The Papain-like Protease Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Conjugated with Human Beta-Defensin 2 and Co1 Induces Mucosal and Systemic Immune Responses against the Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Animals
2.2. Cloning and Expression of Proteins
2.3. Immunization and Challenge of Mice
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Complement-Dependent Cytotoxicity Assay
2.6. Flow Cytometry
2.7. Quantitative RT-PCR Analysis
2.8. Statistical Analysis
3. Results
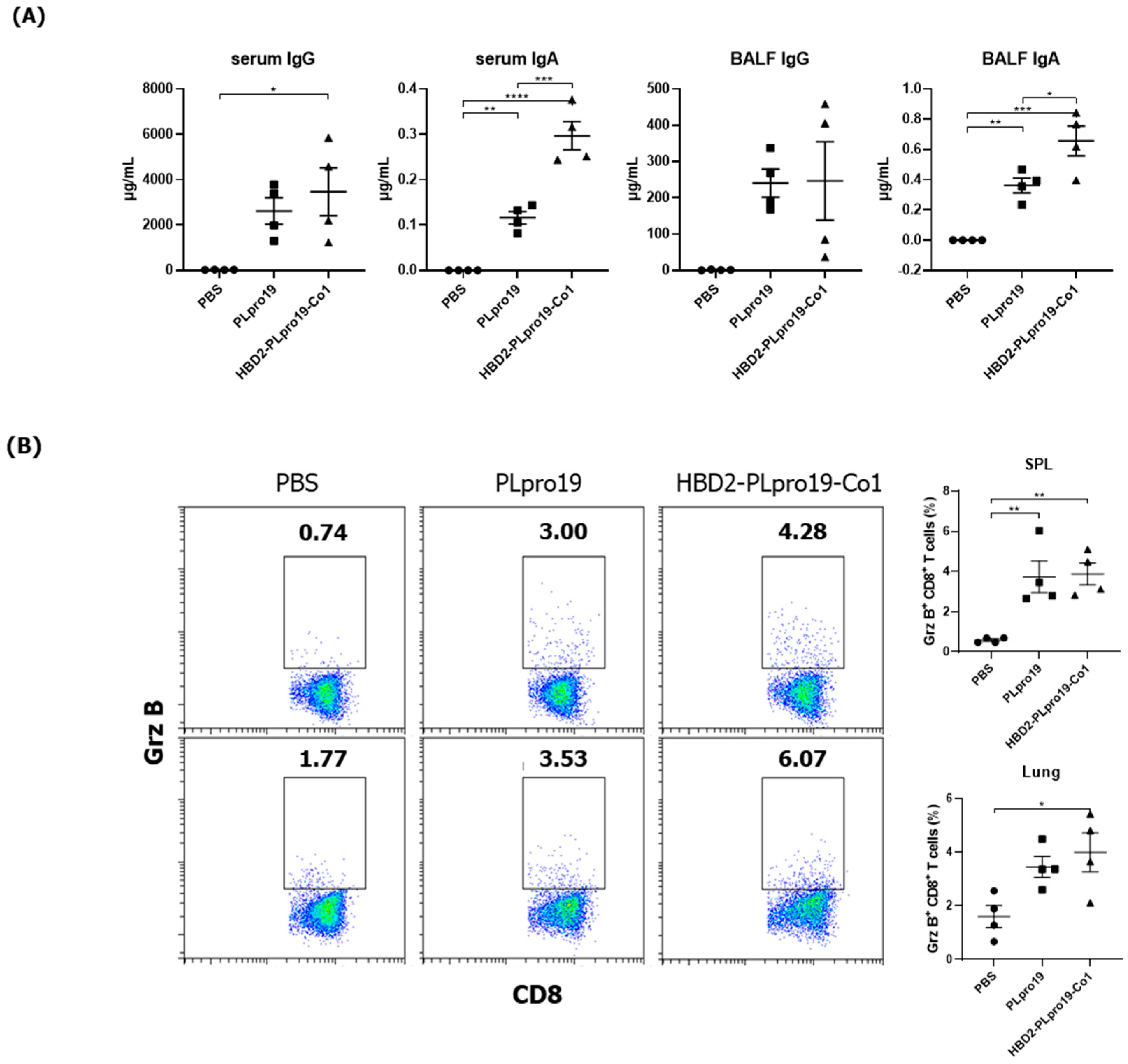
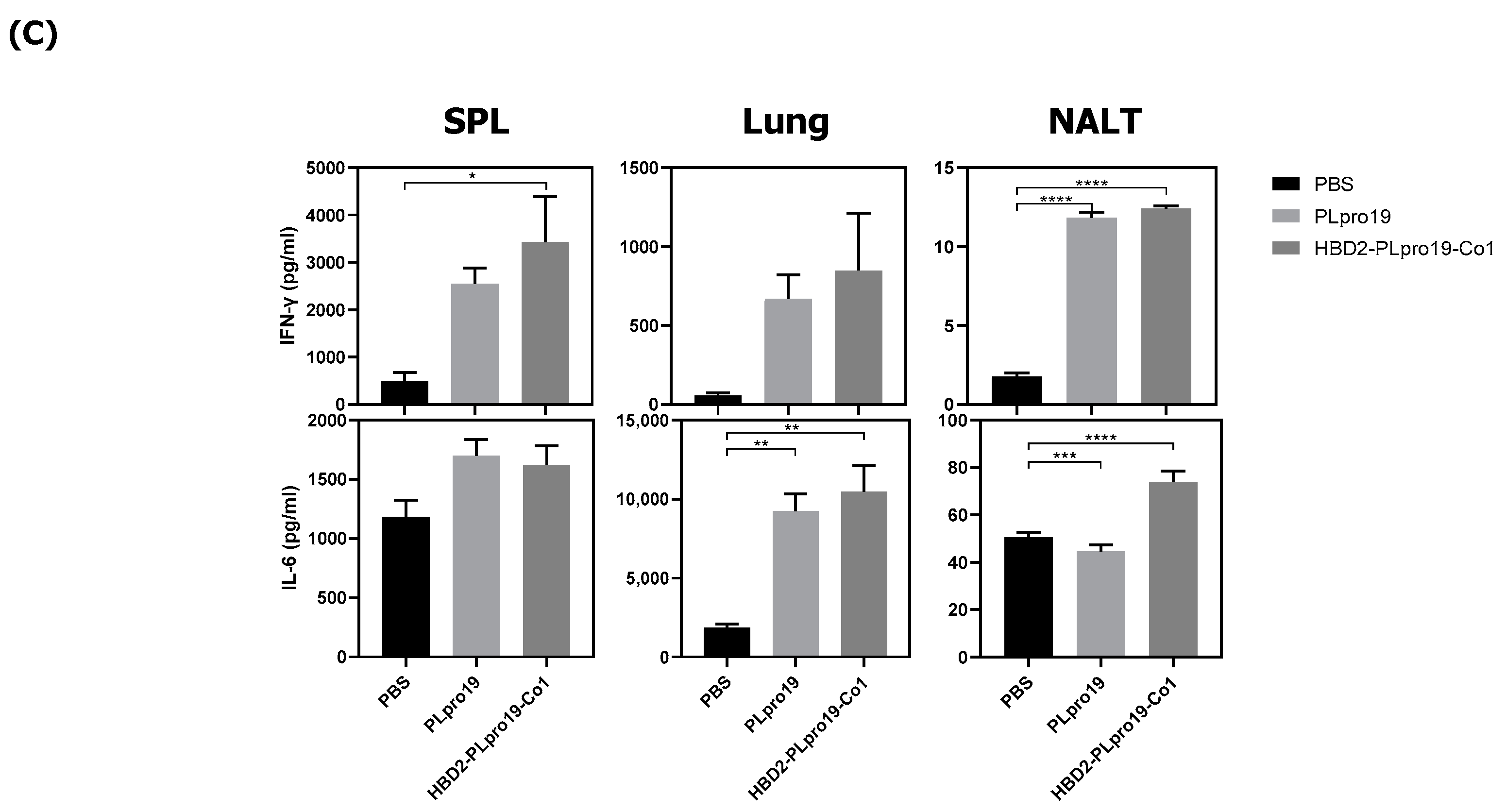
3.1. Intranasal Administration of Recombinant PLpro Conjugate Elicits Mucosal and Systemic Immune Responses in C57BL/6 Mice
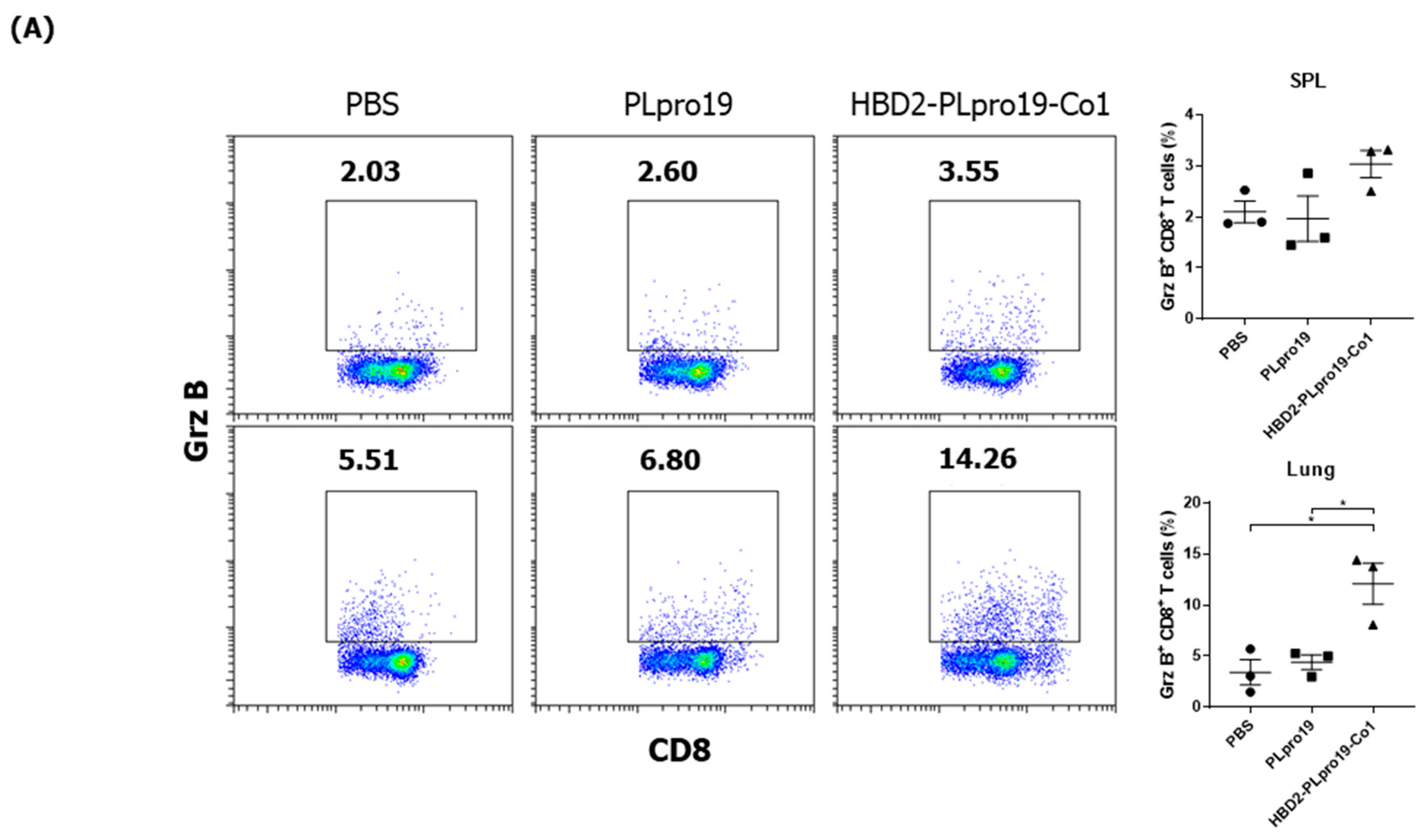
3.2. Recombinant PLpro Conjugate Stimulates Ag-Specific CTL Activation and the Production of Cytokines Involved in T-Cell Immune Responses
3.3. The Persistence of an Ag-Specific Adaptive Immune Response Induced by PLpro Conjugated with HBD2 and Co1 Allows for the Retention of Immune Memory
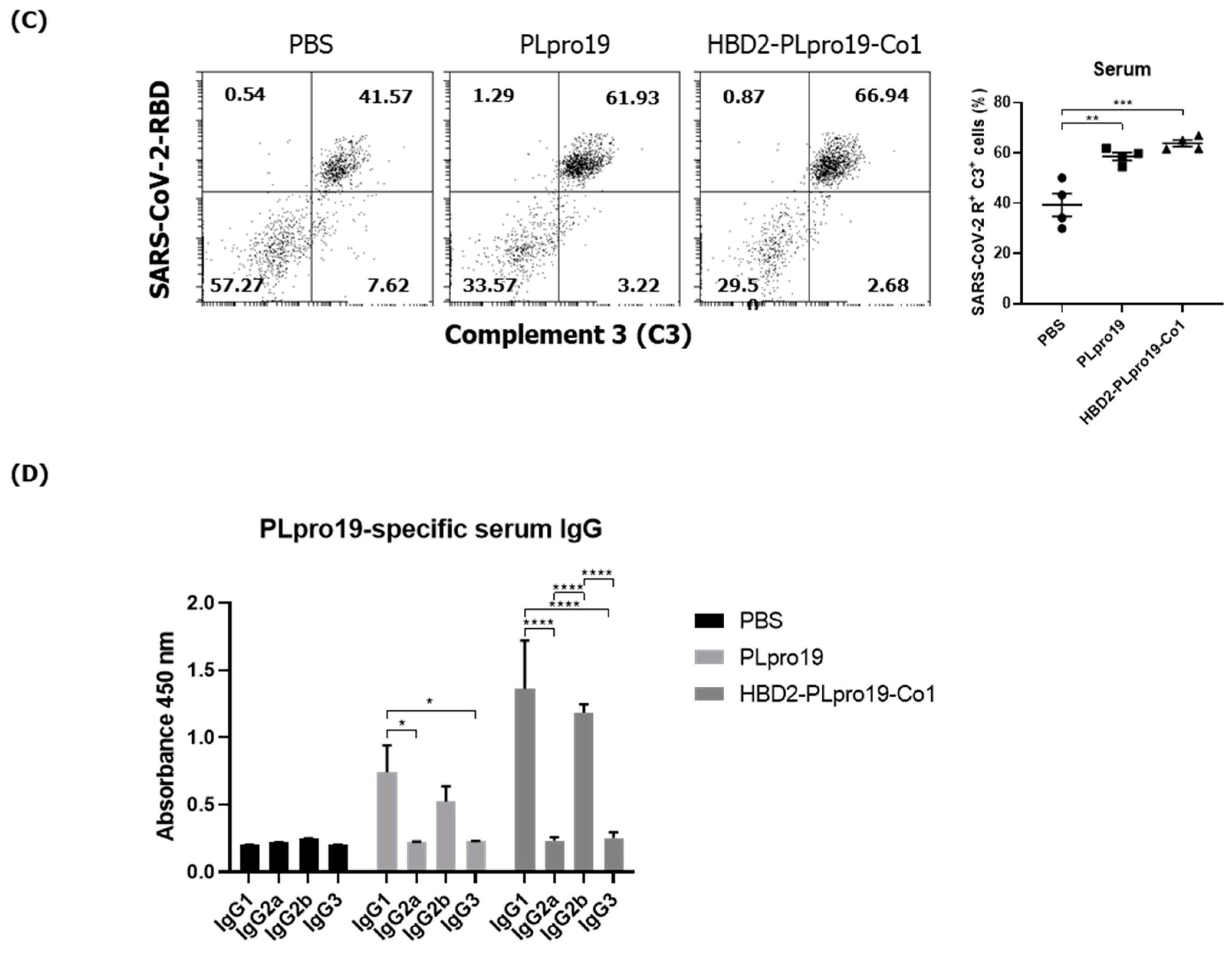
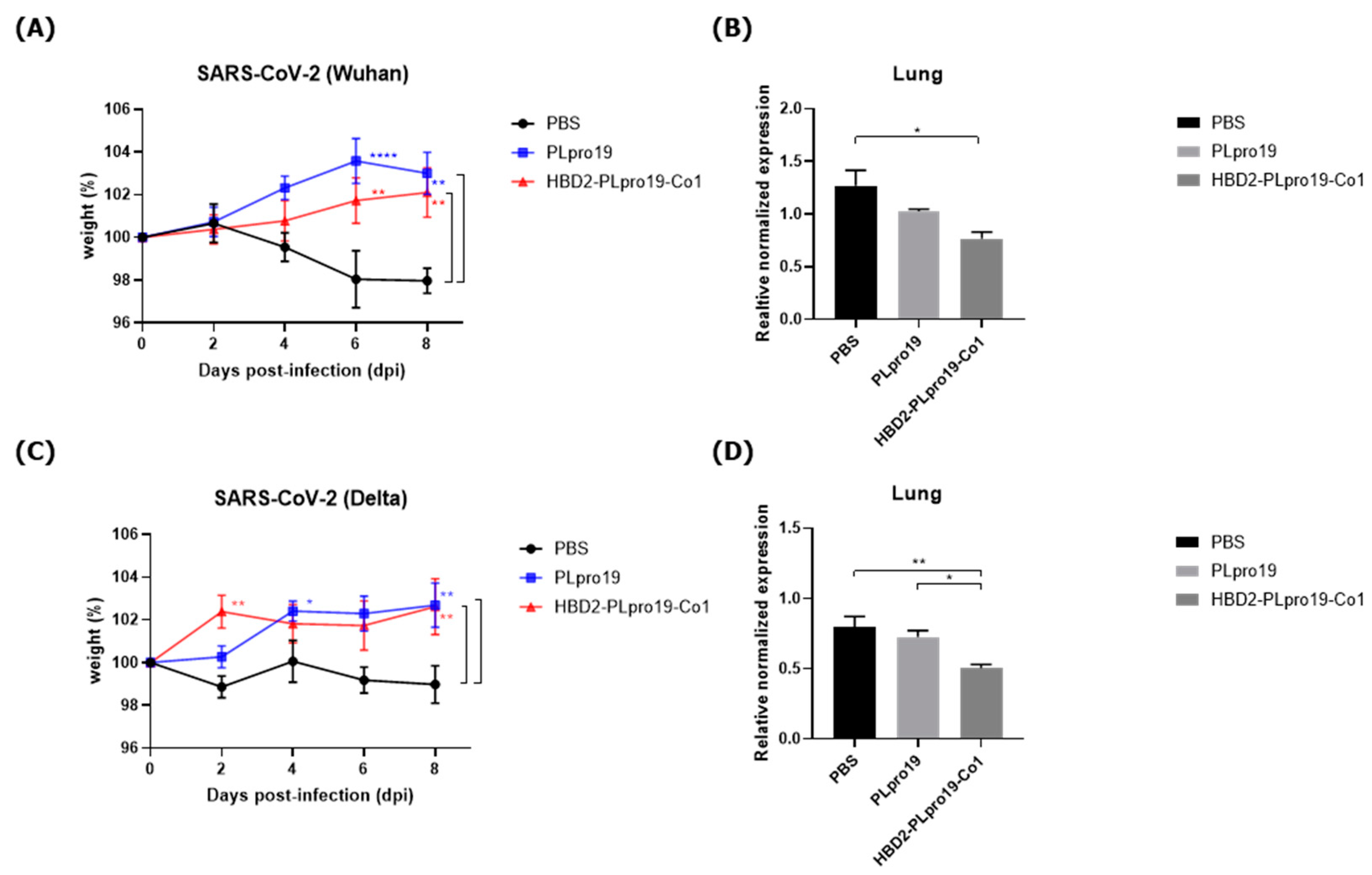
3.4. Intranasal Administration of Recombinant PLpro Conjugate Induces Protective Immune Responses against SARS-CoV-2 in hACE2 KI Mice
3.5. Long-Term Persistence of Ag-Specific Adaptive Immune Response Is Enhanced by PLpro Conjugated with HBD2 and Co1 in hACE2 KI Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 vaccines, vaccine development technologies, and significant efforts in vaccine development during the pandemic: The lessons learned might help to fight against the next pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Li, L.; Yi, L.; Jiang, Y.; Hao, P.; Xu, Z.; Zou, W.; Li, P.; Gao, Z. Immunogenicity and protective potential of chimeric virus-like particles containing SARS-CoV-2 spike and H5N1 matrix 1 proteins. Front. Cell. Infect. Microbiol. 2022, 12, 967493. [Google Scholar] [CrossRef]
- Li, L.; Honda-Okubo, Y.; Huang, Y.; Jang, H.; Carlock, M.A.; Baldwin, J.; Piplani, S.; Bebin-Blackwell, A.G.; Forgacs, D.; Sakamoto, K. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine 2021, 39, 5940–5953. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Zou, J.; Xie, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Kanevsky, I.; Tompkins, K.; Cutler, M.; Cooper, D.; Dormitzer, P.R.; Shi, P.-Y. The effect of SARS-CoV-2 D614G mutation on BNT162b2 vaccine-elicited neutralization. npj Vaccines 2021, 6, 44. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e2379. [Google Scholar] [CrossRef]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; De Oliveira, T.; Vermeulen, M.; Van der Berg, K. SARS-CoV-2 501Y. V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; De Groot, R.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.; Lauber, C.; Leontovich, A.; Neuman, B. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Mielech, A.M.; Kilianski, A.; Baez-Santos, Y.M.; Mesecar, A.D.; Baker, S.C. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014, 450–451, 64–70. [Google Scholar] [CrossRef]
- Isaacson, M.K.; Ploegh, H.L. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe 2009, 5, 559–570. [Google Scholar] [CrossRef]
- Fielding, C.A.; Sabberwal, P.; Williamson, J.C.; Greenwood, E.J.; Crozier, T.W.; Zelek, W.; Seow, J.; Graham, C.; Huettner, I.; Edgeworth, J.D. SARS-CoV-2 host-shutoff impacts innate NK cell functions, but antibody-dependent NK activity is strongly activated through non-spike antibodies. eLife 2022, 11, e74489. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Kim, S.-M.; Jung, M.K.; Noh, J.Y.; Yoo, J.-S.; Kim, E.-H.; Kim, Y.-I.; Yu, K.; Jang, S.-G.; Gil, J. Enhanced antibody responses in fully vaccinated individuals against pan-SARS-CoV-2 variants following Omicron breakthrough infection. Cell Rep. Med. 2022, 3, 100764. [Google Scholar] [CrossRef]
- Diallo, B.K.; Ní Chasaide, C.; Wong, T.Y.; Schmitt, P.; Lee, K.S.; Weaver, K.; Miller, O.; Cooper, M.; Jazayeri, S.D.; Damron, F.H. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines 2023, 8, 68. [Google Scholar] [CrossRef]
- Deng, S.; Liu, Y.; Tam, R.C.-Y.; Chen, P.; Zhang, A.J.; Mok, B.W.-Y.; Long, T.; Kukic, A.; Zhou, R.; Xu, H. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters. Nat. Commun. 2023, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Kozhaya, L.; Placek, L.; Gunter, C.; Yigit, M.; Hardy, R.; Plassmeyer, M.; Coatney, P.; Lillard, K.; Bukhari, Z. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun. Biol. 2021, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Dufloo, J.; Grzelak, L.; Staropoli, I.; Madec, Y.; Tondeur, L.; Anna, F.; Pelleau, S.; Wiedemann, A.; Planchais, C.; Buchrieser, J. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep. Med. 2021, 2, 100275. [Google Scholar] [CrossRef]
- Lee, K.; Wong, T.; Russ, B.; Horspool, A.; Miller, O.; Rader, N.; Givi, J.; Winters, M.; Wong, Z.Y.; Cyphert, H. SARS-CoV-2 Delta variant induces enhanced pathology and inflammatory responses in K18-hACE2 mice. PLoS ONE 2022, 17, e0273430. [Google Scholar] [CrossRef]
- Elmore, S.A. Enhanced histopathology of mucosa-associated lymphoid tissue. Toxicol. Pathol. 2006, 34, 687–696. [Google Scholar] [CrossRef]
- Mutoh, M.; Kimura, S.; Takahashi-Iwanaga, H.; Hisamoto, M.; Iwanaga, T.; Iida, J. RANKL regulates differentiation of microfold cells in mouse nasopharynx-associated lymphoid tissue (NALT). Cell Tissue Res. 2016, 364, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.R.; Franco, L.H.; Zacharia, V.M.; Khan, H.S.; Stamm, C.E.; You, W.; Marciano, D.K.; Yagita, H.; Levine, B.; Shiloh, M.U. Microfold cells actively translocate Mycobacterium tuberculosis to initiate infection. Cell Rep. 2016, 16, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Kraehenbuhl, J.-P.; Schödel, F.; Potts, A.; Peterson, D.; De Grandi, P.; Nardelli-Haefliger, D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 1995, 63, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Seo, K.-W.; Kim, J.; Lee, K.-Y.; Jang, Y.-S. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J. Immunol. 2010, 185, 5787–5795. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jung, D.-I.; Yang, I.-Y.; Jang, S.-H.; Kim, J.; Truong, T.T.; Pham, T.V.; Truong, N.U.; Lee, K.-Y.; Jang, Y.-S. Application of an M-cell-targeting ligand for oral vaccination induces efficient systemic and mucosal immune responses against a viral antigen. Int. Immunol. 2013, 25, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kim, Y.N.; Kim, J.; Jang, Y.-S. C5a receptor targeting of partial non-structural protein 3 of dengue virus promotes antigen-specific IFN-γ-producing T-cell responses in a mucosal dengue vaccine model. Cell. Immunol. 2018, 325, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Cho, B.-H.; Kim, K.S.; Jang, Y.-S. Complement C5a promotes antigen cross-presentation by Peyer’s patch monocyte-derived dendritic cells and drives a protective CD8+ T cell response. Cell Rep. 2021, 35, 108995. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Shim, E.-H.; Kim, D.-J.; Jang, Y.-S. C5aR+ dendritic cells fine-tune the Peyer’s patch microenvironment to induce antigen-specific CD8+ T cells. npj Vaccines 2023, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Boniotto, M.; Jordan, W.J.; Eskdale, J.; Tossi, A.; Antcheva, N.; Crovella, S.; Connell, N.D.; Gallagher, G. Human β-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 2006, 50, 1433–1441. [Google Scholar] [CrossRef]
- Pazgier, M.; Hoover, D.; Yang, D.; Lu, W.; Lubkowski, J. Human β-defensins. Cell. Mol. Life Sci. 2006, 63, 1294–1313. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Wu, Z.; Freeman, T.; Bafna, V.; Zasloff, M.; Wilson, J.M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 1998, 102, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Järvå, M.; Phan, T.K.; Lay, F.T.; Caria, S.; Kvansakul, M.; Hulett, M.D. Human β-defensin 2 kills Candida albicans through phosphatidylinositol 4, 5-bisphosphate–mediated membrane permeabilization. Sci. Adv. 2018, 4, eaat0979. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, J.P.; Sun, L.; Lu, W.; Gartner, S.; Garzino-Demo, A. Human beta-defensin 2 and 3 inhibit HIV-1 replication in macrophages. Front. Cell. Infect. Microbiol. 2021, 11, 535352. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chertov, O.; Bykovskaia, S.; Chen, Q.; Buffo, M.; Shogan, J.; Anderson, M.; Schroder, J.; Wang, J.; Howard, O. β-defensins: Linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999, 286, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Belyakov, I.M.; Chow, Y.-H.; Dimitrov, D.S.; Berzofsky, J.A.; Kwak, L.W. DNA vaccines encoding human immunodeficiency virus–1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 2002, 100, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Biragyn, A.; Ruffini, P.A.; Leifer, C.A.; Klyushnenkova, E.; Shakhov, A.; Chertov, O.; Shirakawa, A.K.; Farber, J.M.; Segal, D.M.; Oppenheim, J.J. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science 2002, 298, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, Y.L.; Jang, S.-H.; Jang, Y.-S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, Y.L.; Jang, Y.-S. Human β-defensin 2 is involved in CCR2-mediated Nod2 signal transduction, leading to activation of the innate immune response in macrophages. Immunobiology 2019, 224, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, Y.L.; Jeong, Y.; Jang, Y.-S. Conjugation of human β-defensin 2 to spike protein receptor-binding domain induces antigen-specific protective immunity against middle east respiratory syndrome coronavirus infection in human dipeptidyl peptidase 4 transgenic mice. Vaccines 2020, 8, 635. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Bangs, D.J.; Sidney, J.; Paul, S.; Peters, B.; de Silva, A.D.; Lindow, J.C.; Diehl, S.A.; Whitehead, S. The human CD8+ T cell responses induced by a live attenuated tetravalent dengue vaccine are directed against highly conserved epitopes. J. Virol. 2015, 89, 120–128. [Google Scholar] [CrossRef]
- Gravina, A.; Tediashvili, G.; Rajalingam, R.; Quandt, Z.; Deisenroth, C.; Schrepfer, S.; Deuse, T. Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression. Nat. Biotechnol. 2023, 41, 717–727. [Google Scholar] [CrossRef]
- Vukovic, N.; Segués, A.; Huang, S.; Waterfall, M.; Sijts, A.J.; Zaiss, D.M. Mouse IgG2a Isotype Therapeutic Antibodies Elicit Superior Tumor Growth Control Compared with mIgG1 or mIgE. Cancer Res. Commun. 2023, 3, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Wang, M.; Wei, G.-W. Mutations strengthened SARS-CoV-2 infectivity. J. Mol. Biol. 2020, 432, 5212–5226. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.; Ding, H.; Triggle, C. A review of the progress and challenges of developing a vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef]
- Corder, B.N.; Bullard, B.L.; Poland, G.A.; Weaver, E.A. A decade in review: A systematic review of universal influenza vaccines in clinical trials during the 2010 decade. Viruses 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Lu, Y.; Zhao, Y.-B.; Shen, F.; Fan, C.-F.; Wang, Q.; He, W.-Q.; He, X.-Y.; Li, Z.-K.; Chen, T.-T. A variant-proof SARS-CoV-2 vaccine targeting HR1 domain in S2 subunit of spike protein. Cell Res. 2022, 32, 1068–1085. [Google Scholar] [CrossRef]
- Wang, C.Y.; Peng, W.-J.; Kuo, B.-S.; Ho, Y.-H.; Wang, M.-S.; Yang, Y.-T.; Chang, P.-Y.; Shen, Y.-H.; Hwang, K.-P. Toward a pan-SARS-CoV-2 vaccine targeting conserved epitopes on spike and non-spike proteins for potent, broad and durable immune responses. PLoS Path. 2023, 19, e1010870. [Google Scholar] [CrossRef]
- Brokstad, K.A.; Cox, R.J.; Olofsson, J.; Jonsson, R.; Haaheim, L.R. Parenteral influenza vaccination induces a rapid systemic and local immune response. J. Infect. Dis. 1995, 171, 198–203. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Tioni, M.F.; Jordan, R.; Pena, A.S.; Garg, A.; Wu, D.; Phan, S.I.; Weiss, C.M.; Cheng, X.; Greenhouse, J.; Orekov, T. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. npj Vaccines 2022, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.-L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2. [Google Scholar] [CrossRef] [PubMed]
- Von Holle, T.A.; Moody, M.A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 2019, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Menezes, J. Antibody-dependent cellular cytotoxicity in HIV infections. FASEB J. 1996, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Görander, S.; Ekblad, M.; Bergström, T.; Liljeqvist, J.-Å. Anti-glycoprotein g antibodies of herpes simplex virus 2 contribute to complete protection after vaccination in mice and induce antibody-dependent cellular cytotoxicity and complement-mediated cytolysis. Viruses 2014, 6, 4358–4372. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Tomioka, Y.; Takakuwa, H.; Uechi, G.-I.; Yabuta, T.; Ozaki, K.; Suyama, H.; Yamamoto, S.; Morimatsu, M.; Mai, L.Q. Cross-protective potential of anti-nucleoprotein human monoclonal antibodies against lethal influenza A virus infection. J. Gen. Virol. 2016, 97, 2104–2116. [Google Scholar] [CrossRef] [PubMed]
- Jegaskanda, S.; Co, M.D.T.; Cruz, J.; Subbarao, K.; Ennis, F.A.; Terajima, M. Induction of H7N9-cross-reactive antibody-dependent cellular cytotoxicity antibodies by human seasonal Influenza A viruses that are directed toward the nucleoprotein. J. Infect. Dis. 2017, 215, 818–823. [Google Scholar] [PubMed]
- Díez, J.M.; Romero, C.; Cruz, M.; Vandeberg, P.; Merritt, W.K.; Pradenas, E.; Trinité, B.; Blanco, J.; Clotet, B.; Willis, T. Anti-severe acute respiratory syndrome coronavirus 2 hyperimmune immunoglobulin demonstrates potent neutralization and antibody-dependent cellular cytotoxicity and phagocytosis through N and S proteins. J. Infect. Dis. 2022, 225, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef]
- Hansen, I.S.; Baeten, D.L.; den Dunnen, J. The inflammatory function of human IgA. Cell. Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar] [CrossRef]
- Decot, V.; Woerly, G.; Loyens, M.; Loiseau, S.; Quatannens, B.; Capron, M.; Dombrowicz, D. Heterogeneity of expression of IgA receptors by human, mouse, and rat eosinophils. J. Immunol. 2005, 174, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, L.; Chen, T. The effects of secretory IgA in the mucosal immune system. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Yue, F.Y.; Cohen, J.C.; Ho, M.; Rahman, A.N.-U.; Liu, J.; Mujib, S.; Saiyed, A.; Hundal, S.; Khozin, A.; Bonner, P. HIV-specific granzyme B-secreting but not gamma interferon-secreting T cells are associated with reduced viral reservoirs in early HIV infection. J. Virol. 2017, 91, e02233-16. [Google Scholar] [CrossRef] [PubMed]
- Ramljak, D.; Vukoja, M.; Curlin, M.; Vukojevic, K.; Barbaric, M.; Glamoclija, U.; Purisevic, B.; Peric, O.; Soljic, V. Early response of CD8+ T cells in COVID-19 patients. J. Pers. Med. 2021, 11, 1291. [Google Scholar] [CrossRef]
- Sun, Q.; Burton, R.L.; Pollok, K.E.; Emanuel, D.J.; Lucas, K.G. CD4+ Epstein–Barr virus-specific cytotoxic T-lymphocytes from human umbilical cord blood. Cell. Immunol. 1999, 195, 81–88. [Google Scholar] [CrossRef]
- Yasukawa, M.; Ohminami, H.; Yakushijin, Y.; Arai, J.; Hasegawa, A.; Ishida, Y.; Fujita, S. Fas-independent cytotoxicity mediated by human CD4+ CTL directed against herpes simplex virus-infected cells. J. Immunol. 1999, 162, 6100–6106. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Mead, H.; Tian, L.; Park, J.-G.; Garcia, J.I.; Jaramillo, S.; Barr, T.; Kollath, D.S.; Coyne, V.K.; Stone, N.E. The K18-human ACE2 transgenic mouse model recapitulates non-severe and severe COVID-19 in response to an infectious dose of the SARS-CoV-2 virus. J. Virol. 2022, 96, e00964-21. [Google Scholar] [CrossRef]
- Winkler, E.S.; Chen, R.E.; Alam, F.; Yildiz, S.; Case, J.B.; Uccellini, M.B.; Holtzman, M.J.; Garcia-Sastre, A.; Schotsaert, M.; Diamond, M.S. SARS-CoV-2 causes lung infection without severe disease in human ACE2 knock-in mice. J. Virol. 2022, 96, e01511-21. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.-H.; Kim, J.; Jang, Y.-S. The Papain-like Protease Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Conjugated with Human Beta-Defensin 2 and Co1 Induces Mucosal and Systemic Immune Responses against the Virus. Vaccines 2024, 12, 441. https://doi.org/10.3390/vaccines12040441
Cho B-H, Kim J, Jang Y-S. The Papain-like Protease Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Conjugated with Human Beta-Defensin 2 and Co1 Induces Mucosal and Systemic Immune Responses against the Virus. Vaccines. 2024; 12(4):441. https://doi.org/10.3390/vaccines12040441
Chicago/Turabian StyleCho, Byeol-Hee, Ju Kim, and Yong-Suk Jang. 2024. "The Papain-like Protease Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Conjugated with Human Beta-Defensin 2 and Co1 Induces Mucosal and Systemic Immune Responses against the Virus" Vaccines 12, no. 4: 441. https://doi.org/10.3390/vaccines12040441
APA StyleCho, B.-H., Kim, J., & Jang, Y.-S. (2024). The Papain-like Protease Domain of Severe Acute Respiratory Syndrome Coronavirus 2 Conjugated with Human Beta-Defensin 2 and Co1 Induces Mucosal and Systemic Immune Responses against the Virus. Vaccines, 12(4), 441. https://doi.org/10.3390/vaccines12040441





