W.; Ralandison, S.; Gasque, P. Emerging Roles of Perivascular Mesenchymal Stem Cells in Synovial Joint Inflammation. J. Neuroimmune Pharmacol. 2020, 15, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, G.; Ah-Pine, F.; Daniel, M.; Bedoui, Y.; Vagner, D.; Frumence, E.; Gasque, P. Perivascular Mesenchymal Stem/Stromal Cells, an Immune Privileged Niche for Viruses? Int. J. Mol. Sci. 2022, 23, 8038. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef]
- Ma, H.; Siu, W.-S.; Leung, P.-C. The Potential of MSC-Based Cell-Free Therapy in Wound Healing—A Thorough Literature Review. Int. J. Mol. Sci. 2023, 24, 9356. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mojsilović, S.; Jauković, A.; Kukolj, T.; Obradović, H.; Đorđević, I.O.; Petrović, A.; Bugarski, D. Tumorigenic Aspects of MSC Senescence—Implication in Cancer Development and Therapy. J. Pers. Med. 2021, 11, 1133. [Google Scholar] [CrossRef]
- Prockop, D.J. “Stemness” Does Not Explain the Repair of Many Tissues by Mesenchymal Stem/Multipotent Stromal Cells (MSCs). Clin. Pharmacol. Ther. 2007, 82, 241–243. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef]
- Han, Y.-D.; Bai, Y.; Yan, X.-L.; Ren, J.; Zeng, Q.; Li, X.-D.; Pei, X.-T.; Han, Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem. Biophys. Res. Commun. 2018, 497, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ju, R.; Wang, Y. Mesenchymal Stem Cell-Derived Extracellular Vesicles for the Treatment of Bronchopulmonary Dysplasia. Front. Pediatr. 2022, 10, 852034. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Santos, C.C.D.; Lopes-Pacheco, M.; English, K.; Enes, S.R.; Krasnodembskaya, A.; Rocco, P.R.M. The MSC-EV-microRNAome: A Perspective on Therapeutic Mechanisms of Action in Sepsis and ARDS. Cells 2024, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef]
- Sun, X.-H.; Wang, X.; Zhang, Y.; Hui, J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 2019, 177, 23–32. [Google Scholar] [CrossRef]
- Yue, R.; Lu, S.; Luo, Y.; Zeng, J.; Liang, H.; Qin, D.; Wang, X.; Wang, T.; Pu, J.; Hu, H. Mesenchymal stem cell-derived exosomal microRNA-182-5p alleviates myocardial ischemia/reperfusion injury by targeting GSDMD in mice. Cell Death Discov. 2022, 8, 202. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Kamm, D.R.; McCommis, K.S. Hepatic stellate cells in physiology and pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res. Ther. 2019, 10, 98. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Y.; Liu, Y.; Li, X.; Tang, L.; Duan, M.; Li, J.; Zhang, G. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res. Ther. 2021, 12, 434. [Google Scholar] [CrossRef]
- Moghadasi, S.; Elveny, M.; Rahman, H.S.; Suksatan, W.; Jalil, A.T.; Abdelbasset, W.K.; Yumashev, A.V.; Shariatzadeh, S.; Motavalli, R.; Behzad, F.; et al. A paradigm shift in cell-free approach: The emerging role of MSCs-derived exosomes in regenerative medicine. J. Transl. Med. 2021, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Oguma, Y.; Kuroda, Y.; Wakao, S.; Kushida, Y.; Dezawa, M. Single-cell RNA sequencing reveals different signatures of mesenchymal stromal cell pluripotent-like and multipotent populations. iScience 2022, 25, 105395. [Google Scholar] [CrossRef] [PubMed]
- Laggner, M.; Gugerell, A.; Bachmann, C.; Hofbauer, H.; Vorstandlechner, V.; Seibold, M.; Gouya Lechner, G.; Peterbauer, A.; Madlener, S.; Demyanets, S.; et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell Res. Ther. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Wan, Z.; Zhao, L.; Lu, F.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Yang, G.; Xing, C.; Liu, L. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 2020, 10, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Y.; Zhu, G.; Zeng, H.; Fan, Y.; Guo, F.; Xu, D.; Wang, B.; Chen, D.; Ge, G. Emerging Pharmacotherapeutic Strategies to Overcome Undruggable Proteins in Cancer. Int. J. Biol. Sci. 2023, 19, 3360–3382. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef]
- Bai, J.; Wei, X.; Zhang, X.; Wu, C.; Wang, Z.; Chen, M.; Wang, J. Microfluidic strategies for the isolation and profiling of exosomes. TrAC Trends Anal. Chem. 2023, 158, 116834. [Google Scholar] [CrossRef]
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369. [Google Scholar] [CrossRef]
- Li, D.; Zhang, C.; Gao, Z.; Xia, N.; Wu, C.; Liu, C.; Tian, H.; Mei, X. Curcumin-Loaded Macrophage-Derived Exosomes Effectively Improve Wound Healing. Mol. Pharm. 2023, 20, 4453–4467. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Zhao, Y.; Harrison, E.B.; Mahajan, V.; Ahmed, S.; He, Z.; Suresh, P.; Hingtgen, S.D.; Klyachko, N.L.; Mosley, R.L.; et al. Specific Transfection of Inflamed Brain by Macrophages: A New Therapeutic Strategy for Neurodegenerative Diseases. PLoS ONE 2013, 8, e61852. [Google Scholar] [CrossRef]
- Zhao, Y.; Haney, M.J.; Gupta, R.; Bohnsack, J.P.; He, Z.; Kabanov, A.V.; Batrakova, E.V. GDNF-Transfected Macrophages Produce Potent Neuroprotective Effects in Parkinson’s Disease Mouse Model. PLoS ONE 2014, 9, e106867. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Koh, H.B.; Kim, H.J.; Kang, S.-W.; Yoo, T.-H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef]
- Marschall, A.L.J. Targeting the Inside of Cells with Biologicals: Chemicals as a Delivery Strategy. BioDrugs 2021, 35, 643–671. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; De Jong, O.G.; Evers, M.J.W.; Nurazizah, M.; Schiffelers, R.M.; Vader, P. Natural or Synthetic RNA Delivery: A Stoichiometric Comparison of Extracellular Vesicles and Synthetic Nanoparticles. Nano Lett. 2021, 21, 1888–1895. [Google Scholar] [CrossRef]
- Hodge, A.L.; Baxter, A.A.; Poon, I.K.H. Gift bags from the sentinel cells of the immune system: The diverse role of dendritic cell-derived extracellular vesicles. J. Leukoc. Biol. 2022, 111, 903–920. [Google Scholar] [CrossRef]
- Segura, E.; Amigorena, S.; Théry, C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells. Mol. Dis. 2005, 35, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Koerhuis, D.G.J.; Borg, E.G.F.; Veld, E.M.T.; Driedonks, T.A.P.; Wubbolts, R.; Stoorvogel, W.; Boes, M. Bystander T-Cells Support Clonal T-Cell Activation by Controlling the Release of Dendritic Cell-Derived Immune-Stimulatory Extracellular Vesicles. Front. Immunol. 2019, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Wubbolts, R.; Borg, E.G.F.; Van ’T Veld, E.M.; Boes, M.; Stoorvogel, W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J. Extracell. Vesicles 2020, 9, 1798606. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.G.; Wang, Z.; DiVito, S.J.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Larregina, A.T.; et al. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J. Immunol. 2008, 180, 3081–3090. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Regulation of Extracellular Vesicle-Mediated Immune Responses against Antigen-Specific Presentation. Vaccines 2022, 10, 1691. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Hurtado-Monzón, A.M.; Gallardo-Flores, C.E.; Alcaraz-Estrada, S.L.; Salas-Benito, J.S.; et al. The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell–Cell Communication: New Insights on Virus–Host Interactions. Viruses 2020, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.R.; Pleet, M.L.; Enose-Akahata, Y.; Erickson, J.; Monaco, M.C.; Akpamagbo, Y.; Velluci, A.; Tanaka, Y.; Azodi, S.; Lepene, B.; et al. Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: Functional role of exosomes. Clin. Transl. Med. 2018, 7, e24. [Google Scholar] [CrossRef] [PubMed]
- Pesce, E.; Manfrini, N.; Cordiglieri, C.; Santi, S.; Bandera, A.; Gobbini, A.; Gruarin, P.; Favalli, A.; Bombaci, M.; Cuomo, A.; et al. Exosomes Recovered From the Plasma of COVID-19 Patients Expose SARS-CoV-2 Spike-Derived Fragments and Contribute to the Adaptive Immune Response. Front. Immunol. 2022, 12, 785941. [Google Scholar] [CrossRef]
- Huda, M.N.; Nurunnabi, M. Potential Application of Exosomes in Vaccine Development and Delivery. Pharm. Res. 2022, 39, 2635–2671. [Google Scholar] [CrossRef] [PubMed]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Nano dimensions/adjuvants in COVID-19 vaccines. J. Mater. Chem. B 2022, 10, 1520–1552. [Google Scholar] [CrossRef] [PubMed]
- Jesus, S.; Soares, E.; Cruz, M.T.; Borges, O. Exosomes as adjuvants for the recombinant hepatitis B antigen: First report. Eur. J. Pharm. Biopharm. 2018, 133, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vallhov, H.; Gutzeit, C.; Johansson, S.M.; Nagy, N.; Paul, M.; Li, Q.; Friend, S.; George, T.C.; Klein, E.; Scheynius, A.; et al. Exosomes Containing Glycoprotein 350 Released by EBV-Transformed B Cells Selectively Target B Cells through CD21 and Block EBV Infection In Vitro. J. Immunol. 2011, 186, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Delcayre, A.; Estelles, A.; Sperinde, J.; Roulon, T.; Paz, P.; Aguilar, B.; Villanueva, J.; Khine, S.; Le Pecq, J.-B. Exosome Display technology: Applications to the development of new diagnostics and therapeutics. Blood Cells. Mol. Dis. 2005, 35, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bliss, C.M.; Parsons, A.J.; Nachbagauer, R.; Hamilton, J.R.; Cappuccini, F.; Ulaszewska, M.; Webber, J.P.; Clayton, A.; Hill, A.V.; Coughlan, L. Targeting Antigen to the Surface of EVs Improves the In Vivo Immunogenicity of Human and Non-human Adenoviral Vaccines in Mice. Mol. Ther.—Methods Clin. Dev. 2020, 16, 108–125. [Google Scholar] [CrossRef]
- Stickney, Z.; Losacco, J.; McDevitt, S.; Zhang, Z.; Lu, B. Development of exosome surface display technology in living human cells. Biochem. Biophys. Res. Commun. 2016, 472, 53–59. [Google Scholar] [CrossRef]
- Zhang, J.; Brown, A.; Johnson, B.; Diebold, D.; Asano, K.; Marriott, G.; Lu, B. Genetically Engineered Extracellular Vesicles Harboring Transmembrane Scaffolds Exhibit Differences in Their Size, Expression Levels of Specific Surface Markers and Cell-Uptake. Pharmaceutics 2022, 14, 2564. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, M.; Bai, Z.; Xie, Y.; Ren, L.; Wei, J.; Zhu, D.; Wang, H.; Liu, Y.; Luo, J.; et al. Huc-MSC-derived exosomes modified with the targeting peptide of aHSCs for liver fibrosis therapy. J. Nanobiotechnology 2022, 20, 432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Hu, S.; Liu, X. Insights into the function of ESCRT and its role in enveloped virus infection. Front. Microbiol. 2023, 14, 1261651. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, I.; Vladimirtsev, D.; Vorontsova, M.; Blatov, I.; Shevchenko, K.; Zvereva, S.; Lunev, E.A.; Faizuloev, E.; Barlev, N. Viral Membrane Fusion Proteins and RNA Sorting Mechanisms for the Molecular Delivery by Exosomes. Cells 2021, 10, 3043. [Google Scholar] [CrossRef]
- Degassart, A.; Trentin, B.; Martin, M.; Hocquellet, A.; Bettebobillo, P.; Mamoun, R.; Vidal, M. Exosomal sorting of the cytoplasmic domain of bovine leukemia virus TM Env protein. Cell Biol. Int. 2009, 33, 36–48. [Google Scholar] [CrossRef]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 61. [Google Scholar] [CrossRef]
- Zheng, W.; Schürz, M.; Wiklander, R.J.; Gustafsson, O.; Gupta, D.; Slovak, R.; Traista, A.; Coluzzi, A.; Roudi, S.; Barone, A.; et al. Surface display of functional moieties on extracellular vesicles using lipid anchors. J. Control. Release 2023, 357, 630–640. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; Van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Uppu, D.S.; Min, Y.; Kim, I.; Kumar, S.; Park, J.; Cho, Y.-K. Glycolipid-Anchored Proteins on Bioengineered Extracellular Vesicles for Lipopolysaccharide Neutralization. ACS Appl. Mater. Interfaces 2021, 13, 29313–29324. [Google Scholar] [CrossRef] [PubMed]
- Anticoli, S.; Falcone, E.; Ruggieri, A.; Federico, M. Engineered exosomes boost the HCV NS3-specific CD8+ T lymphocyte immunity in humans. Trials Vaccinol. 2016, 5, 105–110. [Google Scholar] [CrossRef]
- Anticoli, S.; Manfredi, F.; Chiozzini, C.; Arenaccio, C.; Olivetta, E.; Ferrantelli, F.; Capocefalo, A.; Falcone, E.; Ruggieri, A.; Federico, M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity against Viral Antigens. Biotechnol. J. 2018, 13, 1700443. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Extracellular Vesicle-Based SARS-CoV-2 Vaccine. Vaccines 2023, 11, 539. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Nice, J.B.; Li, Y.; LeClaire, M.J.; Twaddle, R.; Mora, C.L.; Adachi, S.Y.; Chin, E.R.; Young, M.; Angeles, J.; et al. Exosome-Based Multivalent Vaccine: Achieving Potent Immunization, Broadened Reactivity, and Strong T-Cell Responses with Nanograms of Proteins. Microbiol. Spectr. 2023, 11, e00503-23. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; Moatti, A.; Scull, G.; Silkstone, D.; Lutz, H.; Abad, B.L.d.J.; George, A.; Belcher, E.; Zhu, D.; Mei, X.; et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter 2022, 5, 2960–2974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Han, C.; Wen, Z.; Meng, X.; Qi, D.; Wang, N.; Du, H.; Wang, J.; Lu, L.; et al. Intraduodenal Delivery of Exosome-Loaded SARS-CoV-2 RBD mRNA Induces a Neutralizing Antibody Response in Mice. Vaccines 2023, 11, 673. [Google Scholar] [CrossRef]
- Kuate, S.; Cinatl, J.; Doerr, H.W.; Überla, K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 2007, 362, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Karn, V.; Ahmed, S.; Tsai, L.-W.; Dubey, R.; Ojha, S.; Singh, H.N.; Kumar, M.; Gupta, P.K.; Sadhu, S.; Jha, N.K.; et al. Extracellular Vesicle-Based Therapy for COVID-19: Promises, Challenges and Future Prospects. Biomedicines 2021, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Popowski, K.D.; Zhu, D.; Abad, B.L.d.J.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N.; et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef]
- Schorey, J.S.; Cheng, Y.; Singh, P.P.; Smith, V.L. Exosomes and other extracellular vesicles in host–pathogen interactions. EMBO Rep. 2015, 16, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Meyering, S.S.; Lepene, B.; Iordanskiy, S.; van Hoek, M.L.; Hakami, R.M.; Kashanchi, F. Extracellular vesicles from infected cells: Potential for direct pathogenesis. Front. Microbiol. 2015, 6, 1132. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E. Bacteriophage Virus-Like Particles: Platforms for Vaccine Design. In Bacteriophages; Methods in Molecular Biology; Springer: New York, NY, USA, 2024; pp. 411–423. [Google Scholar] [CrossRef]
- Celis-Giraldo, C.T.; López-Abán, J.; Muro, A.; Patarroyo, M.A.; Manzano-Román, R. Nanovaccines against Animal Pathogens: The Latest Findings. Vaccines 2021, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Leonard, J. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals 2013, 6, 659–680. [Google Scholar] [CrossRef]
- Wang, X.; Xia, J.; Yang, L.; Dai, J.; He, L. Recent progress in exosome research: Isolation, characterization and clinical applications. Cancer Gene Ther. 2023, 30, 1051–1065. [Google Scholar] [CrossRef]
- Fu, C.; Zhou, L.; Mi, Q.-S.; Jiang, A. Plasmacytoid Dendritic Cells and Cancer Immunotherapy. Cells 2022, 11, 222. [Google Scholar] [CrossRef]
- Shahraki, K.; Boroumand, P.G.; Lotfi, H.; Radnia, F.; Shahriari, H.; Sargazi, S.; Mortazavi, S.S.; Shirvaliloo, M.; Shirvalilou, S.; Sheervalilou, R. An update in the applications of exosomes in cancer theranostics: From research to clinical trials. J. Cancer Res. Clin. Oncol. 2023, 149, 8087–8116. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Safadi, D.; Mokhtari, A.; Krejbich, M.; Lagrave, A.; Hirigoyen, U.; Lebeau, G.; Viranaicken, W.; Krejbich-Trotot, P. Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design. Vaccines 2024, 12, 280. https://doi.org/10.3390/vaccines12030280
El Safadi D, Mokhtari A, Krejbich M, Lagrave A, Hirigoyen U, Lebeau G, Viranaicken W, Krejbich-Trotot P. Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design. Vaccines. 2024; 12(3):280. https://doi.org/10.3390/vaccines12030280
Chicago/Turabian StyleEl Safadi, Daed, Alexandre Mokhtari, Morgane Krejbich, Alisé Lagrave, Ugo Hirigoyen, Grégorie Lebeau, Wildriss Viranaicken, and Pascale Krejbich-Trotot. 2024. "Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design" Vaccines 12, no. 3: 280. https://doi.org/10.3390/vaccines12030280
APA StyleEl Safadi, D., Mokhtari, A., Krejbich, M., Lagrave, A., Hirigoyen, U., Lebeau, G., Viranaicken, W., & Krejbich-Trotot, P. (2024). Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design. Vaccines, 12(3), 280. https://doi.org/10.3390/vaccines12030280





