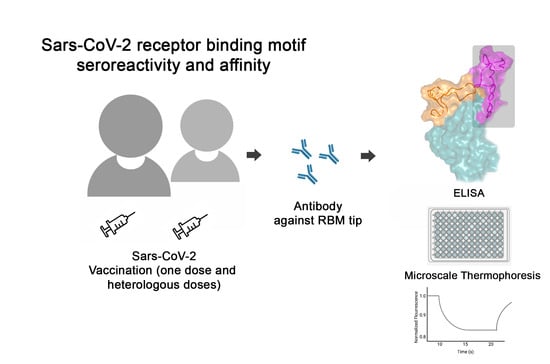
Humoral Immune Response to SARS-CoV-2 Spike Protein Receptor-Binding Motif Linear Epitopes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Samples
2.2. Peptide Synthesis
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Purification of RBM Antibodies
2.5. Microscale Thermophoresis (MST)
2.6. Protein Thermal Shift Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An Update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed]
- Lenharo, M. WHO declares end to COVID-19’s emergency phase. Nature 2023. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Xiang, T.; Wang, J.; Zheng, X. The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages. Virol. Sin. 2022, 37, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.M.; Lai, S.J.; Lin, T.L.; Tseng, T.S. Antigen-Antibody complex-guided exploration of the hotspots conferring the immune-escaping ability of the SARS-CoV-2 RBD. Front. Mol. Biosci. 2022, 9, 797132. [Google Scholar] [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A Neutralizing human antibody binds to the N-terminal domain of the spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Castro Dopico, X.; Dyrdak, R.; Martin, D.P.; Reddy, S.T.; Dillner, J.; Karlsson Hedestam, G.B.; et al. Neutralization sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: A cross-sectional study. Lancet Infect Dis. 2022, 22, 813–820. [Google Scholar] [CrossRef]
- Huang, Q.; Han, X.; Yan, J. Structure-based neutralizing mechanisms for SARS-CoV-2 antibodies. Emerg. Microbes Infect. 2022, 11, 2412–2422. [Google Scholar] [CrossRef]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 Spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef]
- Deshpande, A.; Harris, B.D.; Martinez-Sobrido, L.; Kobie, J.J.; Walter, M.R. Epitope classification and RBD binding properties of neutralizing antibodies against SARS-CoV-2 variants of concern. Front. Immunol. 2021, 12, 691715. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.; Cloutier, M.; Prévost, J.; Fink, C.; Ducas, É.; Ding, S.; Dussault, N.; Landry, P.; Tremblay, T.; Laforce-Lavoie, A.; et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021, 34, 108790. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Beard, L.J.; Feldman, R.G. IgG subclass distribution of antibodies to bacterial and viral antigens. Ped. Infect. Dis. J. 1990, 9, 516. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Zueva, E.V.; Arsentieva, N.A.; Batsunov, O.K.; Liubimova, N.E.; Khamitova, I.V.; Kuznetsova, R.N.; Rubinstein, A.A.; Savin, T.V.; Stanevich, O.V.; et al. Changes in anti-SARS-CoV-2 IgG subclasses over time and in association with disease severity. Viruses 2022, 14, 941. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system? Biomolecules 2023, 13, 1338. [Google Scholar] [CrossRef]
- Almanza, G.; Clark, A.E.; Kouznetsova, V.; Olmedillas, E.; Castro, A.; Tsigelny, I.F.; Wu, Y.; Gao, G.F.; Leibel, S.L.; Bray, W.; et al. Structure-selected RBM immunogens prime polyclonal memory responses that neutralize SARS-CoV-2 variants of concern. PLoS Pathog. 2022, 18, e1010686. [Google Scholar] [CrossRef]
- Pratesi, F.; Errante, F.; Pacini, L.; Peña-Moreno, I.C.; Quiceno, S.; Carotenuto, A.; Balam, S.; Konaté, D.; Diakité, M.M.; Aréva-lo-Herrera, M.; et al. A SARS-CoV-2 spike receptor binding motif peptide induces anti-spike antibodies in mice and is recognized by COVID-19 patients. Front. Immunol. 2022, 13, 879946. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Mohsen, M.O.; Zha, L.; Vogel, M.; Speiser, D.E. SARS-CoV-2 Structural features may explain limited neutralizing-antibody responses. NPJ Vaccines 2021, 6, 2. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; de Pina, J.S.; da Silva, F.R. Epitope mapping of the diphtheria toxin and development of an ELISA-specific diagnostic assay. Vaccines 2021, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.R.; Durans, A.M.; Napoleão-Pêgo, P.; Waterman, J.A.; Freitas, M.S.; De Sá, N.B.R.; Pereira, L.V.; Furtado, J.S.; Aquino, R.G.; Machado, M.C.R.; et al. Multiepitope proteins for the differential detection of IgG antibodies against RBD of the spike protein and non-RBD regions of SARS-CoV-2. Vaccines 2021, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Bottino, C.C.G.; Pinho, R.T.; Provance-Jr, D.W.; De-Simone, S.G. Trypanosoma cruzi presenilin-like transmembrane aspartyl protease: Characterization and cellular localization. Biomolecules 2020, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Nascimento, H.J.; Prado, I.C.; Aguiar, A.S.; Melgarejo, A.R.; Pina, J.L.S.; Ferreira, P.F.; Provance, D.W. Puri-fication of equine IgG3 by lectin affinity and an interaction analysis via microscale thermophoresis. Anal. Biochem. 2018, 561–562, 27–31. [Google Scholar] [CrossRef]
- Guo, Y.; Han, J.; Zhang, Y.; He, J.; Yu, W.; Zhang, X.; Wu, J.; Zhang, S.; Kong, Y.; Guo, Y.; et al. SARS-CoV-2 Omicron variant: Epidemiological features, biological characteristics, and clinical significance. Front. Immunol. 2022, 13, 877101. [Google Scholar] [CrossRef]
- Nchioua, R.; Schundner, A.; Klute, S.; Koepke, L.; Hirschenberger, M.; Noettger, S.; Fois, G.; Zech, F.; Graf, A.; Krebs, S.; et al. Reduced replication but increased interferon resistance of SARS-CoV-2 Omicron BA.1. Life Sci. Alliance 2023, 6, e202201745. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Thorne, L.G.; Bouhaddou, M.; Reuschl, A.K.; Zuliani-Alvarez, L.; Polacco, B.; Pelin, A.; Batra, J.; Whelan, M.V.X.; Hosmillo, M.; Fossati, A.; et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature 2023, 602, 487–495. [Google Scholar] [CrossRef]
- Reuschl, A.K.; Thorne, L.G.; Whelan, M.V.X.; Ragazzini, R.; Furnon, W.; Cowton, V.M.; De Lorenzo, G.; Mesner, D.; Turner, J.L.E.; Dowgier, G.; et al. Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants. Nat. Microbiol. 2024, 9, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, D.; Zhang, H.; Jiang, H.; Tian, X.; Ma, M.; Qi, H.; Meng, Q.; Guo, S.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Makdasi, E.; Levy, Y.; Alcalay, R.; Noy-Porat, T.; Zahavy, E.; Mechaly, A.; Epstein, E.; Peretz, E.; Cohen, H.; Bar-On, L.; et al. Neutralizing monoclonal anti-SARS-CoV-2 antibodies isolated from immunized rabbits define novel vulnerable spike-protein epitope. Viruses 2021, 13, 566. [Google Scholar] [CrossRef]
- Xie, J.; Ding, C.; He, J.; Zhang, Y.; Ni, S.; Zhang, X.; Chen, Q.; Wang, J.; Huang, L.; He, H.; et al. Novel monoclonal antibodies and recombined antibodies against variant SARS-CoV-2. Front. Immunol. 2021, 12, 715464. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.A.; Zhou, D.; Tan, T.K.; Chen, C.; Duyvesteyn, H.M.E.; Zhao, Y.; Ginn, H.M.; Qin, L.; Rijal, P.; Cheng, S.H. Structures and therapeutic potential of anti-RBD human monoclonal antibodies against SARS-CoV-2. Theranostics 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Wilhelm, A.; Widera, M.; Grikscheit, K.; Toptan, T.; Schenk, B.; Pallas, C.; Metzler, M.; Kohmer, N.; Hoehl, S.; Marschalek, R.; et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine 2022, 82, 104158. [Google Scholar] [CrossRef] [PubMed]
- Anzai, I.; Fujita, J.; Ono, C.; Kosaka, Y.; Miyamoto, Y.; Shichinohe, S.; Takada, K.; Torii, S.; Taguwa, S.; Suzuki, K.; et al. Characterization of a neutralizing antibody that recognizes a loop region adjacent to the receptor-binding interface of the SARS-CoV-2 spike receptor-binding domain. Microbiol. Spectr. 2024, e0365523. [Google Scholar] [CrossRef]
- Acharjee, A.; Ray, A.; Salkar, A.; Bihani, S.; Tuckley, C.; Shastri, J.; Agrawal, S.; Duttagupta, S.; Srivastava, S. Humoral Immune Response Profile of COVID-19 Reveals Severity and Variant-Specific Epitopes: Lessons from SARS-CoV-2 Peptide Microarray. Viruses 2023, 15, 248. [Google Scholar] [CrossRef]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Paul Chourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Jacobsen, H.; Sitaras, I.; Katzmarzyk, M.; Jiménez, V.C.; Naughton, R.; Higdon, M.M.; Knoll, M.D. Systematic review and meta-analysis of the factors affecting waning of post-vaccination neutralizing antibody responses against SARS-CoV-2. NPJ Vaccines 2023, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xie, C.; Bu, G.-L.; Zhong, L.-Y.; Zeng, M.-S. Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants. Signal Transduct. Target. Ther. 2022, 7, 202. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Li, M.; Lou, F.; Fan, H. SARS-CoV-2 variant Omicron: Currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct. Target. Ther. 2022, 7, 28. [Google Scholar] [CrossRef]
- Lupala, C.S.; Ye, Y.; Chen, H.; Su, X.-D.; Liu, H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem. Biophys. Res. Commun. 2022, 590, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Hotez, P.J.; Bottazzi, M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum. Vaccine Immunother. 2020, 16, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chang, X.; Rothen, D.; Derveni, M.; Krenger, P.; Roongta, S.; Wright, E.; Vogel, M.; Tars, K.; Mohsen, M.O.; et al. AP205 VLPs based on dimerized capsid proteins accommodate RBM domain of SARS-CoV-2 and serve as an attractive vaccine candidate. Vaccines 2021, 9, 403. [Google Scholar] [CrossRef]
- Castro, A.; Ozturk, K.; Zanetti, M.; Carter, H. In silico analysis suggests less effective MHC-II Presentation of SARS-CoV-2 RBM Peptides: Implication for neutralizing antibody responses. PLoS ONE 2021, 16, e0246731. [Google Scholar] [CrossRef]
- Vainio, O.; Toivanen, P.; Toivanen, A. Major histocompatibility complex and cell cooperation. Poul. Sci. 1987, 66, 795–801. [Google Scholar] [CrossRef]
- Lani, R.; Senin, N.A.; AbuBakar, S.; Hassandarvish, P. Knowledge of SARS-CoV-2 epitopes and population HLA types is important in the design of COVID-19 vaccines. Vaccines 2022, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Bertinetto, F.E.; Magistroni, P.; Mazzola, G.A.; Costa, C.; Elena, G.; Alizzi, S.; Scozzari, G.; Migliore, E.; Galassi, C.; Ciccone, G.; et al. The humoral and cellular response to mRNA SARS-CoV-2 vaccine is influenced by HLA polymorphisms. HLA 2023, 102, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, A.J.; Connor, D.; Bibi, S.; Chelysheva, I.; Clutterbuck, E.A.; Demissie, T.; Dinesh, T.; Edwards, N.J.; Felle, S.; Feng, S.; et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat. Med. 2023, 29, 147–157. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, M.E.S.; Lechuga, G.C.; Napoleão-Pêgo, P.; Carvalho, J.P.R.S.; Gomes, L.R.; Morel, C.M.; Provance, D.W.; De-Simone, S.G. Humoral Immune Response to SARS-CoV-2 Spike Protein Receptor-Binding Motif Linear Epitopes. Vaccines 2024, 12, 342. https://doi.org/10.3390/vaccines12040342
Monteiro MES, Lechuga GC, Napoleão-Pêgo P, Carvalho JPRS, Gomes LR, Morel CM, Provance DW, De-Simone SG. Humoral Immune Response to SARS-CoV-2 Spike Protein Receptor-Binding Motif Linear Epitopes. Vaccines. 2024; 12(4):342. https://doi.org/10.3390/vaccines12040342
Chicago/Turabian StyleMonteiro, Maria E. S., Guilherme C. Lechuga, Paloma Napoleão-Pêgo, João P. R. S. Carvalho, Larissa R. Gomes, Carlos M. Morel, David W. Provance, and Salvatore G. De-Simone. 2024. "Humoral Immune Response to SARS-CoV-2 Spike Protein Receptor-Binding Motif Linear Epitopes" Vaccines 12, no. 4: 342. https://doi.org/10.3390/vaccines12040342
APA StyleMonteiro, M. E. S., Lechuga, G. C., Napoleão-Pêgo, P., Carvalho, J. P. R. S., Gomes, L. R., Morel, C. M., Provance, D. W., & De-Simone, S. G. (2024). Humoral Immune Response to SARS-CoV-2 Spike Protein Receptor-Binding Motif Linear Epitopes. Vaccines, 12(4), 342. https://doi.org/10.3390/vaccines12040342