Cell-Mediated Proteomics, and Serological and Mucosal Humoral Immune Responses after Seasonal Influenza Immunization: Characterization of Serological Responders and Non-Responders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Vaccine
2.2. Total Serum Immunoglobulin A, G and M
2.3. Isolation of Peripheral Blood Mononuclear Cells
2.4. Stimulation of Peripheral Blood Mononuclear Cells with Influenza Vaccine
2.5. Haemagglutination Inhibition Test, HAI
2.6. Influenza-Specific Mucosal Immunoglobulin A (IgA)
2.7. Proximity Extension Assay
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Total Serum Immunoglobulins and Specific Mucosal Immunoglobulin A
3.3. Protein Profiling of Supernatants from PBMCs Stimulated with Influenza Vaccine VaxigripTetra
3.4. Characterisation of Influenza Immunisation Responders and Non-Responders
3.5. Immunosuppressive Medication
3.6. Vaccine Breakthrough
3.7. Age-Associated Immune Response
3.8. Comparison of PBMC Stimulations Using Fresh and Cryopreserved Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. The Immunological Basis for Immunization Series: Module 23: Influenza Vaccines; World Health Organization: Geneva, Switzerland, 2017.
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Folkhälsomyndigheten. Rekommendationer om Influensavaccination Till Riskgrupper. 2021 2021-10-13; 6th. Available online: https://www.folkhalsomyndigheten.se/contentassets/af9f68e3cb324aaf818f8e7d53132090/rekommendationer-influensavaccination-riskgrupper.pdf (accessed on 23 August 2022).
- Buchy, P.; Badur, S. Who and when to vaccinate against influenza. Int. J. Infect. Dis. 2020, 93, 375–387. [Google Scholar] [CrossRef]
- Darvishian, M.; Bijlsma, M.J.; Hak, E.; Heuvel, E.R.v.D. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: A meta-analysis of test-negative design case-control studies. Lancet Infect. Dis. 2014, 14, 1228–1239. [Google Scholar] [CrossRef]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Di Pietrantonj, C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2014, CD001269. [Google Scholar] [CrossRef]
- Committee on Infectious Diseases. Recommendations for Prevention and Control of Influenza in Children, 2022–2023. Pediatrics 2022, 150, e2022059275. [Google Scholar] [CrossRef] [PubMed]
- Castrucci, M.R. Factors affecting immune responses to the influenza vaccine. Hum. Vaccines Immunother. 2018, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Hancock, K.; Xu, X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev. Anti-Infect. Ther. 2011, 9, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Franceschi, C.; Blomberg, B.B.; Pirazzini, C.; Ravaioli, F.; Gentilini, D.; Di Blasio, A.M.; Garagnani, P.; Frasca, D.; Bacalini, M.G. Responders and non-responders to influenza vaccination: A DNA methylation approach on blood cells. Exp. Gerontol. 2018, 105, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, L.; Syedbasha, M.; Vogt, D.; Hollenstein, Y.; Hartmann, J.; Linnik, J.E.; Egli, A. An Optimized Hemagglutination Inhibition (HI) Assay to Quantify Influenza-specific Antibody Titers. J. Vis. Exp. 2017, 130, e55833. [Google Scholar]
- Gianchecchi, E.; Torelli, A.; Montomoli, E. The use of cell-mediated immunity for the evaluation of influenza vaccines: An upcoming necessity. Hum. Vaccines Immunother. 2019, 15, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Gijzen, K.; Liu, W.M.; Visontai, I.; Oftung, F.; van der Werf, S.; Korsvold, G.E.; Pronk, I.; Aaberge, I.S.; Tüttő, A.; Jankovics, I.; et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine 2010, 28, 3416–3422. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Ewen, C.; Zhou, X.; Kane, K.P.; Xie, D.; Hager, W.D.; Barry, M.B.; Kleppinger, A.; Wang, Y.; Bleackley, R.C. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009, 27, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Xie, D.; Hager, W.D.; Barry, M.B.; Wang, Y.; Kleppinger, A.; Ewen, C.; Kane, K.P.; Bleackley, R.C. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 2006, 176, 6333–6339. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Verschoor, C.P.; Andrew, M.K.; Haynes, L.; Kuchel, G.A.; Pawelec, G. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011.
- Fujimoto, C.; Takeda, N.; Matsunaga, A.; Sawada, A.; Tanaka, T.; Kimoto, T.; Shinahara, W.; Sawabuchi, T.; Yamaguchi, M.; Hayama, M.; et al. Induction and maintenance of anti-influenza antigen-specific nasal secretory IgA levels and serum IgG levels after influenza infection in adults. Influ. Other Respir. Viruses 2012, 6, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Kuno-Sakai, H.; Kimura, M.; Oh, Y.; Kim, R.; Kumihashi, H.; Takashima, M.; Fukai, K. Enzyme-Linked Immunosorbent Assay of Influenza Specific IgA Antibody in Nasal Mucus. Pediatr. Int. 1991, 33, 617–622. [Google Scholar] [CrossRef]
- Olink. Olink® Target 96 Immuno-Oncology Panels. Available online: https://www.olink.com/products-services/target/immune-response-panel/ (accessed on 14 September 2022).
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef]
- Folkhälsomyndigheten. Influenza in Sweden–Season 2017–2018; The Public Health Agency: Solna, Sweden, 2018.
- Justel, M.; Socias, L.; Almansa, R.; Ramírez, P.; Gallegos, M.C.; Fernandez, V.; Gordon, M.; Andaluz-Ojeda, D.; Nogales, L.; Rojo, S.; et al. IgM levels in plasma predict outcome in severe pandemic influenza. J. Clin. Virol. 2013, 58, 564–567. [Google Scholar] [CrossRef]
- Qiu, C.; Tian, D.; Wan, Y.; Zhang, W.; Qiu, C.; Zhu, Z.; Ye, R.; Song, Z.; Zhou, M.; Yuan, S.; et al. Early adaptive humoral immune responses and virus clearance in humans recently infected with pandemic 2009 H1N1 influenza virus. PLoS ONE 2011, 6, e22603. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Lindqvist, M.; Heit, A.; Wu, J.E.; Reiss, S.M.; Kendric, K.; Bélanger, S.; Kasturi, S.P.; Landais, E.; Akondy, R.S.; et al. CXCL13 is a plasma biomarker of germinal center activity. Proc. Natl. Acad. Sci. USA 2016, 113, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Kazanietz, M.G.; Durando, M.; Cooke, M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front. Endocrinol. 2019, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, F.; Fraleigh, N.; Vlasschaert, C.; McElhaney, J.; Hanifi-Moghaddam, P. Human granzymes: Related but far apart. Med. Hypotheses 2014, 83, 688–693. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Wojno, E.D.T.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, W.; Zhu, Y. Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems. Viruses 2019, 11, 772. [Google Scholar] [CrossRef] [PubMed]
| Responders | Non-Responders | |||
|---|---|---|---|---|
| Influenza Strain, Median (Range) | One Month (n = 51) | Six Months (n = 29) | One Month (n = 22) | Six Months (n = 44) |
| A/H1N1/09pdm | 160 (5–20,480) | 120 (5–10,240) | 160 (60–5120) | 160 (20–1280) |
| A/H3N2/HK | 80 (5–10,240) | 60 (5–5120) | 80 (5–5120) | 80 (5–2560) |
| B/60/Brisbane2008 | 160 (5–5120) | 100 (5–2560) | 320 (40–1280) | 160 (20–640) |
| Variable | Responders | Non-Responders | p-Value | Total |
|---|---|---|---|---|
| n (%) | 51 (70) | 22 (30) | 73 (100) | |
| Sex n (%) | ||||
| Female | 37 (73) | 15 (68) | 52 (71) | |
| Male | 14 (27) | 7 (32) | 0.781 | 21 (29) |
| Age (yrs) | ||||
| Mean (SD) | 70 (18) | 67 (15) | 69 (17) | |
| Median (Q1–Q3) | 77 (61–84) | 73 (61–81) | 0.243 | 76 (61–81) |
| Age categories n (%) | ||||
| ≤65 | 14 (27) | 9 (41) | 23 (32) | |
| >65 | 37 (73) | 13 (59) | 0.282 | 50 (68) |
| Height (cm) | ||||
| Mean (SD) | 168 (9) | 170 (7) | 168 (9) | |
| Median (Q1–Q3) | 167 (160–175) | 170 (164–175) | 0.367 | 169 (161–175) |
| Weight (kg) | ||||
| Mean (SD) | 76 (15) | 77 (18) | 76 (16) | |
| Median (Q1–Q3) | 75 (66–86) | 75 (67–87) | 0.967 | 75 (66–86) |
| BMI (kg m−2) | ||||
| Mean (SD) | 27 (4) | 27 (6) | 27 (5) | |
| Median (Q1–Q3) | 27 (24–29) | 25 (24–30) | 0.539 | 27 (24–29) |
| Diabetes | ||||
| No | 43 (84) | 16 (76) | 59 (82) | |
| Yes | 8 (16) | 5 (24) | 0.504 | 13 (18) |
| Lung disease | ||||
| No | 45 (88) | 20 (95) | 65 (90) | |
| Yes | 5 (10) | 1 (5) | 6 (8) | |
| Missing | 1 (2) | 0 (0) | 0.662 | 1 (1) |
| Upper respiratory tract infections in last five years | ||||
| 0–3 | 45 (88) | 18 (86) | 63 (88) | |
| 4–6 | 5 (10) | 1 (5) | 6 (8) | |
| >6 | 0 (0) | 1 (5) | 1 (1) | |
| Missing | 1 (2) | 1 (5) | 0.233 | 2 (3) |
| Immune-suppressive medication | ||||
| No | 50 (98) | 21 (100) | 71 (99) | |
| Yes | 1 (2) | 0 (0) | >0.99 | 1 (1) |
| Side effects | ||||
| No | 46 (90) | 19 (86) | 65 (89) | |
| Yes | 5 (10) | 3 (14) | 0.691 | 8 (11) |
| ONE MONTH | ||||
|---|---|---|---|---|
| Variable | Responders | Non-Responders | p-Value | Total |
| n | 51 | 22 | 73 | |
| Total serum IgA (g/L) | ||||
| Mean (SD) | 2.56 (1.09) | 2.54 (1.01) | 2.55 (1.06) | |
| Median (Q1–Q3) | 2.5 (1.6–3.3) | 2.7 (1.9–2.9) | 0.891 | 2.6 (1.9–3.0) |
| Total serum IgG (g/L) | ||||
| Mean (SD) | 10.7 (2.7) | 11.1 (2.0) | 10.8 (2.5) | |
| Median (Q1–Q3) | 11 (9–13) | 11 (10–12) | 0.587 | 11 (10–12) |
| Total serum IgM (g/L) | ||||
| Mean (SD) | 0.88 (0.71) | 1.03 (0.45) | 0.93 (0.64) | |
| Median (Q1–Q3) | 0.7 (0.5–1.0) | 0.9 (0.7–1.3) | 0.053 | 0.8 (0.6–1.2) |
| Mucosal IgA A/Brisbane | ||||
| Mean (SD) | 22.1 (34.1) | 17.7 (17.3) | 20.7 (29.6) | |
| Median (Q1–Q3) | 12.8 (7.0–21.3) | 11.5 (6.6–22.2) | 0.994 | 12.6 (7.0–22.0) |
| Mucosal IgA A/Kansas | ||||
| Mean (SD) | 22.6 (27.8) | 19.0 (17.1) | 21.4 (24.7) | |
| Median (Q1–Q3) | 14.5 (8.3–22.9) | 11.6 (8.7–22.2) | 0.865 | 13.8 (8.5–22.6) |
| Mucosal IgA B/Brisbane-like | ||||
| Mean (SD) | 20.4 (18.5) | 21.0 (19.8) | 20.6 (18.8) | |
| Median (Q1–Q3) | 15.0 (8.0–25.7) | 12.6 (8.2–32.2) | 0.960 | 14.2 (8.2–27.6) |
| Ratio Mucosal IgA A/Brisbane | ||||
| Mean (SD) | 1.81 (1.94) | 1.69 (0.89) | 1.77 (1.67) | |
| Median (Q1–Q3) | 1.2 (0.8–2.2) | 1.7 (0.9–2.5) | 0.365 | 1.2 (0.8–2.3) |
| Ratio Mucosal IgA A/Kansas | ||||
| Mean (SD) | 3.21 (9.44) | 2.10 (1.42) | 2.84 (7.71) | |
| Median (Q1–Q3) | 1.4 (0.9–2.0) | 1.6 (1.1–2.8) | 0.408 | 1.4 (0.9–2.4) |
| Ratio Mucosal IgA B/Brisbane-like | ||||
| Mean (SD) | 2.96 (5.15) | 2.03 (1.61) | 2.66 (4.35) | |
| Median (Q1–Q3) | 1.3 (0.9–2.2) | 1.6 (0.9–2.7) | 0.720 | 1.4 (0.9–2.3) |
| Responders | Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Total | n | (%) | OR (95% Conf Int) | p-Value | OR (95% Conf Int) | p-Value |
| Sex | |||||||
| Female | 52 | 37 | 71 | 1.00 | 1.00 | ||
| Male | 21 | 14 | 67 | 0.81 (0.27–2.45) | 0.707 | 0.94 (0.22–4.03) | 0.936 |
| Age categories n (%) | |||||||
| ≤65 | 23 | 14 | 61 | 1.00 | 1.00 | ||
| >65 | 50 | 37 | 74 | 1.83 (0.63–5.33) | 0.263 | 0.37 (0.07–2.04) | 0.249 |
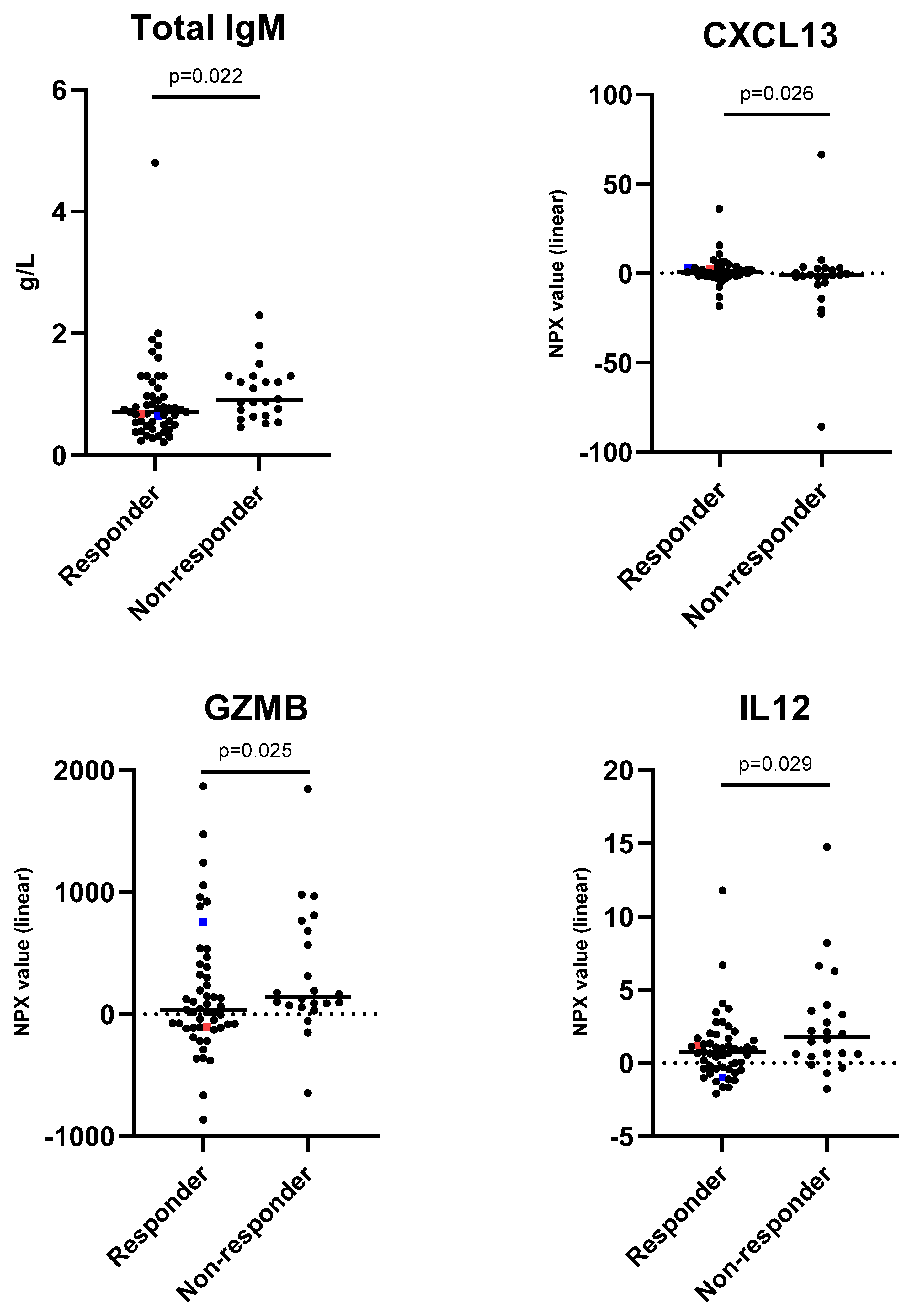
| Total IgM (g/L) | |||||||
| ≤0.5 | 15 | 14 | 93 | 1.00 | 1.00 | ||
| 0.6–0.8 | 26 | 19 | 73 | 0.54 (0.33–0.90) | 0.49 (0.27–0.90) | ||
| 0.9–1.1 | 12 | 7 | 58 | 0.29 (0.11–0.80) | 0.24 (0.07–0.81) | ||
| >1.1 | 20 | 11 | 55 | 0.16 (0.04–0.72) | 0.017 | 0.12 (0.02–0.72) | 0.022 |
| CCL19 | |||||||
| ≤−1.1 | 19 | 13 | 68 | 1.00 | |||
| −1.2–0.6 | 18 | 18 | 100 | 0.62 (0.38–1.00) | |||
| 0.7–2.0 | 17 | 11 | 65 | 0.38 (0.15–0.99) | |||
| >2.0 | 19 | 9 | 47 | 0.24 (0.06–0.99) | 0.048 | - | |
| CD70 | |||||||
| ≤−0.3 | 18 | 13 | 72 | 1.00 | |||
| −0.4–0.2 | 19 | 17 | 89 | 0.70 (0.43–1.13) | |||
| 0.3–0.9 | 19 | 11 | 58 | 0.49 (0.19–1.28) | |||
| >0.9 | 17 | 10 | 59 | 0.34 (0.08–1.45) | 0.143 | - | |
| CXCL13 | |||||||
| ≤−1.7 | 18 | 9 | 50 | 1.00 | 1.00 | ||
| −1.8–−0.4 | 18 | 12 | 67 | 1.48 (0.92–2.38) | 2.06 (1.09–3.88) | ||
| −0.5–1.1 | 18 | 17 | 94 | 2.20 (0.86–5.66) | 4.24 (1.20–15.02) | ||
| >1.1 | 19 | 13 | 68 | 3.26 (0.79–13.46) | 0.100 | 8.73 (1.31–58.19) | 0.026 |
| IL13 | |||||||
| ≤−0.9 | 19 | 18 | 95 | 1.00 | |||
| −0.8–−0.06 | 17 | 10 | 59 | 0.61 (0.38–0.98) | |||
| −0.05–0.7 | 17 | 11 | 65 | 0.37 (0.14–0.96) | |||
| >0.8 | 20 | 12 | 60 | 0.23 (0.05–0.95) | 0.042 | - | |
| GZMB | |||||||
| ≤−135 | 19 | 17 | 89 | 1.00 | 1.00 | ||
| −134–14 | 17 | 12 | 71 | 0.61 (0.38–0.99) | 0.42 (0.19–0.89) | ||
| 15–259 | 19 | 11 | 58 | 0.37 (0.14–0.98) | 0.17 (0.04–0.80) | ||
| >259 | 18 | 11 | 61 | 0.23 (0.05–0.98) | 0.046 | 0.07 (0.01–0.72) | 0.025 |
| IL12 | |||||||
| ≤−0.5 | 18 | 15 | 83 | 1.00 | 1.00 | ||
| −0.4–0.4 | 18 | 12 | 67 | 0.63 (0.39–1.02) | 0.43 (0.21–0.92) | ||
| 0.5–1.2 | 18 | 15 | 83 | 0.40 (0.15–1.05) | 0.19 (0.04–0.84) | ||
| >1.2 | 19 | 9 | 47 | 0.25 (0.06–1.07) | 0.061 | 0.08 (0.01–0.77) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlsson, H.; Brudin, L.; Serrander, L.; Hinkula, J.; Tjernberg, I. Cell-Mediated Proteomics, and Serological and Mucosal Humoral Immune Responses after Seasonal Influenza Immunization: Characterization of Serological Responders and Non-Responders. Vaccines 2024, 12, 303. https://doi.org/10.3390/vaccines12030303
Carlsson H, Brudin L, Serrander L, Hinkula J, Tjernberg I. Cell-Mediated Proteomics, and Serological and Mucosal Humoral Immune Responses after Seasonal Influenza Immunization: Characterization of Serological Responders and Non-Responders. Vaccines. 2024; 12(3):303. https://doi.org/10.3390/vaccines12030303
Chicago/Turabian StyleCarlsson, Hanna, Lars Brudin, Lena Serrander, Jorma Hinkula, and Ivar Tjernberg. 2024. "Cell-Mediated Proteomics, and Serological and Mucosal Humoral Immune Responses after Seasonal Influenza Immunization: Characterization of Serological Responders and Non-Responders" Vaccines 12, no. 3: 303. https://doi.org/10.3390/vaccines12030303






