Inclusion of Anti-Tick Vaccines into an Integrated Tick Management Program in Mexico: A Public Policy Challenge
Abstract
:1. Introduction
2. Biological Diversity and Taxonomy of Ticks
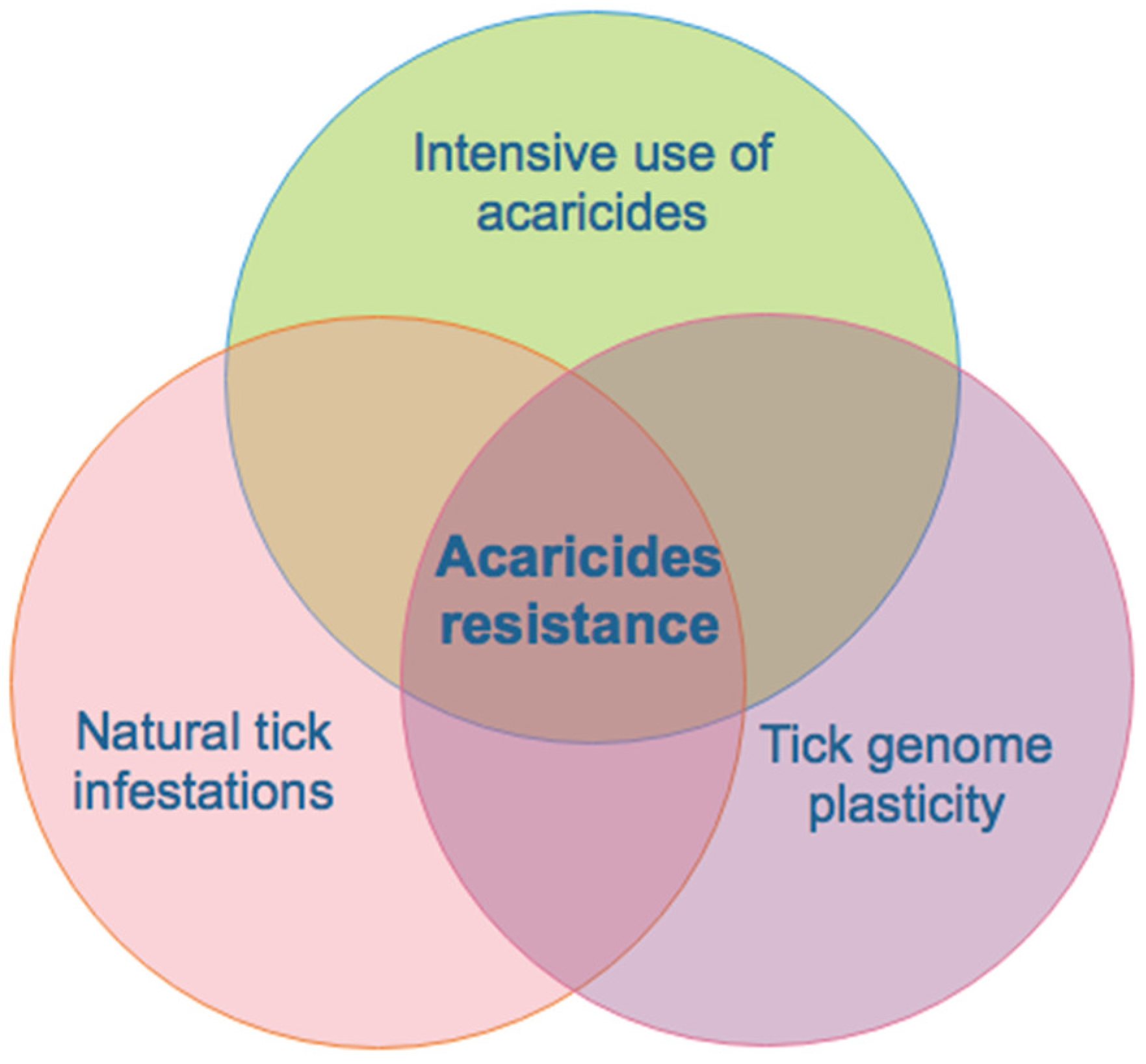
3. Acaricide Resistance and Food Safety
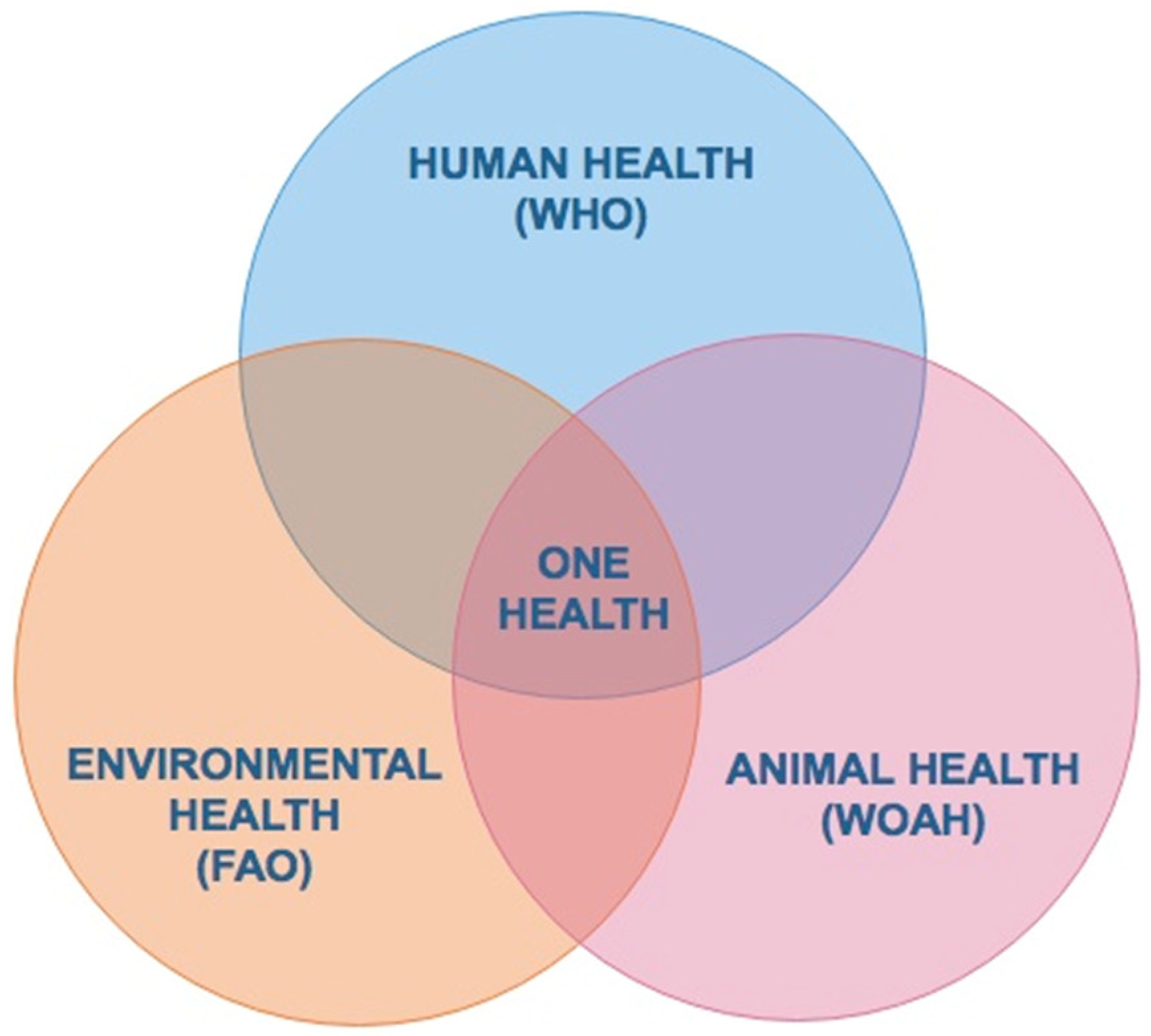
4. Ticks and Tick-Borne Pathogens: A Public Policy Issue
5. The Cattle Tick Eradication Program in Mexico
6. Host Resistance to Ticks and Anti-Tick Vaccines
7. Bm86-Based Vaccines
| Tick Vaccine Antigen | Localization/Function | General Efficacy | References |
|---|---|---|---|
| Bm86/Bm95 | Membrane-bound extracellular protein, intestinal cells | 49–70% | [71,72,73] |
| Subolesin | Highly conserved protein involved in tick feeding and fertility | 51–60% | [74,75] |
| Bm95-msp1a | Tick intestine glycoprotein and Anaplasma marginale msp1a | 64% | [76] |
| Sub-mp1a | Tick feeding and fertility, and A. marginale msp1a | 81% | [76] |
| Ba86 | Bm86 orthologue from R. annulatus, Mercedes strain | 71.5% | [77] |
| Ferritin 2 | Tick iron metabolism, originally identified in Ixodes ricinus | 64–74% | [78] |
| Subolesin peptide | Subolesin immunogenic peptide derived from R. microplus Media Joya strain | 67% | [79] |
| VDAC | Mitochondrial protein with a role as a central component of the apoptotic machinery in R. microplus | 82% | [80] |
| Bm86 polypeptide | Bm86 polypeptide derived from R. microplus Media Joya strain | 58% | [81] |
8. Other Available Proteins
8.1. Subolesin
8.2. Chimeric Proteins
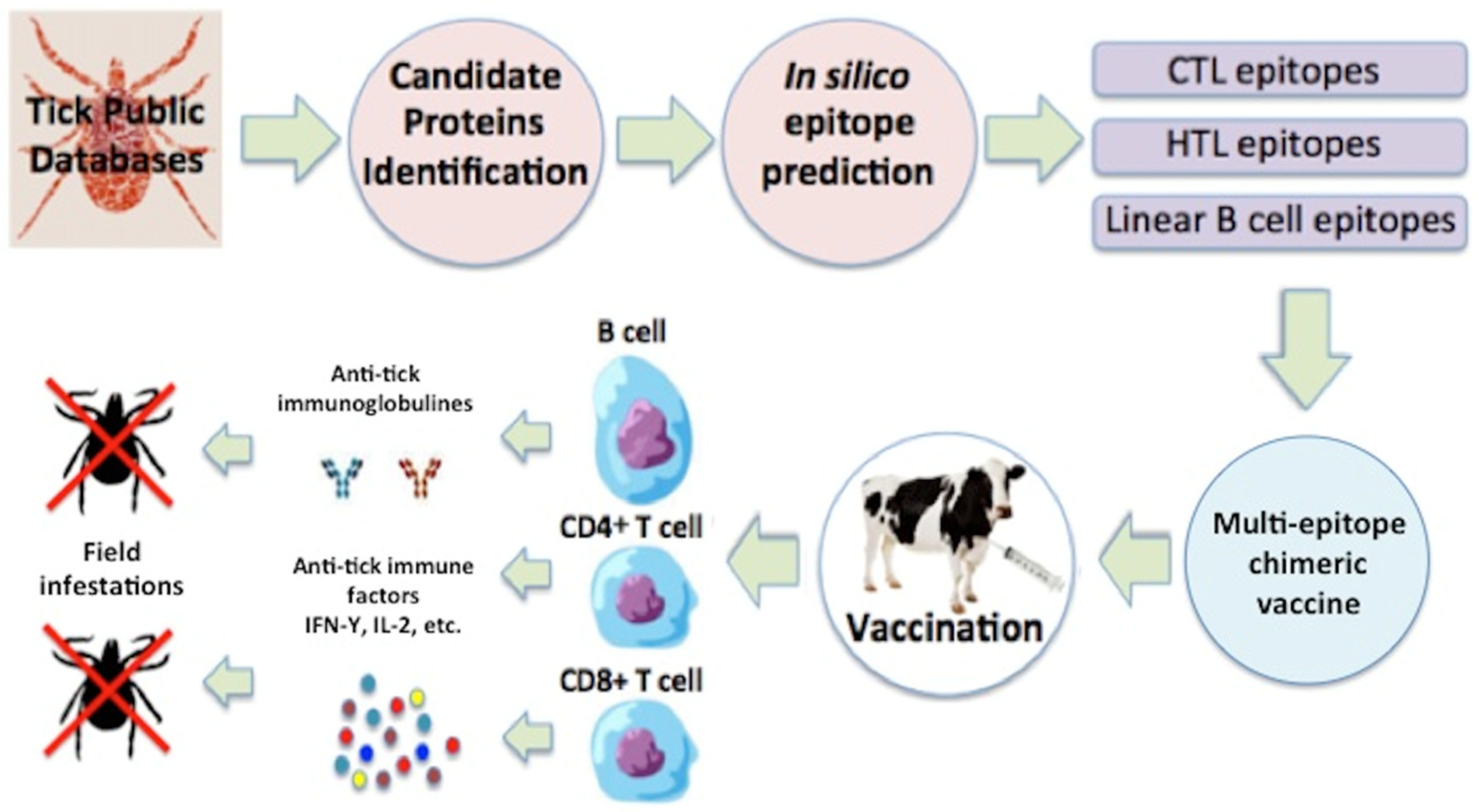
9. Anti-Tick Vaccination Role as a Part of an Integrated Control Program
10. Tick Control and Vaccination Proposal
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, R.; Opdebeeck, J.P. Arthropod vaccines. Infect. Dis. Clin. N. Am. 1999, 13, 209–226. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Estrada-Pena, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosc. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- FAO. The state of food and agriculture. In Climate Change, Agriculture and Food Security; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/i6030e/i6030e.pdf (accessed on 20 March 2024).
- Parola, P.; Raoult, D. Ticks and tick borne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.P.; Loomis, E.C. The Ticks of California (Acari: Ixodidae). In University of California Publications, Bulletin of the California Insect Survey; University of California Press: Berkeley, CA, USA, 1984; Volume 25, pp. 1–35. ISBN 0-520-09685-1. [Google Scholar]
- Tahir, D.; Meyer, L.; Fourie, J.; Jongejan, F.; Mather, T.; Choumet, V.; Blagburn, B.; Straubinger, R.K.; Varloud, M. Interrupted blood feeding in Ticks: Causes and consequences. Microorganisms 2020, 8, 910. [Google Scholar] [CrossRef] [PubMed]
- Sharifah, N.; Heo, C.C.; Ehlers, J.; Houssaini, J.; Tappe, D. Ticks and tick-borne pathogens in animals and humans in the island nations of Southeast Asia: A review. Acta Trop. 2020, 209, 105527. [Google Scholar] [CrossRef] [PubMed]
- Buczek, A.; Bartosik, K. Tick-host interactions. Prz. Epidemiol. 2006, 60 (Suppl. S1), 28–33. [Google Scholar]
- Vieira, L.L.; Canever, M.F.; Cardozo, L.L.; Cardozo, C.P.; Herkenhoff, M.E.; Neto, A.T.; Vogel, C.; Milleti, L.C. Prevalence of Anaplasma marginale, Babesia bovis and Babesia bigemina in the Cattle in the campos de Lages región Santa Catarina State, Brazil estimated by multiplex-PCR. Parasite Epidemiol. Control. 2001, 6, e00114. [Google Scholar] [CrossRef] [PubMed]
- Shyma, K.P.; Gupta, J.P.; Singh, V. Breeding strategies for tick resistance in tropical cattle: A sustainable approach for tick control. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2015, 39, 1–6. [Google Scholar] [CrossRef]
- Raijput, Z.I.; Hu, S.H.; Chen, W.J.; Arijo, A.G.; Xiao, C.W. Importance of ticks and their chemical and immunological control in livestock. J. Zhejiang Univ. Sci. B Biomed Biotechnol. 2006, 7, 912–921. [Google Scholar] [CrossRef]
- van Oosterwijk, J.G.; Wikel, S.K. Resistance to Ticks and the Path to Anti-Tick and Transmission Blocking Vaccines. Vaccines 2021, 9, 725. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Laboratory identification of arthropod ectoparasites. Clin. Microbiol. Rev. 2014, 27, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J. Physician’s Guide to Arthropods of Medical Importance, 6th ed.; Taylor and Francis Group; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Barker, S.C.; Murrell, A. Systematics and evolution of ticks with a list of valid genus and species names. In Ticks: Biology, Disease and Control; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–39. [Google Scholar] [CrossRef]
- Durden, L.A.; Beati, L. Modern tick systematics. In Biology of Ticks; Sonenshine, D.E., Roe, R.M., Eds.; Oxford University Press: New York, NY, USA, 2014; Volume 1, pp. 17–58. ISBN 978-0199744060. [Google Scholar]
- Peñalver, E.; Arillo, A.; Delclòs, X. Ticks parasitised feathered dinosaurs as revealed by Cretaceous amber assemblages. Nat. Commun. 2017, 8, 19–24. [Google Scholar] [CrossRef]
- Oliver, J.H., Jr. Biology and Systematics of Ticks (Acari:Ixodida). Annu. Rev. Ecol. Evol. Syst. 1989, 20, 397–430. [Google Scholar] [CrossRef]
- Sonenshine, D.E. The biology of tick vectors of human disease. In Tick-Borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; American Society for Microbiology: Washington, DC, USA, 2005; pp. 12–36. [Google Scholar] [CrossRef]
- Vail, L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 2009, 16, 191–202. [Google Scholar] [CrossRef]
- Apanaskevich, D.A.; Oliver, J.H., Jr. Life cycles and natural history of ticks. In Ticks: Biology, Disease and Control; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 59–73. [Google Scholar]
- Bergman, D.K. Mouthparts and feeding mechanisms of haematophagous arthropods. In The Immunology of Host-Ectoparasitic Arthropod Relationships; Wikel, S.K., Ed.; CAB International: Wallingford, UK, 1996; pp. 30–61. [Google Scholar]
- Binnington, K.C.; Kemp, D.H. Role of tick salivary glands in feeding and disease transmission. Adv. Parasitol. 1980, 18, 315–339. [Google Scholar] [CrossRef]
- Kemp, D.H.; Stone, B.F.; Binnington, K.C. Tick attachment and feeding: Role of the mouthparts, feeding apparatus, salivary gland secretions and the host response. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Peragon Press: Oxford, UK, 1982; Volume 1, pp. 119–168. [Google Scholar] [CrossRef]
- Moorhouse, D.E. The attachment of some ixodid ticks to their natural hosts. In Proceedings of the Second International Congress of Acarology, Sutton Bonington, UK, 19–25 July 1967; Hungarian Academy of Sciences: Budapest, Hungary, 1969; pp. 319–327. [Google Scholar]
- Cooley, R.A.; Kohls, G.M. The Argasidae of North America, Central America and Cuba. Am. Midl. Nat. J. 1944, 1, 1–152. Available online: https://www.biodiversitylibrary.org/item/23448#page/7/mode/1up (accessed on 20 March 2024).
- Kaufman, W.R. Tick-host interaction: A synthesis of current concepts. Parasitol. Today 1989, 5, 47–56. [Google Scholar] [CrossRef]
- George, J.E.; Pound, J.M.; Davey, R.B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129, S353–S366. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Rosario-Cruz, R.; Almazan, C.; Miller, R.J.; Domínguez-Garcia, D.I.; Hernandez-Ortiz, R.; de la Fuente, J. Genetic basis and impact of tick acaricide resistance. Front. Biosc. 2009, 14, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Fred, F.; Wang, H.-S. Suresh Menon. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Rosario-Cruz, R.; Domínguez-García, D.I. Rhipicephalus (Boophilus) microplus: Molecular evolution of Acaricide Resistance. In The Entomological Guide to Rhipicephalus; Nova Publisher: Hauppauge, NY, USA, 2021; pp. 224–246. ISBN 978-1-53619-619-1. [Google Scholar]
- Prudencio, C.R.; Marra, A.O.; Cardoso, R.; Goulart, L.R. Recombinant peptides as new immunogens for the control of the bovine tick, Rhipicephalus (Boophilus) microplus. J. Vet. Parasitol. 2010, 172, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Mallón, A.R.; Bermúdez, S.; de la Fuente, J.; Domingos, A.; García, M.P.E.; Labruna, M.B.; Merino, O.; Mosqueda, J.; Nava, S.; et al. One Health Approach to Identify Research Needs on Rhipicephalus microplus Ticks in the Americas. Pathogens 2022, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. [Google Scholar] [CrossRef] [PubMed]
- Colunga-Salas, P.; Sánchez-Montes, S.; Volkow, P.; Ruíz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.C.; Pak, A.W. Multidisciplinary, interdisciplinary and transdisciplinarity in health research, services, education and policy: 1 Definitions, objectives, and evidence of effectiveness. Clin. Investig. Med. 2006, 29, 351–364. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Bonfoh, B.; Fooks, A.R.; Kasymbekov, J.; Waltner-Toews, D.; Tanner, M. Towards a “One Health” research and application toolbox. Vet. Ital. 2009, 45, 119–131. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-Peña, A.; Horak, I.; Latif, A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A guide to identification of species. In Bioscience Reports; Edinburgh: Scotland, UK, 2003; ISBN 0-9545173-0-X. [Google Scholar]
- Estrada-Peña, A.; García, Z.; Sánchez, H.F. The distribution and ecological preferences of Boophilus microplus (Acari: Ixodidae) in Mexico. Exp. Appl. Acarol. 2006, 38, 307–316. [Google Scholar] [CrossRef]
- Guerrero, F.D.; Bendele, K.G.; Chen, A.C.; Li, A.Y.; Miller, R.J.; Pleasance, E.; Varhol, R.; Rousseau, M.E.; Nene, V.M. Serial analysis of gene expression in the southern cattle tick following acaricide treatment of larvae from organophosphate resistant and susceptible strains. Insect Mol. Biol. 2007, 16, 49–60. [Google Scholar] [CrossRef]
- Dennis, D.T.; Piesman, J.F. Overview of tick-borne infections of humans. In Tick-borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; American Society for Microbiology: Washington, DC, USA, 2005; pp. 3–11. [Google Scholar]
- Kiss, T.; Cadar, D.; Spînu, M. Tick prevention at a crossroad: New and renewed solutions. Vet. Parasitol. 2012, 187, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Salman, M. Current limitations in the control and spread of ticks that affect livestock: A review. Agriculture 2013, 3, 221–235. [Google Scholar] [CrossRef]
- Baneth, G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Messenger, T.M. Cattle Fever Tick Eradication Program–Tick Control Barrier Maverick, Starr, Webb, and Zapata Counties, Texas. Cattle Fever Tick Eradication Program. Veterinary Services Animal and Plant Health Inspection Service. USDA. 2013. Available online: https://www.aphis.usda.gov/animal_health/animal_diseases/tick/downloads/draft_eis_document.pdf (accessed on 20 March 2024).
- Mallori, P. Recent Study Highlights the Importance of Testing Stray Mexico Origin Cattle for Tick Borne Pathogens. Texas A&M, Veterinary Medical Diagnostic Laboratory. Available online: https://tvmdl.tamu.edu/2021/06/28/recent-study-highlights-the-importance-of-testing-stray-mexico-origin-cattle-for-tick-borne-pathogens/ (accessed on 20 March 2024).
- George, J.E. The effects of global change on the threat of exotic arthropods and arthropod-borne pathogens to livestock in the United States. Ann. N. Y. Acad. Sci. 2008, 1149, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Trapaga, B. The Campaign against Boophilus Microplus in Mexico, Benefit, Problems and Prospects; FAO: Mexico, 1989; pp. 50–75. [Google Scholar]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Zoosanitary Campaigns. Mexico. Available online: https://www.gob.mx/senasica/acciones-y-programas/campana-nacional-para-el-control-de-la-garrapata-boophilus-spp (accessed on 20 March 2024).
- United States Department of Agriculture. U.S.-Mexico Binational Committee for Tuberculosis, Brucellosis, and Cattle Fever Tick; United States Department of Agriculture USDA: Riverdale, CA, USA, 2020. [Google Scholar]
- Bautista-Garfias, C.R.; Castañeda-Arriola, R.; Álvarez-Martínez, J.A.; Rojas-Martínez, C.; Figueroa-Millán, J.V.; Rodríguez-Lozano, A. The simultaneous vaccination of bovines with Lactobacillus casei and the bivalent vaccine against bovine babesiosis induces a better protection against Babesia bovis and B. bigemina transmitted by ticks in extreme field conditions. Vet. Méx. 2012, 43, 189–200. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0301-50922012000300001&lng=es&tlng=es (accessed on 20 March 2024).
- Pérez de León, A.A.; Teel, P.D.; Auclair, A.N.; Messenger, M.T.; Guerrero, F.D.; Schuster, G.; Miller, R.J. Integrated Strategy for Sustainable Cattle Fever Tick Eradication in USA is Required to Mitigate the Impact of Global Change. Front. Physiol. 2012, 3, 195. [Google Scholar] [CrossRef]
- Thomas, D.B.; Klafke, G.; Busch, J.D.; Olafson, P.U.; Miller, R.A.; Mosqueda, J.; Stone, N.E.; Scoles, G.; Wagner, D.M.; Perez-De-Leon, A. Tracking the Increase of Acaricide Resistance in an Invasive Population of Cattle Fever Ticks (Acari: Ixodidae) and Implementation of Real-Time PCR Assays to Rapidly Genotype Resistance Mutations. Ann. Entomol. Soc. Am. 2020, 113, 298–309. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service. US Department of Agriculture. Available online: https://www.ars.usda.gov/data-products/livestock-and-meat-international-trade-data/ (accessed on 20 March 2024).
- Animal and plant health inspection service. US Department of Agriculture. Available online: https://www.aphis.usda.gov/aphis/ourfocus/Animalhealth/animal-and-animal-product-import-information/live-animal-imports/import-live-animals (accessed on 20 March 2024).
- González Sáenz Pardo, J.R.; Hernández Ortiz, R. Boophilus microplus: Current status of resistance to acaricides on the Mexico-United States border and its impact on the trade relationship. Rev. Mex. Cienc Pecu. 2012, 3, 1–8. [Google Scholar]
- Esteve-Gasent, M.D.; Rodríguez-Vivas, R.I.; Medina, R.F.; Ellis, D.; Schwartz, A.; Cortés Garcia, B.; Hunt, C.; Tietjen, M.; Bonilla, D.; Thomas, D.; et al. Research on Integrated Management for Cattle Fever Ticks and Bovine Babesiosis in the United States and Mexico: Current Status and Opportunities for Binational Coordination. Pathogens 2020, 9, 871. [Google Scholar] [CrossRef]
- Giles, J.R.; Peterson, A.T.; Busch, J.D.; Olafson, P.U.; Scoles, G.A.; Davey, R.B.; Pound, J.M.; Kammlah, D.M.; Lohmeyer, K.H.; Wagner, D.M. Invasive potential of cattle fever ticks in the southern United States. Parasites Vect. 2014, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.H.; Bancroft, M.J. A tick resistant condition in cattle. Proc. R. Soc. Qld. 1918, 30, 219–317. [Google Scholar] [CrossRef]
- Trager, W. Acquired immunity to ticks. J. Parasitol. 1939, 25, 57–81. [Google Scholar] [CrossRef]
- Pimentel, D. Green revolution agriculture and chemical hazards. Sci. Total Environ. 1996, 188, S86–S98. [Google Scholar] [CrossRef]
- Allen, J.R.; Humphreys, S.J. Immunisation of guinea pigs and cattle against ticks. Nature 1979, 280, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.H.; Agbede, R.I.; Johnston, L.A.; Gough, J.M. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Feeding and survival of the parasite on vaccinated cattle. Int. J. Parasitol. 1986, 16, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, P.; Kemp, D.H. Vaccination with ‘concealed’ antigens for tick control. Parasitol. Today 1988, 4, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.N.; Moore, T.; Sriskantha, A.; Spring, K.; Tellam, R.; Willadsen, P.; Cobon, G.S. Cloning and expression of a protective antigen from the cattle tick Boophilus Microplus. Proc. Natl. Acad. Sci. USA 1989, 86, 9657–9661. [Google Scholar] [CrossRef]
- Johnston, L.A.Y.; Kemp, D.H.; Pearson, R.D. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Effects of induced immunity on tick populations. Int. J. Parasitol. 1986, 16, 27–34. [Google Scholar] [CrossRef]
- Agbede, R.I.S.; Kemp, D.H. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Histopathology of ticks feeding on vaccinated cattle. Int. J. Parasitol. 1986, 16, 35–41. [Google Scholar] [CrossRef]
- Andreotti, R.; Pedroso, M.S.; Caetano, A.R.; Martins, N.F. Comparison of predicted binders in Rhipicephalus (Boophilus) microplus intestine protein variants Bm86 Campo Grande strain, Bm86 and Bm95. Rev. Bras. Parasitol. Vet. 2008, 17, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Canales, M.; Moreno-Cid, J.A.; Almazán, C.; Villar, M.; de la Fuente, J. Bioprocess design and economics of recombinant BM86/BM95 antigen production for anti-tick vaccines. Biochem. Eng. J. 2010, 52, 79–90. [Google Scholar] [CrossRef]
- Vanegas, L.F.; Parra, S.A.; Vanegas, C.G.; de la Fuente, J. Commercialization of the recombinant vaccine Gavac against Boophilus microplus in Colombia. In Recombinant Vaccines for the Control of Cattle Tick; De la Fuente, J., Ed.; Elfos Scientiae: La Habana, Cuba, 1995; pp. 195–199. [Google Scholar]
- Merino, O.; Antunes, S.; Mosqueda, J.; Moreno-Cid, J.A.; de la Lastra, J.M.; Rosario-Cruz, R.; Rodríguez, S.; Domingos, A.; de la Fuente, J. Vaccination with proteins involved in tick–pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013, 31, 5889–5896. [Google Scholar] [CrossRef]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Moreno-Cantú, O.; Moreno-Cid, J.A.; Galindo, R.C.; Canales, M.; Villar, M.; De la Fuente, J. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine 2012, 30, 265–272. [Google Scholar] [CrossRef]
- Canales, M.; Almazán, C.; Naranjo, V.; Jongejan, F.; de la Fuente, J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Hajdusek, O.; Almazán, C.; Loosova, G.; Villar, M.; Canales, M.; Grubhoffer, L.; De la Fuente, J. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010, 28, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Martínez, N.; Alonso Díaz, M.A.; Merino, O.; Fernández-Salas, A.; Lagunes-Quintanilla, R. Protective efficacy of the peptide Subolesin antigen against the cattle tick Rhipicephalus microplus under natural infestation. Vet. Parasitol. 2021, 299, 109577. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sánchez, R.; Camacho-Nuez, M.; Castañeda-Ortiz, E.J.; Martínez-Benítez, M.B.; Hernández-Silva, D.J.; Aguilar-Tipacamú, G.; Mosqueda, J. Vaccine efficacy of recombinant BmVDAC on Rhipicephalus microplus fed on Babesia bigemina-infected and uninfected cattle. Vaccine 2020, 38, 3618–3625. [Google Scholar] [CrossRef]
- Coate, R.; Alonso-Díaz, M.Á.; Martínez-Velázquez, M.; Castro-Saines, E.; Hernández-Ortiz, R.; Lagunes-Quintanilla, R. Testing Efficacy of a Conserved Polypeptide from the Bm86 Protein against Rhipicephalus microplus in the Mexican Tropics. Vaccines 2023, 11, 1267. [Google Scholar] [CrossRef]
- Willadsen, P.; Bird, P.; Cobon, G.S.; Hungerford, J. Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitol. 1995, 110, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Rubiera, R.; Penichet, M.; Montesinos, R.; Cremata, J.; Falcón, V.; Sánchez, G.; Bringas, R.; Cordovés, C.; Mario Valdés, M.; et al. High level expression of the B. microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J. Biotechnol. 1994, 33, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Playford, M. Review of Research Needs for Cattle Tick Control—Phases I and II. In Meat & Livestock; Australia Ltd.: Sydney, Australia, 2005. [Google Scholar]
- de la Fuente, J.; Almazán, C.; Canales, M.; de la Lastra, J.M.P.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.C.; Montero, C.; Redondo, M.; Vargas, M.; Canales, M.; Boue, O.; Rodríguez, M.; Joglar, M.; Machado, H.; González, I.L.; et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine 2000, 18, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.C.; Gonzalez, I.L.; González, D.M.; Valdés, M.; Méndez, L.; Lamberti, J.; De La Fuente, J. sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Exp. Appl. Acarol. 1999, 23, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Kocan, K.; Bergman, D.; Garcia-Garcia, J.; Blouin, E.; de la Fuente, J. Identification of protective antigens for the control of Ixodes scapularis infestations using cDNA expression library immunization. Vaccine 2003, 21, 1492–1501. [Google Scholar] [CrossRef]
- Galindo, R.C.; Doncel-Perez, E.; Zivkovic, Z.; Naranjo, V.; Gortazar, C.; Mangold, A.J.; de la Fuente, J. Tick subolesin is an ortholog of the akirins described in insects and vertebrates. Dev. Comp. Immunol. 2009, 33, 612–617. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Almazán, C.; Blouin, E.F.; Naranjo, V.; Kocan, K.M. RNA interference screening in ticks for identification of protective antigens. Parasitol. Res. 2005, 96, 137–141. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Garcia-Garcia, J.C.; Blouin, E.F.; Kocan, K.M. Differential adhesion of major surface proteins 1a and 1b of the ehrlichial cattle pathogen Anaplasma marginale to bovine erythrocytes and tick cells. Int. J. Parasitol. 2001, 31, 145–153. [Google Scholar] [CrossRef]
- Canales, M.; de la Lastra JM, P.; Naranjo, V.; Nijhof, A.M.; Hope, M.; Jongejan, F.; de la Fuente, J. Expression of recombinant Rhipicephalus (Boophilus) microplus, R. annulatus and R. decoloratus Bm86 orthologs as secreted proteins in Pichia pastoris. BMC Biotechnol. 2008, 8, 1–12. [Google Scholar] [CrossRef]
- Gallardo, N.L.; Villamar, A.L.; Olivera, C.E. Current Situation and Perspective of Bovine Milk Production in Mexico. 2005. SAGARPA. Available online: https://www.cofemersimir.gob.mx/expediente/4606/mir/12233/anexo/447373 (accessed on 20 March 2024).
- Gallardo, N.L.; Villamar, A.L. Current Situation and Perspective of Beef Production in Mexico. SAGARPA. 2004. Available online: https://www.ipcva.com.ar/files/mexico.pdf (accessed on 20 March 2024).
- Magaña-Monforte, J.G.; Rios-Arjona, G.; Martínez-González, J.C. Dual purpose cattle production systems and the challenges of the tropics of Mexico. Arch. Latinoam. Prod. Anim. 2006, 14, 105–114. [Google Scholar]
- Padilla, E.G.; Vivas, R.I.R.; de Villegas, G.A.; Quero, C.A.R.; García, R.A.; Fernández, S.A.; Benítez, G.A.; Villa, G.A.; Ruiz, M.A.; Sifuentes, R.A.M.; et al. Current Situation and Perspective of State-of-the-Art Production on Research and Technological Innovation in Tropical Bovine Livestock, 2nd ed.; CONACyT-UNAM; Bioempresor Digital RECA S.A. de C.V: Ciudad de Mexico, Mexico, 2018; pp. 130–345. ISBN 978-607-37-0556-1. [Google Scholar]
- Redondo, M.; Fragoso, H.; Montero, C.; Lona, J.; Medellín, J.A.; Fría, R.; De La Fuente, J. Integrated control of acaricide-resistant Boophilus microplus populations on grazing cattle in Mexico using vaccination with Gavac™ and amidine treatments. Exp. Appl. Acarol. 1999, 23, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, H.; Rad, P.H.; Ortiz, M.; Rodriguez, M.; Redondo, M.; Herrera, L.; De la Fuente, J. Protection against Boophilus annulatus infestations in cattle vaccinated with the B. microplus Bm86-containing vaccine Gavac. Vaccine 1998, 16, 1990–1992. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Estrada-Peña, A.; Almazán, C.; Allen, A.; Jory, L.; Yeater, K.; de León, A.A.P. Exploring the use of an anti-tick vaccine as a tool for the integrated eradication of the cattle fever tick, Rhipicephalus (Boophilus) annulatus. Vaccine 2012, 30, 5682–5687. [Google Scholar] [CrossRef] [PubMed]
- Arocho, R.C.M.; Miller, R.J.; Klafke, G.M.; Coates, C.; Grant, W.E.; Samenuk, G.; Teel, P.D. Interaction between anti-tick vaccine and a macrocyclic lactone improves acaricidal efficacy against Rhipicephalus (Boophilus) microplus (Canestrini) (Acari: Ixodidae) in experimentally infested cattle. Vaccine 2022, 40, 6795–6801. [Google Scholar] [CrossRef]
- Rosario-Cruz, R.; Torres-Agaton, F.; Dominguez-Garcia, D.I. Use of Recombinant Proteins in the integrated control of Boophilus microplus, affecting the bovine cattle industry at the pacific coastal state of Guerrero. FESGRO 2020, 5, 738–742. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosario-Cruz, R.; Domínguez-García, D.I.; Almazán, C. Inclusion of Anti-Tick Vaccines into an Integrated Tick Management Program in Mexico: A Public Policy Challenge. Vaccines 2024, 12, 403. https://doi.org/10.3390/vaccines12040403
Rosario-Cruz R, Domínguez-García DI, Almazán C. Inclusion of Anti-Tick Vaccines into an Integrated Tick Management Program in Mexico: A Public Policy Challenge. Vaccines. 2024; 12(4):403. https://doi.org/10.3390/vaccines12040403
Chicago/Turabian StyleRosario-Cruz, Rodrigo, Delia Inés Domínguez-García, and Consuelo Almazán. 2024. "Inclusion of Anti-Tick Vaccines into an Integrated Tick Management Program in Mexico: A Public Policy Challenge" Vaccines 12, no. 4: 403. https://doi.org/10.3390/vaccines12040403






