Estimating All-Cause Deaths Averted in the First Two Years of the COVID-19 Vaccination Campaign in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Sources
2.3. Estimating the Expected Excess All-Cause Deaths
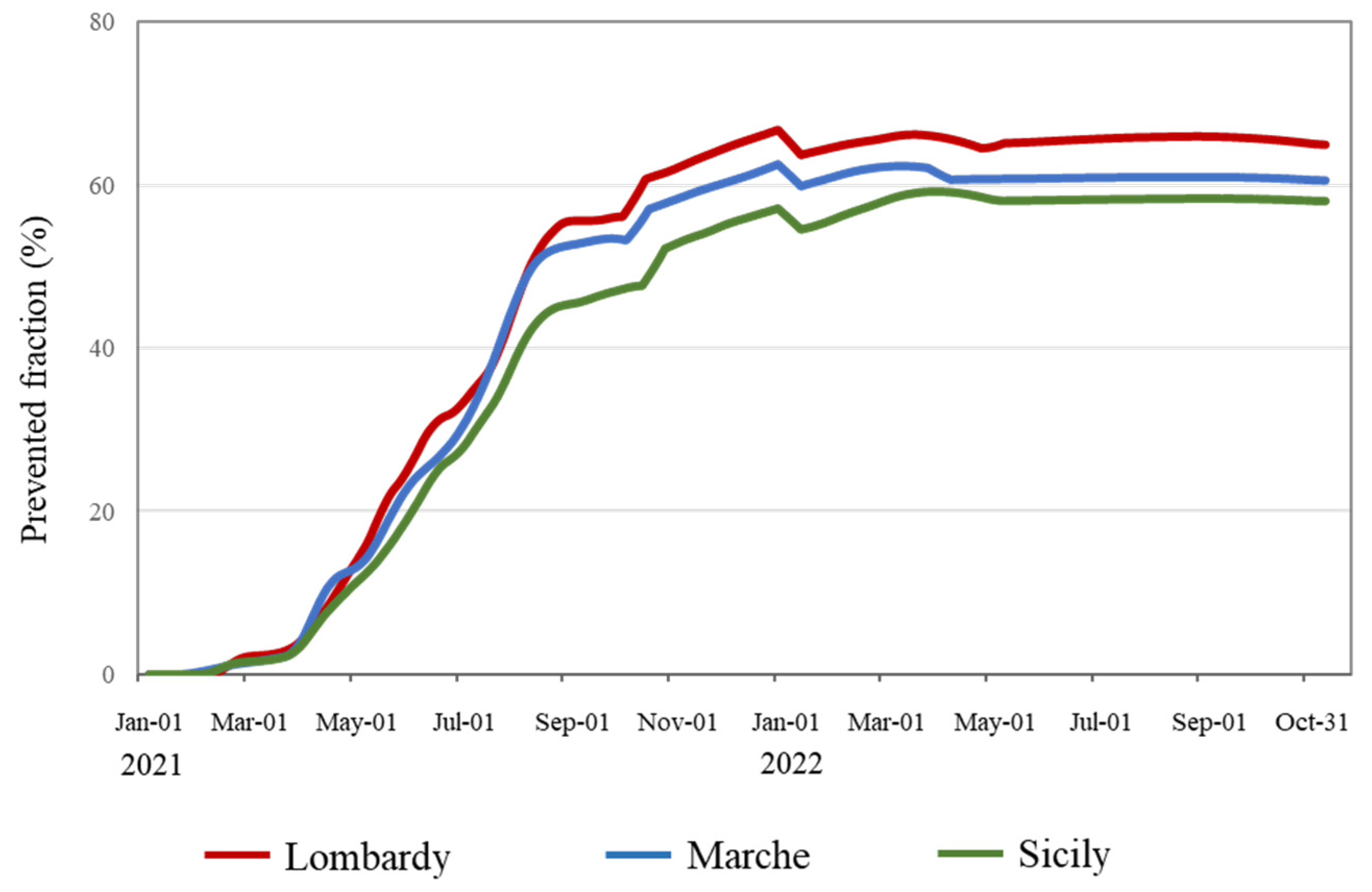
2.4. Prevented Fraction: Rationale and Estimate
2.5. Estimating the Rate of All-Cause Deaths Avoided
3. Results
3.1. Trends in SARS-CoV-2 and Excess Deaths
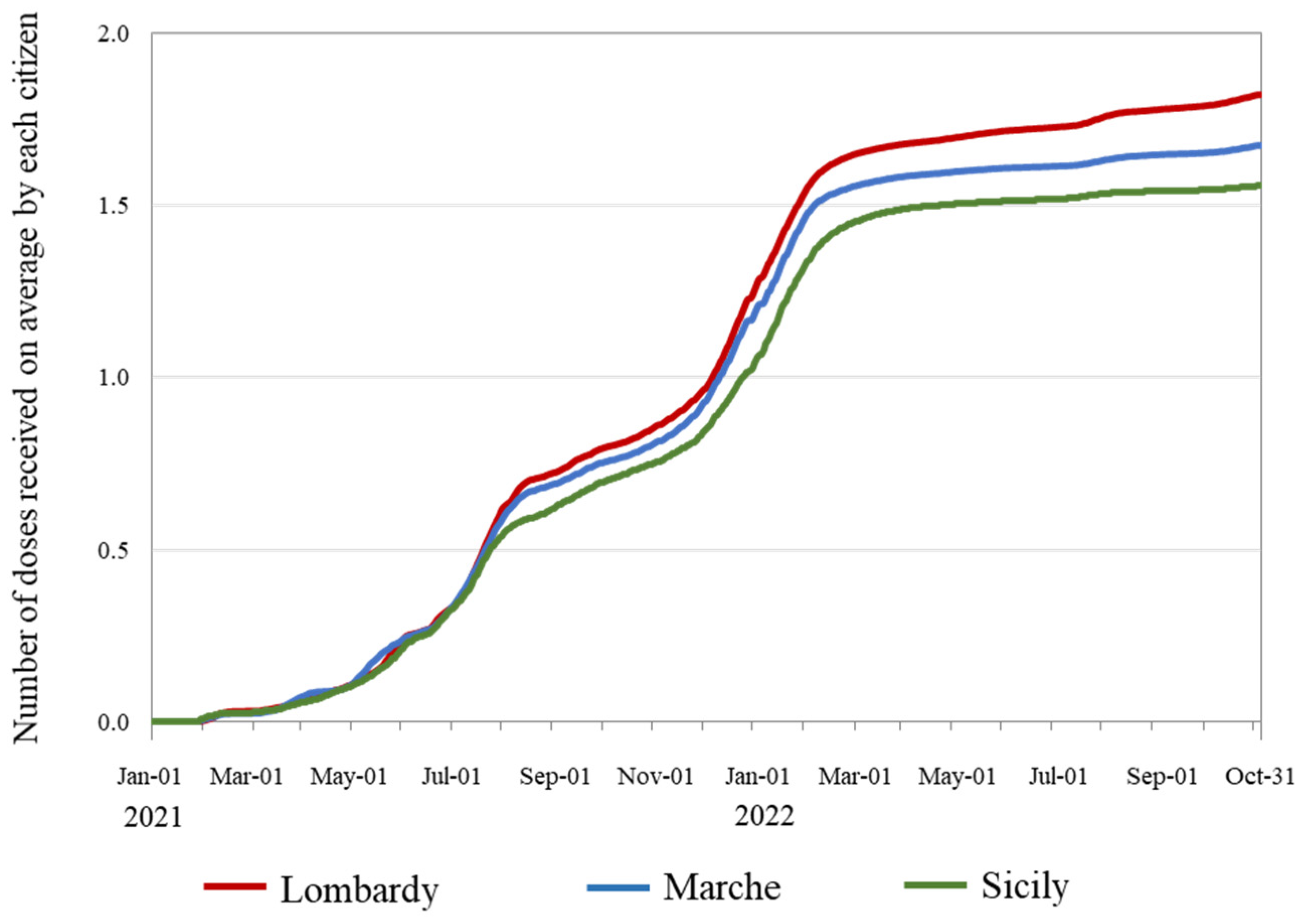
3.2. Trends in the Speed of Vaccination Campaigns and Prevented Fraction
3.3. Trends in the Rate of All-Cause Deaths Avoided
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, G.M.; Conti, S.; Wolters, A.T.; Steventon, A. Evaluating the impact of healthcare interventions using routine data. BMJ 2019, 365, l2239. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on Covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Cegolon, L.; Magnano, G.; Negro, C.; Larese Filon, F. ORCHESTRA Working Group SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses 2023, 15, 1551. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Franchi, M.; Cereda, D.; Bortolan, F.; Zoli, A.; Leoni, O.; Borriello, C.R.; Valle GP, D.; Tirani, M.; Pavesi, G.; et al. Persistence of protection against SARS-CoV-2 clinical outcomes up to 9 months since vaccine completion: A retrospective observational analysis in Lombardy, Italy. Lancet Infect. Dis. 2022, 22, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg. Microbes Infect. 2022, 11, 585–592. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Real-life effectiveness of COVID-19 vaccine during the Omicron variant-dominant pandemic: How many booster doses do we need? Emerg. Microbes Infect. 2023, 12, 2174779. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G.; Franchi, M.; Rea, F.; Cereda, D.; Barone, A.; Borriello, C.R.; Della Valle, P.G.; Ercolanoni, M.; Fortino, I.; Jara, J.; et al. Protective action of natural and induced immunization against the occurrence of delta or alpha variants of SARS-CoV-2 infection: A test-negative case-control study. BMC Med. 2022, 20, 5. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis. 2022, 22, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; A Hernán, M.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 5 April 2023).
- European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccine-tracker (accessed on 5 April 2023).
- Our World in Data. Statistics and Research. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 5 April 2023).
- Presidenza del Consiglio dei Ministri. Report Vaccini Anti-Covid-19. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 5 April 2023).
- Corrao, G.; Bertolaso, G.; Pavesi, G.; Moratti, L. Eight Good Reasons for Careful Monitoring and Evaluation of the Vaccine Campaign against COVID-19, Lessons Learned through the Lombardy Experience for Dealing with Next Challenges. Int. J. Environ. Res. Public Health 2022, 19, 1073. [Google Scholar] [CrossRef] [PubMed]
- Rao, C. Medical certification of cause of death for COVID-19. BullWorld Health Organ. 2020, 98, 298–298A. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Lawlor, D.A.; Brickley, E.B. Comparisons between countries are essential for the control of COVID-19. Int. J. Epidemiol. 2020, 49, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Vandenbroucke, J.P.; VanderWeele, T.J.; Greenland, S. Accurate statistics on COVID-19 are essential for policy guidance and decisions. Am. J. Public Health 2020, 110, 949–951. [Google Scholar] [CrossRef]
- Hasell, J.; Mathieu, E.; Beltekian, D.; Macdonald, B.; Giattino, C.; Ortiz-Ospina, E.; Roser, M.; Ritchie, H. A cross-country database of COVID-19 testing. Sci. Data 2020, 7, 345. [Google Scholar] [CrossRef]
- Pathak, E.B.; Garcia, R.B.; Menard, J.M.; Salemi, J.L. Out-of-hospital COVID-19 deaths: Consequences for quality of medical care and accuracy of cause of death coding. Am. J. Public 2021, 111, S101–S106. [Google Scholar] [CrossRef] [PubMed]
- Labib, P.L.; Aroori, S. Expanding the definition of covid-19 deaths will show the true effect of the pandemic. BMJ 2020, 369, m2153. [Google Scholar] [CrossRef] [PubMed]
- WHO. Revealing the Toll of COVID-19: A Technical Package for Rapid Mortality Surveillance and Epidemic Response. Available online: https://www.who.int/publications/i/item/revealing-the-toll-of-covid-19 (accessed on 5 April 2023).
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Guo, Y.; Gasparrini, A.; Armstrong, B.; Li, S.; Tawatsupa, B.; Tobias, A.; Lavigne, E.; Coelho, M.D.S.Z.S.; Leone, M.; Pan, X.; et al. Global variation in the effects of ambient temperature on mortality: A systematic evaluation. Epidemiology 2014, 25, 781–789. [Google Scholar] [CrossRef]
- Tulimilli, S.V.; Dallavalasa, S.; Basavaraju, C.G.; Kumar Rao, V.; Chikkahonnaiah, P.; Madhunapantula, S.V.; Veeranna, R.P. Variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Vaccine Effectiveness. Vaccines 2022, 10, 1751. [Google Scholar] [CrossRef] [PubMed]
- Elements of Preparation and Implementation of the Vaccination Strategy. Available online: https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=24/03/2021&redaz=21A01802&artp=1&art=1&subart=1&subart1=10&vers=1&prog=001 (accessed on 25 March 2024).
- Homan, T.; Mazzilli, S.; Chieti, A.; Musa, A.; Roth, A.; Fortunato, F.; Bisceglia, L.; Prato, R.; Lopalco, P.L.; Martinelli, D. COVID-19 vaccination programme effectiveness against SARS-CoV-2 related infections, hospital admissions and deaths in the Apulia region of Italy: A one-year retrospective cohort study. Sci. Rep. 2022, 12, 18597. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Pellegrini, G.; Cereda, D.; Bortolan, F.; Leoni, O.; Pavesi, G.; Galli, M.; Valenti, G.; Corrao, G. Natural and vaccine-induced immunity are equivalent for the protection against SARS-CoV-2 infection. J. Infect. Public Health 2023, 16, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Mateo-Urdiales, A.; Sacco, C.; Rota, M.C.; Fotakis, E.A.; Petrone, D.; Del Manso, M.; Siddu, A.; Stefanelli, P.; Bella, A.; et al. Relative effectiveness of bivalent Original/Omicron BA.4-5 mRNA vaccine in preventing severe COVID-19 in persons 60 years and above during SARS-CoV-2 Omicron XBB.1.5 and other XBB sublineages circulation, Italy, April to June 2023. Eurosurveillance 2023, 28, 2300397. [Google Scholar] [CrossRef]
- Mateo-Urdiales, A.; Sacco, C.; Petrone, D.; Bella, A.; Riccardo, F.; Del Manso, M.; Bressi, M.; Siddu, A.; Brusaferro, S.; Palamara, A.T.; et al. Estimated Effectiveness of a Primary Cycle of Protein Recombinant Vaccine NVX-CoV2373 Against COVID-19. JAMA Netw. Open 2023, 6, e2336854. [Google Scholar] [CrossRef]
- Sacco, C.; Mateo-Urdiales, A.; Rota, M.C. Infezioni da SARS-CoV-2, Ricoveri e Decessi Associati a COVID-19 Direttamente Evitati dalla Vaccinazione. Italia 27/12/2020–31/01/2022. Nota Tecnica. Roma: Istituto Superiore di Sanità. 2022. Available online: https://www.iss.it/documents/20126/6703853/NT_Eventi+evitati+COVID19_LAST.pdf/a140e155-bd62-adcd-1b29-d1be3464ed48?t=1649832260103 (accessed on 28 March 2024).
- National Institute of Statistics. Mortality Data. Available online: https://www.istat.it/en/archive/deaths (accessed on 5 April 2023).
- Further Urgent Civil Protection Interventions in Relation to the Emergency Relating to the Health Risk Associated with the Onset of Pathologies Resulting from Transmissible Viral Agents. Available online: https://www.gazzettaufficiale.it/eli/id/2020/02/28/20A01348/SG (accessed on 28 March 2024).
- Sistema Nazionale per la Protezione dell’Ambiente. Available online: www.snpambiente.it/chi-siamo/i-nodi-del-sistema/i-siti-web (accessed on 5 April 2023).
- Korstanje, J. The SARIMAX Model. In Advanced Forecasting with Python; Apress: Berkeley, CA, USA, 2021. [Google Scholar]
- Porta, M. A Dictionary of Epidemiology, 6th ed.; Oxford University Press: Cambrige, MA, USA, 2014; ISBN 13:9780199976720. [Google Scholar]
- Strain, T.; Brage, S.; Sharp, S.; Richards, J.; Tainio, M.; Ding, D.; Benichou, J.; Kelly, P. Use of the prevented fraction for the population to determine deaths averted by existing prevalence of physical activity: A descriptive study. Lancet Glob. Health 2020, 8, e920–e930. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.; Huang, F.; Zhang, K. Effectiveness of mRNA and viral-vector vaccines in epidemic period led by different SARS-CoV-2 variants: A systematic review and meta-analysis. J. Med. Virol. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; Oliveira, V.d.A.; Boaventura, V.S.; Pescarini, J.M.; Júnior, J.B.; Machado, T.M.; Flores-Ortiz, R.; Penna, G.O.; Ichihara, M.Y.; de Barros, J.V.; et al. Influence of age on the effectiveness and duration of protection of Vaxzevria and CoronaVac vaccines: A population-based study. Lancet Reg. Health Am. 2022, 6, 100154. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Mazagatos, C.; Dijkstra, F.; Kislaya, I.; Gherasim, A.; McDonald, S.A.; Kissling, E.; Valenciano, M.; Meijer, A.; Hooiveld, M.; et al. Impact of influenza vaccination programmes among the elderly population on primary care, Portugal, Spain and the Netherlands: 2015/16 to 2017/18 influenza seasons. Eurosurveillance 2019, 24, 1900268. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, L.; Li, M.; Xie, B.; He, L.; Wang, M.; Zhang, R.; Hou, N.; Zhang, Y.; Jia, F. Real-Word Effectiveness of Global COVID-19 Vaccines Against SARS-CoV-2 Variants: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 820544. [Google Scholar] [CrossRef] [PubMed]
- Meslé, M.M.; Brown, J.; Mook, P.; Hagan, J.; Pastore, R.; Bundle, N.; Spiteri, G.; Ravasi, G.; Nicolay, N.; Andrews, N.; et al. Estimated number of deaths directly averted in people 60 years and older as a result of COVID-19 vaccination in the WHO European Region, December 2020 to November 2021. Eurosurveillance 2021, 26, 2101021. [Google Scholar] [CrossRef]
- Cameron-Blake, E.; Tatlow, H.; Andretti, B.; Boby, T.; Green, K.; Hale, T.; Petherick, A.; Phillips, T.; Pott, A.; Wade, A.; et al. A panel dataset of COVID-19 vaccination policies in 185 countries. Nat. Hum. Behav. 2023, 7, 1402–1413. [Google Scholar] [CrossRef]
- Markel, H.; Lipman, H.B.; Navarro, J.A.; Sloan, A.; Michalsen, J.R.; Stern, A.M.; Cetron, M.S. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007, 298, 644–654. [Google Scholar] [CrossRef]
- Ramalingam, S.; Graham, C.; Dove, J.; Morrice, L.; Sheikh, A. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci. Rep. 2019, 9, 1015. [Google Scholar] [CrossRef]
- Cegolon, L.; Mastrangelo, G.; Emanuelli, E.; Camerotto, R.; Spinato, G.; Frezza, D. Early Negativization of SARS-CoV-2 Infection by Nasal Spray of Seawater plus Additives: The RENAISSANCE Open-Label Controlled Clinical Trial. Pharmaceutics 2022, 14, 2502. [Google Scholar] [CrossRef]
- Lin, J.L.; Zhang, F.; Li, Y.B.; Yuan, S.H.; Wu, J.H.; Zhang, J.; Zhang, L.; He, Y.; Chen, J.; Yin, Y. Efficacy of physiological seawater nasal irrigation for the treatment of children with SARS-CoV-2 Omicron BA.2 variant infection: A randomized controlled trial. World J. Pediatr. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Pirrotta, L.; Guidotti, E.; Tramontani, C.; Bignardelli, E.; Venturi, G.; De Rosis, S. COVID-19 vaccinations: An overview of the Italian national health system’s online communication from a citizen perspective. Health Policy 2022, 126, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Alabdulrazzaq, H.; Alenezi, M.N.; Rawajfih, Y.; Alghannam, B.A.; Al-Hassan, A.A.; Al-Anzi, F.S. On the accuracy of ARIMA based prediction of COVID-19 spread. Results Phys. 2021, 27, 104509. [Google Scholar] [CrossRef]
- Box, G.E.; Jenkins, G.M.; Reinsel, G.C.; Ljung, G.M. Time Series Analysis: Forecasting and Control; Holden-Day: San Francisco, CA, USA, 1976. [Google Scholar]
- ArunKumar, K.E.; Kalaga, D.V.; Kumar CM, S.; Chilkoor, G.; Kawaji, M.; Brenza, T.M. Forecasting the dynamics of cumulative COVID-19 cases (confirmed. recovered and deaths) for top-16 countries using statistical machine learning models: Auto-Regressive Integrated Moving Average (ARIMA) and Seasonal Auto-Regressive Integrated Moving Average (SARIMA). Appl. Soft Comput. 2021, 103, 107161. [Google Scholar] [PubMed]
- Cong, J.; Ren, M.; Xie, S.; Wang, P. Predicting Seasonal Influenza Based on SARIMA Model. in Mainland China from 2005 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 4760. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Ahmed, S.; Uddin, M.J. Impact of weather on COVID-19 transmission in south Asian countries: An application of the ARIMAX model. Sci Total Environ. 2021, 761, 143315. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhu, H.; Yin, X.; Ke, T.; Gu, Y.; Li, S.; Li, Y.; Zheng, G. Exploration of influenza incidence prediction model based on meteorological factors in Lanzhou, China. 2014-2017. PLoS ONE 2022, 17, e0277045. [Google Scholar] [CrossRef] [PubMed]
- Alencar, C.H.; Cavalcanti, L.P.d.G.; Almeida, M.M.d.; Barbosa, P.P.L.; Cavalcante, K.K.d.S.; Melo, D.N.d.; de Brito Alves, B.C.F.; Heukelbach, J. High Effectiveness of SARS-CoV-2 Vaccines in Reducing COVID-19-Related Deaths in over 75-Year-Olds. Ceará State. Brazil. Trop Med Infect Dis. 2021, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Sergienko, R.; Friger, M.; Peretz, A.; Beckenstein, T.; Yaron, S.; Netzer, D.; Hammerman, A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022, 28, 1486–1490. [Google Scholar] [CrossRef]
- Anderegg, N.; Althaus, C.L.; Colin, S.; Hauser, A.; Laube, A.; Mäusezahl, M.; Wagner, M.; Zaffora, B.; Riou, J. Assessing real-world vaccine effectiveness against severe forms of SARS-CoV-2 infection: An observational study from routine surveillance data in Switzerland. Swiss Med Wkly. 2022, 152, w30163. [Google Scholar] [CrossRef]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Effectiveness of COVID-19 vaccines in older adults in Colombia: A retrospective population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022, 3, e242–e252, Erratum in: Lancet Healthy Longev. 2022, 3, e518. [Google Scholar] [CrossRef]
- Kislaya, I.; Machado, A.; Magalhães, S.; Rodrigues, A.P.; Franco, R.; Leite, P.P.; Dias, C.M.; Nunes, B. COVID-19 mRNA vaccine effectiveness (second and first booster dose) against hospitalisation and death during Omicron BA.5 circulation: Cohort study based on electronic health records, Portugal, May to July 2022. Euro Surveill. 2022, 27, 2200697. [Google Scholar] [CrossRef] [PubMed]
- Glatman-Freedman, A.; Bromberg, M.; Dichtiar, R.; Hershkovitz, Y.; Keinan-Boker, L. The BNT162b2 vaccine effectiveness against new COVID-19 cases and complications of breakthrough cases: A nation-wide retrospective longitudinal multiple cohort analysis using individualised data. EBioMedicine 2021, 72, 103574. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Woodbridge, Y.; Fluss, R.; Novikov, I.; Yaari, R.; Ziv, A.; Freedman, L.; Huppert, A. Similarity of Protection Conferred by Previous SARS-CoV-2 Infection and by BNT162b2 Vaccine: A 3-Month Nationwide Experience From Israel. Am. J. Epidemiol. 2022, 191, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Z.; Wittmann, I.; Polivka, L.; Surján, G.; Surján, O.; Barcza, Z.; Molnár, G.A.; Nagy, D.; Müller, V.; Bogos, K.; et al. Nationwide Effectiveness of First and Second SARS-CoV2 Booster Vaccines During the Delta and Omicron Pandemic Waves in Hungary (HUN-VE 2 Study). Front Immunol. 2022, 13, 905585. [Google Scholar] [CrossRef]
- Nunes, B.; Rodrigues, A.P.; Kislaya, I.; Cruz, C.; Peralta-Santos, A.; Lima, J.; Pinto Leite, P.; Sequeira, D.; Matias Dias, C.; Machado, A. mRNA vaccine effectiveness against COVID-19-related hospitalisations and deaths in older adults: A cohort study based on data linkage of national health registries in Portugal, February to August 2021. Eurosurveillance 2021, 26, 2100833. [Google Scholar] [CrossRef]
- Lytras, T.; Kontopidou, F.; Lambrou, A.; Tsiodras, S. Comparative effectiveness and durability of COVID-19 vaccination against death and severe disease in an ongoing nationwide mass vaccination campaign. J. Med. Virol. 2022, 94, 5044–5050. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection. hospitalisation. and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective. total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
- Saciuk, Y.; Kertes, J.; Mandel, M.; Hemo, B.; Shamir Stein, N.; Ekka Zohar, A. Pfizer-BioNTech vaccine effectiveness against Sars-Cov-2 infection: Findings from a large observational study in Israel. Prev. Med. 2022, 155, 106947. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Mok, A.H.Y.; Yan, V.K.C.; Chan, C.I.Y.; Wang, B.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Lau, C.S.; et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against SARS-CoV-2 omicron infection in people aged 60 years or above: A case-control study. J. Travel Med. 2022, 29, taac119. [Google Scholar] [CrossRef]
- Sheikh, A.; Robertson, C.; Taylor, B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. N. Engl. J. Med. 2021, 385, 2195–2197. [Google Scholar] [CrossRef] [PubMed]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Emborg, H.-D.; Valentiner-Branth, P.; Schelde, A.B.; Nielsen, K.F.; Gram, M.A.; Moustsen-Helms, I.R.; Chaine, M.; Seidelin, U.H.; Nielsen, J. Vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine against RT-PCR confirmed SARS-CoV-2 infections. hospitalisations and mortality in prioritised risk groups. medRxiv 2021. [Google Scholar] [CrossRef]
- Zeng, B.; Gao, L.; Zhou, Q.; Yu, K.; Sun, F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.B.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw Open 2022, 5, e2232760. [Google Scholar] [CrossRef] [PubMed]
- Gram, M.A.; Emborg, H.D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha. Delta. or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef]
- Stefanelli, P.; Trentini, F.; Petrone, D.; Mammone, A.; Ambrosio, L.; Manica, M.; Guzzetta, G.; d’Andrea, V.; Marziano, V.; Zardini, A.; et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy. December 2021 to January 2022. Eurosurveillance 2022, 27, 2200125. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, G.; Porcu, G.; Tratsevich, A.; Cereda, D.; Pavesi, G.; Bertolaso, G.; Franchi, M. Estimating All-Cause Deaths Averted in the First Two Years of the COVID-19 Vaccination Campaign in Italy. Vaccines 2024, 12, 413. https://doi.org/10.3390/vaccines12040413
Corrao G, Porcu G, Tratsevich A, Cereda D, Pavesi G, Bertolaso G, Franchi M. Estimating All-Cause Deaths Averted in the First Two Years of the COVID-19 Vaccination Campaign in Italy. Vaccines. 2024; 12(4):413. https://doi.org/10.3390/vaccines12040413
Chicago/Turabian StyleCorrao, Giovanni, Gloria Porcu, Alina Tratsevich, Danilo Cereda, Giovanni Pavesi, Guido Bertolaso, and Matteo Franchi. 2024. "Estimating All-Cause Deaths Averted in the First Two Years of the COVID-19 Vaccination Campaign in Italy" Vaccines 12, no. 4: 413. https://doi.org/10.3390/vaccines12040413
APA StyleCorrao, G., Porcu, G., Tratsevich, A., Cereda, D., Pavesi, G., Bertolaso, G., & Franchi, M. (2024). Estimating All-Cause Deaths Averted in the First Two Years of the COVID-19 Vaccination Campaign in Italy. Vaccines, 12(4), 413. https://doi.org/10.3390/vaccines12040413






