Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review
Abstract
:1. Introduction
1.1. HPV-Associated Cervical Malignancies
1.2. Therapeutic Vaccines for HPV-Associated Cervical Malignancies
1.3. Importance and Objectives of the Review
2. Methods
2.1. Eligibility Criteria
2.2. Search Protocol
2.3. Study Selection
2.4. Data Collection
2.5. Risk-of-Bias Assessment
2.6. Data Synthesis
2.7. Certainty of Evidence Assessment
3. Results
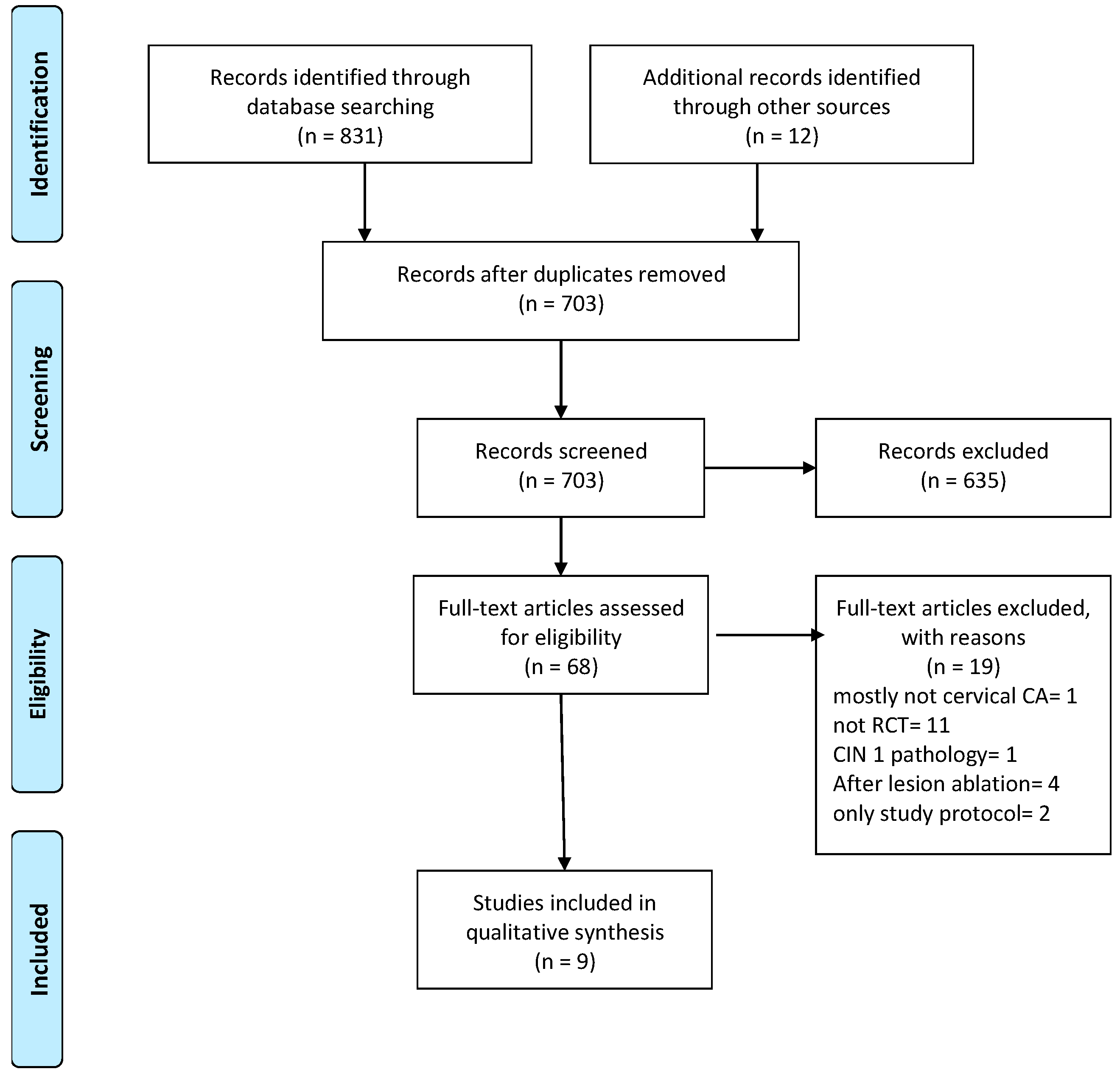
3.1. Search Results and Study Selection
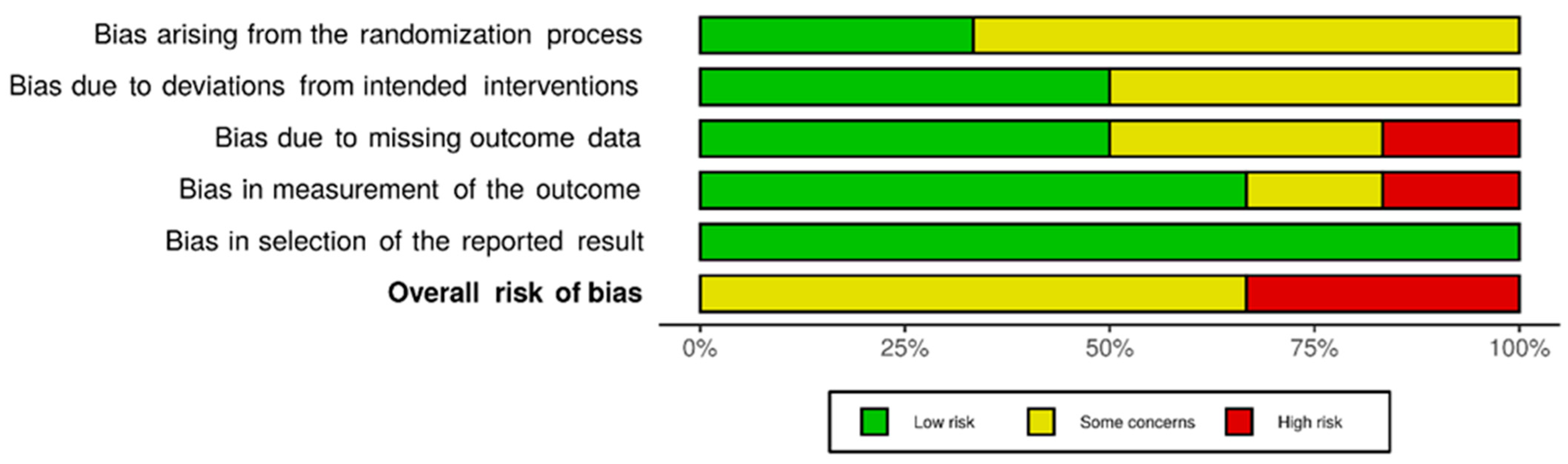
3.2. Risk-of-Bias Assessment
3.3. Description of Included Studies
3.4. Effects of Interventions
3.5. Lesion Regression
3.6. Other Vaccine Efficacy Endpoints
4. Discussion
4.1. Summary of Main Results
4.2. Lesion Regression
4.3. Other Vaccine Efficacy Endpoints
4.4. Comparison of Studies Grouped According to Efficacy Endpoints
4.5. Adverse Events
4.6. Overall Completeness and Applicability of Evidence
4.7. Certainty of the Evidence
4.8. Risk of Bias in and across Studies
4.9. Quality of Evidence
4.10. Limitations
4.10.1. Potential Biases in the Review Process
4.10.2. Agreements and Disagreements with Other Studies or Reviews
5. Conclusions
5.1. Implications for Practice
5.2. Implications for Research
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. S5), F12–F23. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.A.O.; Aaltonen, R.; Cárdenas, J.; Hernándes; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ (Clin. Res. Ed.) 2018, 360, k499. [Google Scholar] [CrossRef]
- Seton-Rogers, S. A vaccine to prevent HPV-related cancers. Nat. Milest. Vaccines 2020. Available online: https://www.nature.com/articles/d42859-020-00019-2.pdf (accessed on 12 February 2024).
- Oyervides-Muñoz, M.A.; Pérez-Maya, A.A.; Rodríguez-Gutiérrez, H.F.; Gómez-Macias, G.S.; Fajardo-Ramírez, O.R.; Treviño, V.; Barrera-Saldaña, H.A.; Garza-Rodríguez, M.L. Understanding the HPV integration and its progression to cervical cancer. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 61, 134–144. [Google Scholar] [CrossRef]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [PubMed]
- Groves, I.J.; Coleman, N. Pathogenesis of human papillomavirus-associated mucosal disease. J. Pathol. 2015, 235, 527–538. [Google Scholar] [CrossRef]
- Schiller, J.; Lowy, D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine 2018, 36, 4768–4773. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yeo-Teh, N.S.L.; Ito, Y.; Jha, S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018, 19, 1706. [Google Scholar] [CrossRef]
- Frazer, I.H.; Quinn, M.; Nicklin, J.L.; Tan, J.; Perrin, L.C.; Ng, P.; Oconnor, V.; White, O.; Wendt, N.; Martin, J.; et al. Phase 1 study of HPV16-specific immunotherapy with E6E7 fusion protein and ISCOMATRIX adjuvant in women with cervical intraepithelial neoplasia. Vaccine 2004, 23, 172–181. [Google Scholar] [CrossRef]
- Kaufmann, A.M.; Nieland, J.D.; Jochmus, I.; Baur, S.; Friese, K.; Gabelsberger, J.; Gieseking, F.; Gissmann, L.; Glasschröder, B.; Grubert, T.; et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int. J. Cancer 2007, 121, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Clin. Res. Ed) 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ (Clin. Res. Ed.) 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
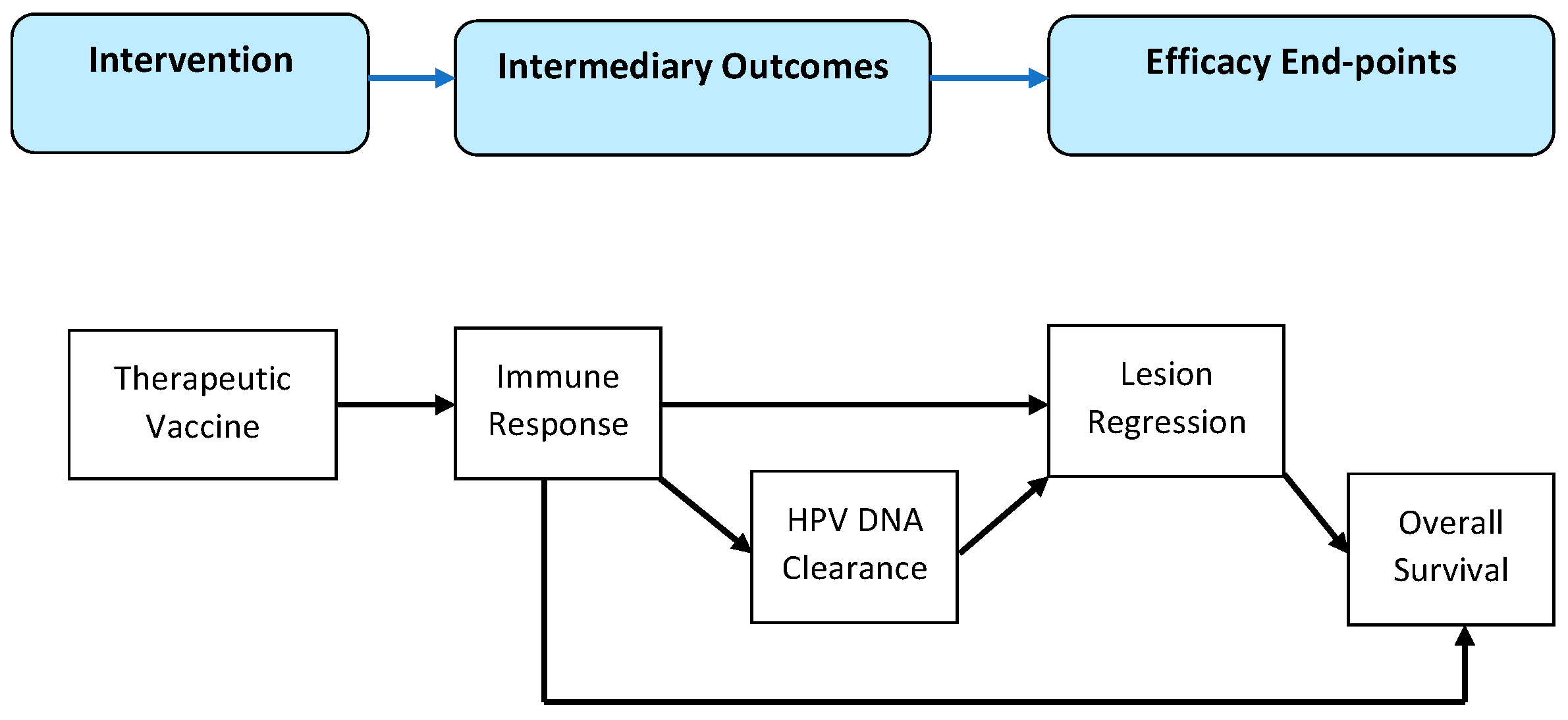
- Anderson, L.M.; Petticrew, M.; Rehfuess, E.; Armstrong, R.; Ueffing, E.; Baker, P.; Francis, D.; Tugwell, P. Using logic models to capture complexity in systematic reviews. Res. Synth. Methods 2011, 2, 33–42. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin. Res. Ed.) 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Kobayashi, O.; Ikeda, Y.; Yahata, H.; Iwata, T.; Satoh, T.; Akiyama, A.; Maeda, D.; Hori-Hirose, Y.; Uemura, Y.; et al. Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3. JNCI Cancer Spectr. 2023, 7, pkad101. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hur, S.Y.; Kim, T.J.; Hong, S.R.; Lee, J.K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.-M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes-Listeriolysin O Immunotherapy with or without Cisplatin in Treatment of Advanced Cervical Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, P.; Ganeshrajah, S.; Raghanvan, R.K.; Singh, S.S.; Thangarajan, R. Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer—A feasibility study. Asian Pac. J. Cancer Prev. 2014, 15, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- De Vos van Steenwijk, P.J.; Ramwadhdoebe, T.H.; Löwik, M.J.; van der Minne, C.E.; Berends-van der Meer, D.M.; Fathers, L.M.; Valentijn, A.R.; Oostendorp, J.; Fleuren, G.J.; Hellebrekers, B.W.; et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol. Immunother. 2012, 61, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Luís, Â.; Soares, C.P.; Venuti, A.; Paolini, F.; Pereira, L.; Sousa, Â. The Effectiveness of Therapeutic Vaccines for the Treatment of Cervical Intraepithelial Neoplasia 3: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Smalley Rumfield, C.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic Vaccines for HPV-Associated Malignancies. ImmunoTargets Ther. 2020, 9, 167–200. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, E.; Modic, Ž.; Cid-Arregui, A. Recent developments in immunotherapy of cancers caused by human papillomaviruses. Immunology 2020, 163, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Van Der Jeught, K.; De Koker, S.; Bialkowski, L.; Heirman, C.; Tjok Joe, P.; Perche, F.; Maenhout, S.; Bevers, S.; Broos, K.; Deswarte, K.; et al. Dendritic Cell Targeting mRNA Lipopolyplexes Combine Strong Antitumor T—Cell Immunity with Improved Inflammatory Safety. ACS Nano 2018, 12, 9815–9829. [Google Scholar] [CrossRef] [PubMed]
- Grunwitz, C.; Salomon, N.; Vascotto, F.; Selmi, A.; Bukur, T.; Diken, M.; Kreiter, S.; Türeci, Ö.; Sahin, U.; Grunwitz, C.; et al. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology 2019, 8, e1629259. [Google Scholar] [CrossRef]
- Bevers, S.; Kooijmans, S.A.A.; Van de Velde, E.; Evers, M.J.W.; Seghers, S.; Gitz-Francois, J.J.J.M.; van Kronenburg, N.C.H.; Fens, M.H.A.M.; Mastrobattista, E.; Hassler, L.; et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef]
| Authors | Study Design | Participants | Interventions | Outcomes | Overall Bias |
|---|---|---|---|---|---|
| Kawana et al., 2023 [19] | Phase I and II, double-blind RCT | 165 patients with HPV16-positive CIN2-3 | IGMKK16E7 (lacticaseibacillus paracasei expressing cell surface, full-length HPV16 E7 was orally administrated to 4 groups: Placebo or low, intermediate, or high doses | Lesion regression, immune response, adverse events | Some concerns |
| Choi et al., 2020 [20] | Phase II, open-label RCT | 72 patients with CIN2-3 | GX-188E vaccine 4 mg, DNA vaccine (encoding HPV16 and HPV18 E6 and E7) | Lesion regression, HPV clearance, immune response, adverse events | Some concerns |
| Harper et al., 2019 [21] | Phase II, double-blind RCT | 192 patients with CIN2 or CIN3 | modified vaccinia Ankara (MVA) viral vector encoding IL-2 and HPV16 E6 and E7 | Lesion regression, HPV clearance, adverse events | Some concerns |
| Basu et al., 2018 [22] | Phase II, RCT | 109 patients with recurrent/refractory cervical cancer | ADXS11-001 monotherapy, Listeria monocytogenes containing the fusion protein Lm-LLO-E7 | Overall survival, immune response, adverse events | Some concerns |
| Trimble et al., 2015 [23] | Phase II, double-blind RCT | 169 patients with CIN2 or CIN3 | VGX-3100 vaccine, DNA-based (mix of 2 plasmids encoding E6 and E7 genes from HPV16 and HPV18) | Lesion regression, HPV clearance, immune response, adverse events | Some concerns |
| Ramanathan et al., 2014 [24] | Phase I, RCT | 14 patients with cervical cancer | cell-based (autologous tumor-lysate-primed mature dendritic cells) | Clinical response, adverse events | Not conducted (no statistical analysis) |
| De Vos van Steenwijk et al., 2012 [25] | Phase II, blinded RCT | 9 patients with HSIL a | HPV16 E6/E7 peptide | Lesion regression, immune response, adverse events | Not conducted (no statistical analysis) |
| Kaufmann et al., 2007 [12] | Double-blind RCT | 39 patients with CIN2 or CIN3 | CVLP vaccine 250 and 75 microg, virus-like particles (CVLP)- HPV16 L1E7 chimeric virus-like particles | Lesion regression, HPV DNA clearance, immune response, adverse events | High |
| Frazer et al., 2004 [11] | Phase I, double-blind RCT | 31 subjects with CIN1, CIN2, or CIN3 | HPV 16 E6E7 vaccine, 3 groups: 20 µg X3 60 µg X3 200 µg X1 | Lesion regression, HPV DNA clearance, immune response, adverse events | High |
| Outcome | Result | Number of Participants (Studies) | Certainty of the Evidence (GRADE) | Comments |
|---|---|---|---|---|
| Lesion regression to ≤CIN1 | Combined p-value = 0.007 | 594 (4) | ⊕⊕⊕⊝ Moderate | Kawana et al. [19], 2023, IC: 0.5–37.8 Choi et al. [20] 2020 (p = 0.135) Frazer et al. [11] 2004 (p = 0.12) Harper et al. [21] 2019 (p = 0.049) Trimble et al. [23] 2015 (p = 0.034) |
| HPV DNA clearance | Variably present | 512 (6) | ⊕⊕⊕⊝ Moderate | |
| Immune response | Variably present | 687 (8) | ⊕⊕⊕⊝ Moderate | |
| Clinical response | Not significant | 14 (1) | ⊕⊝⊝⊝ Very low | |
| Overall survival | Not significant | 109 (1) | ⊕⊝⊝⊝ Very Low | |
| Adverse events | Well tolerated | 796 (9) | ⊕⊕⊕⊝ Moderate | |
| GRADE: Working group grades of evidence | ||||
| High quality: Further research is very unlikely to change our confidence in the estimation of the effect | ||||
| Moderate quality: Further research is very likely to have an important impact on our confidence in the estimation of the effect and may change the estimated effect | ||||
| Very low quality: Very little confidence in the effect estimated | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alouini, S.; Pichon, C. Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review. Vaccines 2024, 12, 428. https://doi.org/10.3390/vaccines12040428
Alouini S, Pichon C. Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review. Vaccines. 2024; 12(4):428. https://doi.org/10.3390/vaccines12040428
Chicago/Turabian StyleAlouini, Souhail, and Chantal Pichon. 2024. "Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review" Vaccines 12, no. 4: 428. https://doi.org/10.3390/vaccines12040428
APA StyleAlouini, S., & Pichon, C. (2024). Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review. Vaccines, 12(4), 428. https://doi.org/10.3390/vaccines12040428







