Abstract
A systematic review and meta-analysis was designed in order to ascertain the effectiveness of respiratory syncytial virus (RSV) vaccination in preventing lower respiratory tract diseases (LRTD) in older adults (age ≥ 60 years). Studies reporting on randomized controlled trials (RCTs) were searched for in three databases (PubMed, Embase, and Scopus) and the preprint repository medRxiv until 31 March 2024. A total of nine studies were eventually included, two of which were conference proceedings. Our analysis included five RCTs on five RSV vaccines (RSVpreF, RSVPreF3, Ad26.RSV.preF, MEDI7510, and mRNA-1345). The meta-analysis documented a pooled vaccine efficacy of 81.38% (95% confidence interval (95% CI) 70.94 to 88.06) for prevention of LRTD with three or more signs/symptoms during the first RSV season after the delivery of the vaccine. Follow-up data were available for RSVPreF3 (2 RSV seasons), RSVpreF (mid-term estimates of second RSV season), and mRNA-1345 (12 months after the delivery of the primer), with a pooled VE of 61.15% (95% CI 45.29 to 72.40). After the first season, the overall risk for developing RSV-related LRTD was therefore substantially increased (risk ratio (RR) 4.326, 95% CI 2.415; 7.748). However, all estimates were affected by substantial heterogeneity, as suggested by the 95% CI of I2 statistics, which could be explained by inconsistencies in the design of the parent studies, particularly when dealing with case definition. In conclusion, adult RSV vaccination was quite effective in preventing LRTD in older adults, but the overall efficacy rapidly decreased in the second season after the delivery of the vaccine. Because of the heterogenous design of the parent studies, further analyses are required before tailoring specific public health interventions.
1. Introduction
Human respiratory syncytial virus (RSV) is a pleomorphic, enveloped, single-stranded negative-sense RNA virus (15 to 16 kb) of medium size (120–300 nm diameter) [1,2,3,4]. RSV belongs to the order of mononegavirales and the genus orthopneumovirus (family Pneumoviridae) [4]. RSV is usually acknowledged as a highly contagious pathogen [5,6]: before 2020 and the COVID-19 pandemic, RSV was by far the single most common viral cause of acute respiratory infections (ARIs) and lower respiratory tract disease (LRTD) [3], accounting for around 33 million cases every year [2,7]. Until recently, cases occurred during seasonal epidemics that, in the northern hemisphere, extensively overlap with other respiratory viruses such as influenza, adenovirus, and SARS-CoV-2 [8,9]. In countries with temperate climates, seasonal epidemics occurred during the winter season, peaking between December and January [2,7], while in tropical countries, RSV outbreaks are associated with the summer season because of the hot, humid, and rainy climate [10,11,12].
According to available estimates, every year RSV causes around 3.5 million hospital admissions in infants aged 0 to 60 months [1,3], even without noticeable comorbidities [2,13,14], with high hospital admission rates [15,16,17,18,19], recently estimated at around 5.3 per 1000 people (95% confidence interval (95% CI)) and 4.2–6.8 at a global level [1,3]. Nonetheless, with around 158,229 hospitalizations occurring annually in adults aged ≥18 years, 92% of which are from adults aged ≥65 years [20], with a corresponding hospitalization rate of 157 per 100,000 [21], RSV also affects older individuals [13,22,23,24]. It is increasingly acknowledged as a cause of respiratory illnesses and severe lower respiratory tract infections in adults [10], mostly older adults [25,26], in whom it causes high morbidity and mortality [24,27,28], particularly among institutionalized subjects [2,29,30,31], resulting in a significant public health impact [32,33,34,35,36,37]. Until 2023, preventive and treatment options for older adults were limited, as several RSV candidate vaccines ultimately failed to achieve targeted efficacy. In children, however, these disease candidates were initially identified as having a greater than 70% vaccine efficacy (VE) against confirmed severe RSV disease over at least one year post vaccination, as well as the secondary target of 50% VE against confirmed severe RSV [38]. Since then, several vaccines have been able to complete phase III clinical trials, and two preventive vaccines were ultimately licensed for human use: Abrysvo from Pfizer Inc. (Pfizer Europe MA EEIG, Brussels, Belgium) and Arexvy from GlaxoSmithKline LLC (GlaxoSmithKline Biologicals SA, Rixensart, Belgium) [39,40]. Both of them are subunit vaccines based on pre-fusion F protein, but while Abrysvo is a divalent, non-adjuvated formulate, Arexvy is a monovalent formulate based on the pre-fusion protein F of RSV from group A [39,40]. Their overall efficacy in older adults has been documented in specifically designed randomized controlled trials (RCTs) [41,42], leading to official recommendations for their use in individuals aged ≥60 years [43]. However, other RSV vaccines are or have been in the pipeline for use in older adults [5,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Even though some high-quality systematic reviews on the VE of RSV vaccines in pregnant women have been published [65], to the best of our knowledge no studies have to date tried to summarize the available evidence on RSV vaccines for older adults. Therefore, this study was designed in order to systematically evaluate the VE of RSV vaccines in older adults, providing valuable evidence and possibly guidance for clinical use.
2. Materials and Methods
2.1. Research Concept
This systematic review with meta-analysis was designed in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) statement [66] (see Supplementary File S1). Prior to conducting the study, it was registered in the PROSPERO database (progressive registration number CRD42023475548 https://www.crd.york.ac.uk/prospero/, accessed on 21 March 2024) [67].
Research concepts were defined by means of the “PECO” strategy (i.e., patient/population/problem, exposure, control/comparator, outcome) [68,69] (Table A1). More precisely, the assessment concerned whether adults aged 60 years or older having received at least one dose of any RSV vaccine (P), upon exposure to RSV infection during the subsequent RSV season (E), incurred LRTD or ARI (O) in reduced occurrence compared to adults aged 60 years or more who were not vaccinated against RSV (placebo) (C).
2.2. Research Strategy
The systematic retrieval was performed across three databases (Pubmed; EMBASE; and Scopus) and the preprint repository medRxiv from their inception until 31 March 2024 (Table A2). The search strategy was obtained by modifying the blueprint originally recommended by Ma et al. [65] for maternal RSV vaccine studies and resulted in the combination of specifically designed search strings provided in Table A2. Where allowed by the searched database, all inquiries were restrictive to individuals aged 60 years or older.
2.3. Selection Criteria
The criteria for being included in the retrieved studies were as follows:
(1) Vaccination of older adults (age ≥ 60 years);
(2) RSV vaccine administration (any);
(3) Comparison of RSV vaccine efficacy with placebo in a randomized controlled trial (RCT);
(4) Reporting on the efficacy of the RSV vaccine in terms of ARI or LRTD.
The following exclusion criteria were then applied:
(1) Vaccination of children and/or younger adults (age < 60 years);
(2) Secondary studies (i.e., systematic reviews, meta-analysis, letters, editorial comments, case reports, preprint from repositories other than medRxiv);
(3) Studies not designed as RCTs;
(4) Studies on animals (including non-human primates) or preclinical testing;
(5) Outcomes other than clinical efficacy;
(6) full text not available either through online repositories or through inter-library loan, or the main text written in a language different from English, Italian, German, French, Spanish, or Portuguese;
(7) lack of details about geographical setting and corresponding time frame;
(8) laboratory diagnosis of respiratory infections performed with methods other than real-time quantitative polymerase chain reaction (RT-qPCR) or non-RT-qPCR nucleic acid amplification tests (NAATs).
In case of cross-publication and/or duplicated series, only the most recent publication was included, and if it proved to be feasible, duplicated data were removed from both qualitative and quantitative analysis.
2.4. Selection Criteria
By using inclusion and exclusion criteria, retained articles were then screened to ascertain their relevance for the research question. The articles were initially title screened using the title alone and all the items were positively assessed; hence, they were screened by reading the whole abstract [66,70]. All the retained entries were eventually full-text screened and independently rated by two investigators (S.C. and F.M.). Disagreements were resolved primarily by consensus between investigators and, when it was not reached, by involving as a third person the chief investigator (M.R.).
2.5. Data Extraction
The extracted data were retrieved from both the main text and the Supplementary Material (where available) and included:
(a) Main characteristics of the study: first author’s name, year of publication, RCT registration number, assessed vaccine;
(b) Characteristics of the RCT: sample size of the study groups (recipients of vaccine and placebo), baseline data (demographics and comorbidities), number of countries involved in the study, timeframe of the study;
(c) Outcome data: definition of primary and secondary outcome; case definition. The main outcome encompassed the reported ARI and LRTD.
2.6. Quality Assessment (Risk of Bias)
To cope with potential risk of bias (ROB) due to research practices [71,72,73], the ROB tool from the National Toxicology Program (NTP)’s Office of Health Assessment and Translation (OHAT) (now the Health Assessment and Translation (HAT) group) [73,74] was used. According to its original design, the OHAT ROB evaluates the internal validity of a given study by weighting 6 potential sources of bias: participant selection (D1), confounding factors (D2), attrition/exclusion (D3), detection (D4), selective reporting (D5), and other sources of bias (D6). All sources of bias are rated on a 4-point scale ranging from “definitely low,” “probably low,” “probably high,” to “definitely high.” The OHAT ROB was prioritized over other similar instruments, as it does not provide an overall rating for each study nor require that studies affected by a certain degree of ROB be removed from the pooled analyses [74].
2.7. Data Analysis
As a preliminary step, individual estimates for vaccine efficacy (VE) were calculated for each study. VE can be defined as the percentage reduction of disease cases in a vaccinated group of people compared to an unvaccinated group. Mathematically, VE was defined as:
VE = (1 − Relative Risk) × 100%
Meta-analysis of demographic data from individual studies was performed through calculation of the risk ratio (RR) as an effect index. Similarly, comparison of breakthrough infections in follow-up studies was performed by calculation of the RR. Pooled estimates of RR and VE were calculated through a random-effects model (REM) meta-analysis of retrieved studies and reported as point estimates with their 95% confidence intervals (95% CIs). As the search strategy likely included both primary studies (i.e., studies performed on subjects never vaccinated against RSV in their first post-vaccine RSV season) and follow-up studies (i.e., studies performed on subjects previously vaccinated, after the first RSV season), two distinctive pooled VE estimates were calculated for primary and follow-up studies, respectively. The REM was preferred over a fixed-effects model, as it is considered more effective in dealing with the genuine differences underlying the results of the studies (heterogeneity) [75,76].
The heterogeneity of the literature (i.e., the inconsistency of the effects between the included studies) was defined as the percentage of total variation across studies likely due to heterogeneity rather than chance [71], and was quantified by means of the I2 statistic through the following categories: 0 to 25%, low heterogeneity; 26% to 50%, moderate heterogeneity; and ≥50%, substantial heterogeneity. Because of the presumptive small size of the meta-analyses, with likely underestimation of actual heterogeneity by point estimate of I2, 95% CIs were provided [71].
Sensitivity analysis (i.e., the study of how the uncertainty in the output of a mathematical model or system can be apportioned to different sources of uncertainty in its inputs) was performed to evaluate the effect of each study on the pooled estimates by excluding one study at a time.
Publication bias was visually assessed through the calculation of contour-enhanced funnel plots, and their asymmetry was eventually assessed by means of the Egger’s test for all outcomes with three or more included studies [66,77]. Small-study bias was assessed by generating corresponding radial plots. A p-value < 0.05 was considered statistically significant for both publication and small-study bias.
All calculations were performed by means of R (version 4.3.1) [78] and Rstudio (version 2023.06.0 Build 421; Rstudio, PBC; Boston, MA, USA) software by means of the packages meta (version 7.0) and fmsb (version 0.7.5). The Prisma2020 flow diagram was designed by means of the PRISMA2020 package [79].
3. Results
3.1. Descriptive Analysis
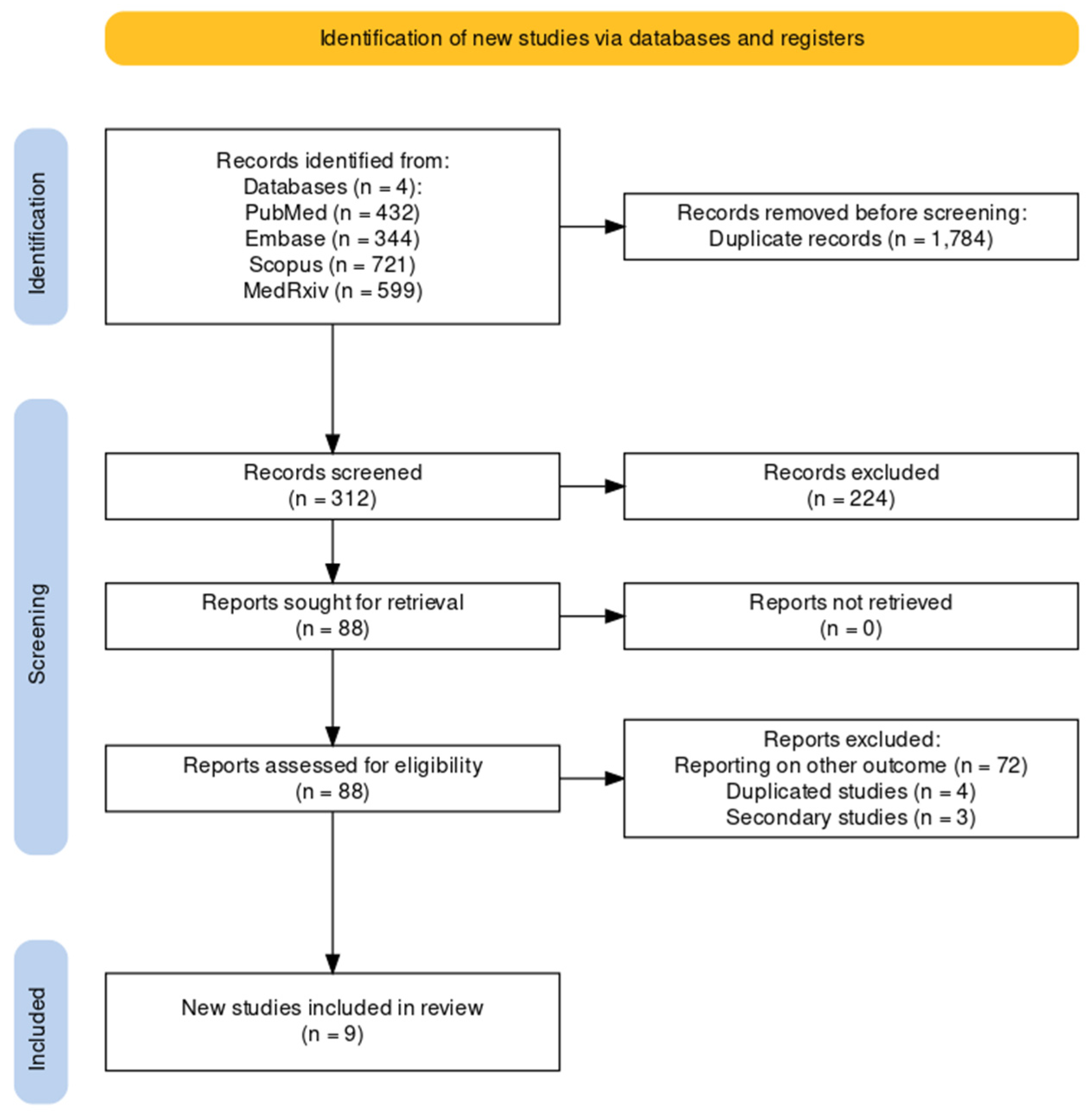
As shown by the flowchart of the search and selection process (Figure 1), a total of 2096 entries were retrieved, including 432 studies from PubMed (20.61%), 344 from EMBASE (16.41%), 721 from Scopus (34.40%), and 599 from MedRxiv (28.58%). After the removal of duplicates, 312 records were screened for title and abstract (14.89% of the original pool).
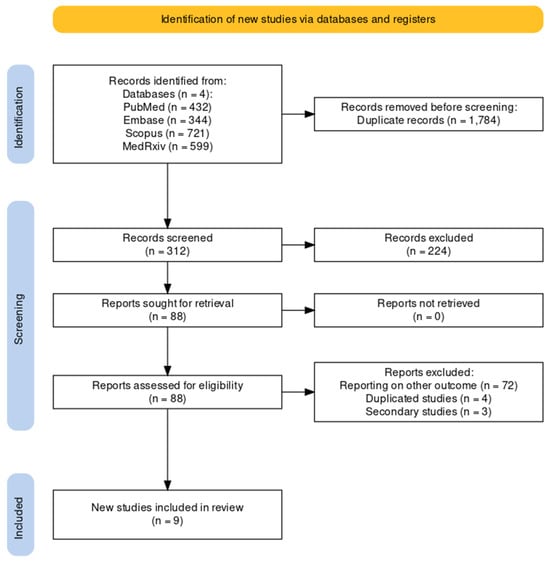
Figure 1.
Flowchart of included studies.
After the removal of duplicates and the full-text screening, nine articles were included in the pooled analysis, from five RCTs (NCT04886596, NCT05035212, NCT05127434, NCT03982199, NCT02508194) on five RSV vaccines (RSVPreF3, RSVpreF, mRNA-1345, Ad26.RSV.preF, MEDI7510; see Table A3). Of the retrieved studies, six reported on the first season after the delivery of the RSV vaccine [51,52,80,81,82,83], two studies reported on follow-up studies [51,61], and one study reported on both primary and follow-up studies [48]. Overall, seven studies were published as peer-reviewed reports [48,51,52,80,81,82,83], while two studies were reported as conference abstracts [51,61]. A summary of the retrieved studies is provided in Table 1.

Table 1.
Summary of randomized controlled trials (RCTs) on vaccines against respiratory syncytial virus (RSV), including the summary of the risk of bias assessment according to the risk of bias (ROB) tool from the National Toxicology Program (NTP)’s Office of Health Assessment and Translation (OHAT) handbook [74,84] on observational studies included in the meta-analysis. Details on individual characteristics of the vaccines are provided in Table A3. Note: D1: possibility of selection bias; D2: exposure assessment; D3: outcome assessment; D4: confounding factors; D5: reporting bias; D6: other bias; PreF = prefusion, PostF = post-fusion; Adj = adjuvated; FP = full paper; AR = abstract report.
As the studies of Ison et al. [48], Feldman et al. [80], and Papi et al. [81] for the first RSV season insisted on using the very same population, pooled estimates were calculated from the study of Ison et al. [48]. Finally, the studies involved a total of 101,931 subjects older than 60 years (51,055 vaccinated vs. 50,876 placebo) for the first season, and 33,130 vaccinated vs. 38,068 placebo individuals for the follow-up. In this regard, while the study from Ison et al. [48] (RSVPreF3) reported on the full second season, to date only mid-season 2 data have been published for RCT NCT05035212 [61] (RSVpreF) and estimates for 12 months after the delivery of the first dose for NCT04886596 [85].
Assessed outcomes are summarized in Table 2. Briefly, four out of five RCT studies and seven out of nine studies only included RT-qPCR confirmed RSV cases [48,51,80,81,82,85,86], while in the RCT on RSVpreF, RSV infection was confirmed through either RT-qPCR or NAAT [52,61].

Table 2.
Summary of outcome definition for lower respiratory tract disease (LRTD). Note: RT-qPCR = real-time quantitative polymerase chain reaction; NAAT = nucleic acid amplification test (i.e., isothermal amplification tests). Likewise: nicking endonuclease amplification reaction, NEAR; transcription mediated amplification, TMA; loop-mediated isothermal amplification, LAMP; helicase-dependent amplification, HAD; clustered regularly interspaced short palindromic repeats, CRISPR; strand displacement amplification, SDA.
Even though all studies reported on the cases of symptomatic ARI, the main outcome of the included RCTs was the first episode of RSV-associated LRTD. While three RCTs (NCT04886596, NCT05035212, and NCT03982199) reported on LRTD defined by either two or more than three signs/symptoms [51,61,82,85,87], and NCT02508194 [83] reported on cases of LRTD with two or more signs/symptoms and on cases of ARI with one symptom or more from two or three different locations, RCT on RSVPreF3 (NCT04886596) implemented a different case definition (see Table A4). To begin with, researchers strictly dichotomized RSV-associated findings in signs and symptoms. Then, an LRTD was defined by either one sign and one symptom, or by a combination of three signs and/or symptoms, but discrete data were not provided. For the aim of the present review, LRTD cases from NCT04886596 were therefore pooled together with LRTD cases with three signs and/or symptoms rather than with cases with two signs/symptoms.
3.2. Risk of Bias
The overall quality of the included studies is detailed in Table 1 and summarized in Figure A1. Overall, all studies were characterized by high quality and low risk of bias. A partial exception is represented by the studies from the RCT NCT04886596 [48,80,81] because of the inconsistencies in the case definition when compared to other cases and the potential inclusion of more severe cases (i.e., those with three or more signs/symptoms) with milder ones, as well as by the limited information conveyed by conference reports [61,85].
3.3. Demographic Characteristics of the Included Studies
As shown in Table 3, there were no statistically significant differences between vaccine recipients and subjects treated with placebo in terms of prevalence of white ethnicity (74.50% in vaccinated, 74.64% in placebo, RR 0.996, 95% CI 0.990 to 1.003; I 0.0%, 95% CI 0.0 to 79.2; Q 2.09, p = 0.300) and subjects older than 70 years (or 75 years according to the age groups reported; 37.84% vs. 37.77%, RR 1.000, 95% CI 0.984 to 1.015; I 0.0%, 95% CI 0.0 to 79.2; Q 0.18, p = 0.996). On the contrary, while no substantial difference in terms of pooled prevalence of male gender was identified among the included studies (49.72% vs. 49.25%, RR 1.024, 95% CI 0.987 to 1.061), these estimates were affected by substantial heterogeneity (71.2%, 95% CI 27.1 to 88.6; Q 13.89, p = 0.008). As summarized in Table A5, the proportion of males ranged from 41.90% (vaccine group) vs. 34.45% (placebo) in the study by Falloon et al. [83] to 51.12% vs. 50.39% (for the vaccine and placebo groups, respectively) in the study by Walsh et al. [52].

Table 3.
Demographics and clinical characteristics of the subjects included in the primary studies (only cases in the first season).
Moreover, a detailed report on comorbidities was provided by four RCTs for COPD and congestive heart disease [48,51,52,82], and for asthma by three studies [48,52,82], while the remaining studies more properly included a description of “respiratory” and “cardiovascular” disorders (Table A6). Again, no substantial differences were identified.
3.4. Effectiveness of RSV Vaccine
3.4.1. First Season
The effectiveness of the assessed vaccines is summarized in Table 4, while individual estimates are reported in forest plots included as Figure A2, Figure A3, Figure A4, Figure A5, Figure A6, Figure A7, Figure A8, Figure A9, Figure A10, Figure A11, Figure A12, Figure A13, Figure A14, Figure A15 and Figure A16. Regarding LRTD, distinctive estimates were calculated for LRTD with two symptoms and with three or more symptoms (see Table A4 for details).

Table 4.
Summary of main outcomes of vaccine efficacy (VE) from collected studies (Note: 95% CI = 95% confidence interval; ARI = acute respiratory illness; LRTD = lower respiratory tract disease).
VE on ARI
The reported VE for preventing ARI ranged from −5.08% (95% CI −106.67 to 46.57) of MEDI7510 to 69.66% (95% CI 43.70 to 83.65), with a pooled estimate of 59.88% (95% CI 41.17 to 72.64), which was affected by substantial heterogeneity (I2 61.0%, 95% CI 0.0 to 85.2). Estimates for the VE on RSV A and B were provided for RSVpreF, mRNA-1345, and MEDI7510 [51,52,83] (Figure A3 and Figure A4): Individual efficacy for RSV A was 78.50% (95% CI 58.77 to 88.79), 66.95% (95% CI −2.46 to 89.34), and −5.08% (95% CI −106.67 to 46.57) for mRNA-1345, RSVpreF, and MEDI7510, respectively, with a pooled estimate of 57.17% (95% CI −16.28 to 84.23). Individual VE for RSV B was 51.77% (95% CI 10.69 to 73.95), 82.37% (95% CI 62.62 to 91.69), and −34.86% (95% CI −50.11 to 84.37) for mRNA-1345, RSVpreF, and MEDI7510, respectively, with a pooled estimate of 51.56% (95% CI −50.11 to 84.37). Both estimates were affected by substantial heterogeneity (I2 82.2%, 95% CI 45.3 to 94.2, and 85.4%, 95% CI 57.1 to 95.0 for RSV A and RSV B, respectively). Calculation of RR for ARI associated with RSV A vs. RSV B ruled out significant differences in the corresponding VE (RR 0.849, 95% CI 0.529 to 1.363; I2 0.0%, 95% CI 0.0 to 89.6, Q = 1.56, p = 0.458) (Table 5).

Table 5.
Summary of risk ratio (RR) for main outcomes from collected studies by serogroup of RSV infections (note: 95% CI = 95% confidence interval; ARI = acute respiratory illness; LRTD = lower respiratory tract disease).
When focusing on adults older than 70/75 years at the time of recruitment [51,52,82], individual estimates were available for RSVpreF, mRNA-1345, and Ad26.RSV.preF. As shown in Figure A6, VE ranged from 60.3% (95% CI −25.99 to 87.50) for Ad26.RSV.preF, to 62.31% (95% CI 14.98 to 83.29) for RSVpreF, and peaked at 84.23% (95% CI 62.71 to 93.33) for mRNA-1345, with a pooled estimate (72.31%, 95% CI 45.58 to 85.09) and a heterogeneity of 22.1% (95% CI 0.0 to 91.9).
VE on LRTD with 2 Symptoms
Point estimates (Figure A7) ranged from −34.86% (95% CI −192.00 to 37.72) for MEDI7510, to 66.95% (95% CI 34.63 to 83.29) for RSVpreF, to 74.91% (95% CI 49.93 to 87.43) for Ad26.RSV.preF, and peaked at 83.67% (95% CI 67.01 to 91.94) for mRNA-1345. A pooled estimate of 63.66% (95% 12.35 to 84.93) was therefore calculated, with substantial heterogeneity (I2 82.5%, 95% CI 55.0 to 93.2, p < 0.001). A subanalysis for individuals aged >70/75 years at the time of the recruitment (see Figure A8) was provided from three studies [51,52,82], and identified a VE ranging from 78.80% (95% CI 26.27 to 93.91 for RSVpreF) to 80.16 (95% CI 9.74 to 95.64) for Ad26.RSV.preF and 95.46% (95% CI 66.32 to 99.39) for mRNA-1345. The corresponding pooled estimate was 84.46% (95% CI 63.00 to 93.47).
VE on LRTD with 3 Symptoms or More
Point estimates (Figure A9) were provided for Ad26.RSV.preF (79.93%, 95% CI 51.85 to 91.63), RSVPreF3 (80.91%, 95% CI 64.62 to 89.70), and mRNA-1345 (82.41%, 95% CI 39.99 to 94.84), peaking at 85.84% (37.69 to 96.78) for RSVpreF, with a pooled VE of 81.38% (95% CI 70.94 to 88.06). Even though the point estimate suggested a low heterogeneity (I2 = 0.0%), a contradictory report was provided by 95% CI (0.0 to 84.7). The pooled efficacy against RSV A was 83.76% (95% CI 52.95 to 94.40; Figure A10), while the pooled VE for RSV B was estimated as 80.72% (95% CI 58.79 to 90.98; Figure A11), with no substantial differences, as shown in Table 5 (RR 0.505; 95% CI 0.150 to 1.706 for LRTD with three or more symptoms in RSV A vs. RSV B; Figure A12).
Finally, subanalysis for individuals aged 70/75 years or more identified a pooled VE of 83.78% (95% CI 61.43 to 93.18) that resulted from four point estimates, i.e., RSVPreF3 (84.18%, 95% CI 46.95 to 95.32) [81], mRNA-1345 (92.32%, 95% CI −36.39 to 99.57) [51], RSVpreF (91.01%, 95% CI −62.62 to 99.50) [52], and Ad26.RSV.preF (75.20%, 95% CI −16.42 to 94.72) [82].
Comparison of Main Outcome by Age Groups
As shown in Table 6, the risk for all assessed outcomes was similar in subjects aged 70/75 years or more and among younger subjects (i.e., <70/75 year of age: RR 0.800, 95% CI 0.459 to 1.392; RR 0.522, 95% CI 0.211 to 1.291; RR 0.819, 95% CI 0.304 to 2.207 for ARI, LRTD with two symptoms, and LRTD with three or more symptoms, respectively) (Figure A15 and Figure A16).

Table 6.
Summary of risk ratio (RR) for main outcomes from collected studies by age groups of participants (note: 95% CI = 95% confidence interval; ARI = acute respiratory illness; LRTD = lower respiratory tract disease).
3.4.2. Follow-Up Studies
The three studies providing data on the follow-up of vaccinated individuals [48,61,85] provided pooled estimates for VE regarding the occurrence of ARI and LRTD with three or more symptoms (see Table A4 for case definition). More precisely, the VE on ARI was estimated as 46.64% (95% CI 35.94 to 55.55) and as 61.15% (45.29 to 72.40) for LRTD with three or more symptoms. Individual estimates for ARI ranged from 40.04% (95% CI 18.90 to 55.67) for RSVPreF3 to 41.38% (95% CI 16.97 and 58.62) for RSVpreF, and peaked at 53.69% (95% CI 40.24 to 64.11) for mRNA-1345, while estimates for LRTD with three or more symptoms was 55.83% (95% CI 28.41 to 72.75), 78.65% (95% CI 25.72 to 93.86), and 62.88% (95% CI 37.17 to 78.07) for RSVpreF3, RSVpreF, and mRNA-1345, respectively (Figure A17 and Figure A18). Heterogeneity among the retrieved studies was reportedly low in point estimates, while the 95% CI for I2 exceeded the cutoff for substantial heterogeneity (0.0 to 89.9 and 0.0 to 89.6, respectively). Subanalyses were performed on the efficacy for the prevention of LRTD with three symptoms or more in the RSV serogroup (Figure A19 and Figure A20), and the corresponding pooled estimates for VE were 70.55%, 95% CI 45.51 to 84.08) for RSV A compared to 50.25% (95% CI 22.62 to 68.02) for RSV B. Not coincidentally (Figure A21), the point estimates for RSVpreF and mRNA-1345 were significantly similar for RSV A and RSV B (RR 1.000, 95% CI 0.063 to 15.985).
The risk for breakthrough infections during the follow-up period is summarized in Table 7 and detailed in Figure A22 and Figure A23. As shown, the risk substantially increased for both ARI (RR 3.740, 95% CI 2.875 to 4.866) and LRTD with three or more symptoms (RR 4.326, 95% CI 2.415 to 7.748). Even though heterogeneity was not substantial when taken into account as a point estimate (I2 = 0.0% for both ARI and LRTD with three or more symptoms), a contradictory appraisal was suggested by the corresponding 95% CI that in both cases exceeded the cutoff value of 60%. When focusing on the individual point estimates, no significant differences were identified for the primary season vs. follow-up season for RSVpreF (RR 2.575, 95% CI 0.430 to 15.410).

Table 7.
Summary of risk ratio (RR) for main outcomes from breakthrough infections by RSV in follow-up time period compared to primary season (note: 95% CI = 95% confidence interval; ARI = acute respiratory illness; LRTD = lower respiratory tract disease).
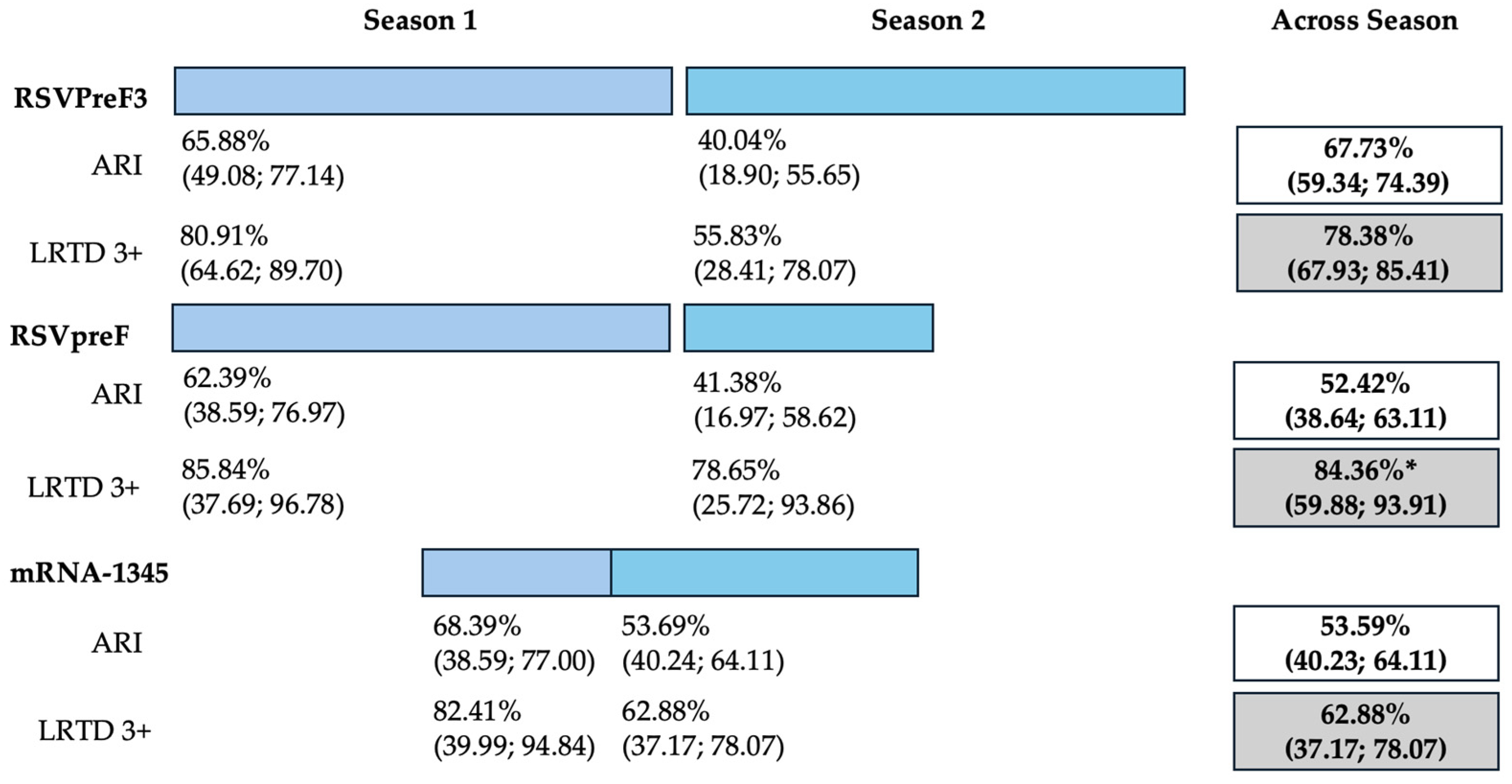
Data on cumulative estimates were similarly provided. However, as data on RSVpreF only included 6 months of season two (representing “mid-season” estimates), and the RCT on mRNA-1345 eventually reported on a total of 12 months across two RSV seasons, a pooled meta-analysis was not performed. However, cumulative estimates across two seasons (Figure 2) were then calculated as 67.73% (59.34 to 74.39), 52.42% (95% CI 38.64 to 63.11), and 53.59% (95% CI 40.23 to 64.11) for RSVPreF3, RSVpreF, and mRNA-1345, respectively, on the prevention of ARI. On the other hand, corresponding estimates for the prevention of LRTD with three or more symptoms were 78.38% (95% CI 67.93 to 85.41), 84.36% (59.88 to 93.91), and 62.88% (37.17 to 78.07) for RSVPreF3, RSVpreF, and mRNA-1345, respectively.
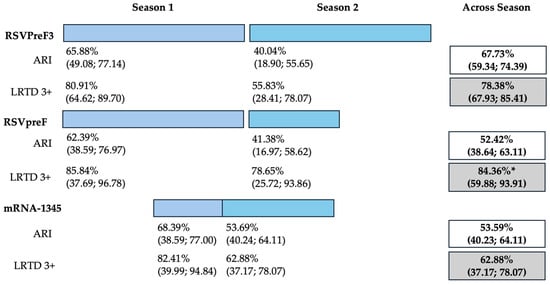
Figure 2.
Follow-up studies and vaccine efficacy estimates for season 1, season 2, and across seasons on the prevention of acute respiratory infection (ARI) and lower respiratory tract disease (LRTD) with 3 or more signs/symptoms. All results are reported as point estimates in percent value and corresponding 95% confidence intervals. * = provisionary estimate for end of season 2 equals 77.8% (95.0% CI: 51.4, 91.1) [88].
3.5. Sensitivity Analysis
Sensitivity analysis required the removal of single studies at a time, and resulting pooled estimates are reported in Figure A24, Figure A25, Figure A26, Figure A27, Figure A28, Figure A29, Figure A30, Figure A31, Figure A32, Figure A33, Figure A34, Figure A35 and Figure A36. Where included in the pooled analyses, the removal of the study by Falloon et al. [83] on MEDI7510 led to a substantial increase in VE (ARI: 66.43%, 95% CI 57.55 to 73.45; LRTD with two symptoms 79.99%, 95% CI 64.18 to 83.91), and heterogeneity decreased consistently (0.0% for ARI, 1% for LRTD with two symptoms). Contrarily, the removal of the study by Wilson et al. on mRNA-1345 [85] from follow-up pooled estimates led to a reduction in VE (40.62%, 95% CI 25.40 to 52.73) for ARI, while the removal of the study by Walsh et al. on RSVpreF [61] reduced the VE to 59.20% (95% CI 41.77 to 71.42).
3.6. Publication Bias
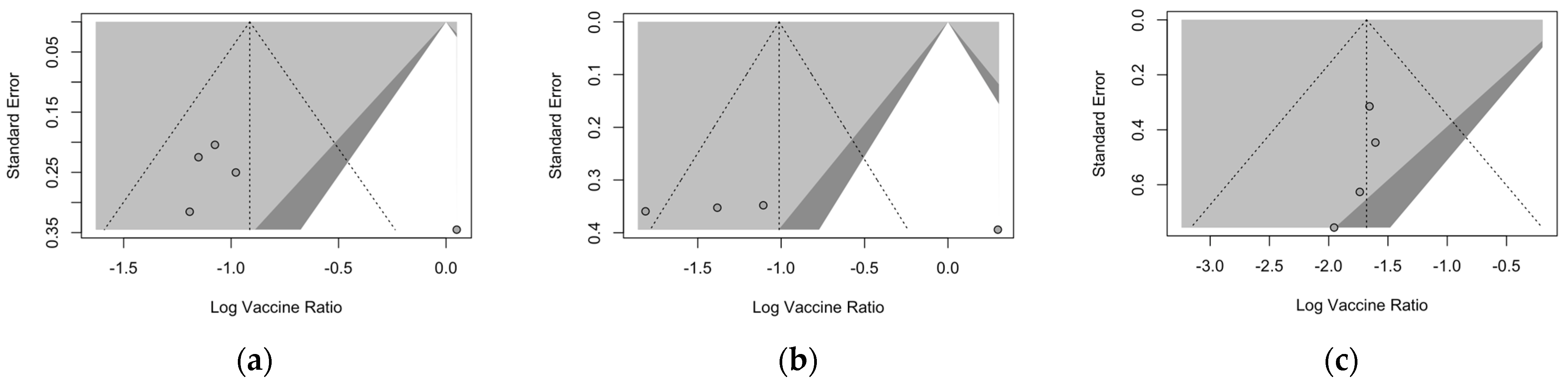
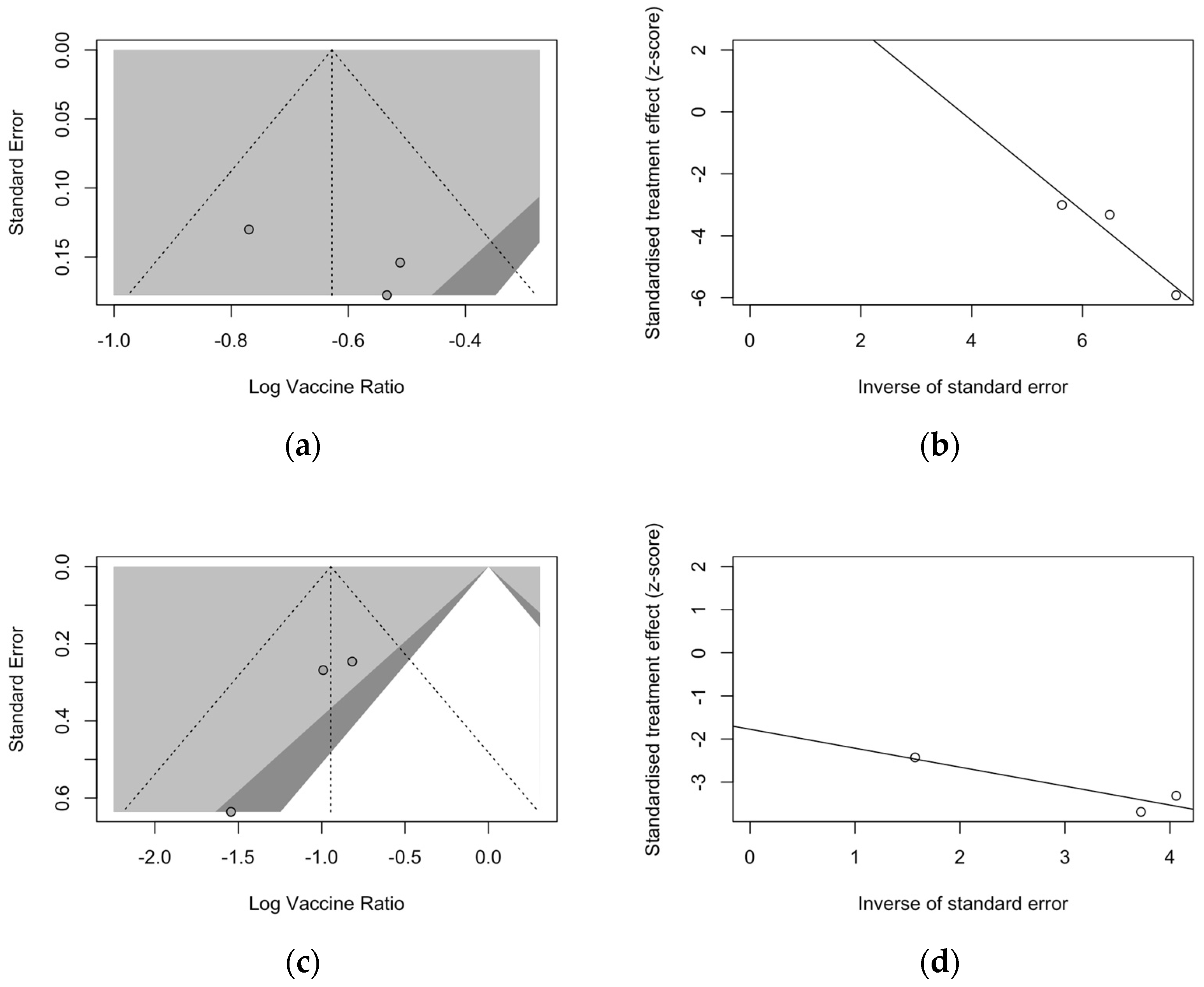
Publication bias was initially ascertained by calculation and visual inspection of funnel plots. In funnel plots, the sample size is plotted against the effect size they report. As the size of the sample increases, individual estimates of the effect are likely to converge around the true underlying estimate [63,66,73]. Funnel plots for the primary season (for ARI and LRTD with two and three or more symptoms) are reported in Figure 3.
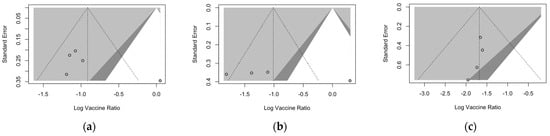
Figure 3.
Funnel plots for randomized controlled trials included in the analyses. (a), acute respiratory illness; (b), lower respiratory tract disease with two symptoms; (c), lower respiratory tract diseases with three or more symptoms.
All funnel plots were substantially asymmetrical, as all the points did point towards the left half of the plot, suggesting the presence of publication bias with a high share of lower prevision studies. However, Egger’s test (Table 8) substantially ruled out potential publication bias, suggesting that other potential sources of asymmetry (e.g., heterogeneity due to different choices in the outcome measure, differences in the underlying risk, etc.) should be ascertained.

Table 8.
Summary of Egger’s test for publication bias in sampled studies.
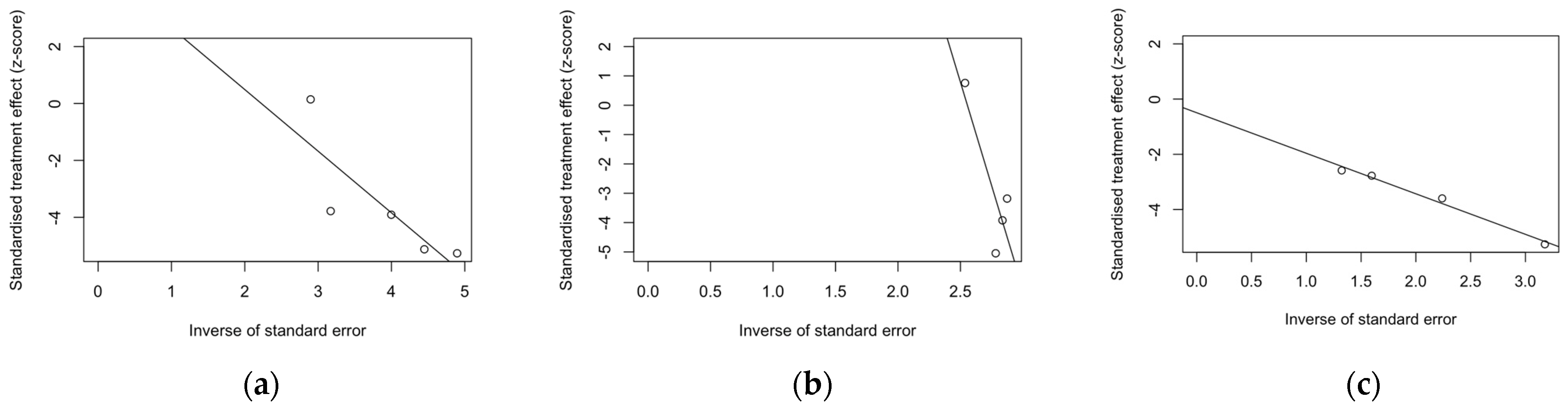
Despite the reduced number of included studies, radial plots were seemingly spared by the clustering of retrieved data, with individual estimates scattered across both sides of the regression lines (Figure 4).
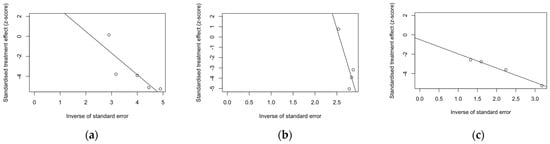
Figure 4.
Radial plots for randomized controlled trials included in the analyses. (a), acute respiratory illness; (b), lower respiratory tract disease with two symptoms; (c), lower respiratory tract disease with three or more symptoms.
Regarding follow-up studies (Figure 5), the analysis of funnel plots for ARI (Figure 5a) and LRTD with three or more symptoms (Figure 5c) suggested a similar asymmetry of estimates, but Egger’s test substantially ruled out the potential publication bias as an explanation of these findings. Also, radial plots (Figure 5b,d) were somehow scattered across the regression line, but the limited number of observations suggested a cautious appraisal of visual inspection.
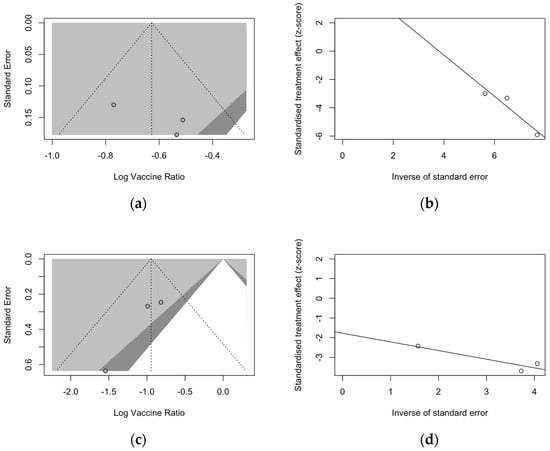
Figure 5.
Funnel and radial plots and for randomized controlled trials included in the analyses and dealing with follow-up studies for ARI (a,b) and for lower respiratory tract diseases with three or more symptoms (c,d).
4. Discussion
4.1. Summary of Main Findings and Their Generalizability
In this systematic review with meta-analysis, we retrieved and ultimately included a total of nine studies on five RSV vaccines (RSVpreF, RSVPreF3, MEDI7510, Ad.26.RSV.preF, and mRNA-1345). Of those, four formulates were based on prefusion F protein (RSVpreF, RSVPreF3, Ad.26.RSV.preF, and mRNA-1345). The only post-fusion F protein-based vaccine to date has been officially discontinued (MEDI7510) [83,86,89].
All the full papers included in this review were of high or even very high quality, and the risk of literature bias was substantially low. Collected data were meta-analyzed, focusing on the VE for the prevention of LRTD in older adults compared with that in the placebo groups, and sub-analyses were specifically performed on adults aged more than 70 or 75 years, according to the age groups included in the primary studies. Heterogenous definitions of LRTD were used in the included studies, particularly when dealing with symptoms that were included in the respective definition (see Table A4). Taking into account this potential source of heterogeneity, a pooled estimate for VE of 81.38% (95% CI 70.94 to 88.06) for LRTD with three or more symptoms was eventually calculated for mRNA-1345, RSVpreF, RSVPreF3, and Ad.26.RSV.preF, with no substantial difference for RSV A (83.76%, 95% CI 52.95 to 94.40) or RSV B (80.72%, 95% CI 58.79 to 90.98), as the RR for LRTD with three or more symptoms associated with RSV A rather than RSV B was equal to 0.505 (95% CI 0.150; 1.706).
Interestingly enough, the final efficacy in adults aged 70/75 years or older was estimated as 83.78% (95% CI 61.43 to 93.18), with no substantial differences in younger individuals (RR 0.522, 95% CI 0.211 to 1.291). In other words, the four assessed formulates were not only quite effective in preventing more severe cases of RSV-associated diseases in older adults, but sensitivity analysis also suggests similar VE estimates.
The assessed RSV vaccines appeared to be significantly less effective against ARI than against LRTD, as the pooled efficacy was estimated in 59.88% (95% CI 41.17 to 72.64), which was improved to 66.43% (95% CI 57.55 to 73.45) after the removal of the report on MEDI7510. In this regard, it should be stressed that the WHO preferred product characteristics for RSV vaccines [38,90] were initially designed for infants and newborns, and a 50% VE in the prevention of severe RSV disease due to LRTD was considered an acceptable target.
Follow-up data were available for RSVpreF, RSVPreF3, and mRNA-1345, including a full report on the second RSV season after the delivery of the vaccine for RSVPreF3 [48], a mid-term report on the second RSV season for RSVpreF [61], and a report on the first calendar year after the delivery of the RSV vaccine for mRNA-1345 [85]. However, despite the promising results from the first RSV season [82,91], no updates on the efficacy of Ad26.RSV.preF vaccine have been published since 2023 [82,92,93]. As the Ad26.RSV.preF was designed as an adenovirus vector-based vaccine, we cannot rule out that it may have been affected by the “fallout” that followed COVID-19 vaccination campaigns. The vaxzevria vaccine for SARS-CoV-2 is similarly based on replication-defective adenovirus (ChAdOx1), and claims for increased risk for thromboembolic events in patients having received this immunization [94] have possibly forced the strengthening of its preventive assessment [82,92,93,95,96]. Moreover, follow-up data on RSVpreF and mRNA-1345 have only been published as conference abstracts [61,85]. Despite their potential significance from a public health point of view, the content they conveyed should be quite cautiously appraised, as conference proceedings should be acknowledged as a “primer” for more detailed primary research, thereby emphasizing the need for more studies. This may explain the lack of information in publications that must be acknowledged as preliminary reports. Taking into account all of the aforementioned potential shortcomings, the pooled VE for ARI was 46.64% (35.95 to 55.55), compared to 61.15% (45.29 to 72.40) for LRTD with three or more symptoms [48,61,85]. In other words, the risk for breakthrough infections was significantly higher after the primary season not only for ARI (RR 3.740, 95% CI 2.875 to 4.866) but also for LRTD (RR 4.326, 95% CI 2.415 to 7.748). When data were considered across the whole of the assessed time period, mRNA-1345 was associated with a cumulative VE of 53.59% (40.23 to 64.11) for ARI and 62.88% (37.17 to 78.07) for LRTD with three or more symptoms compared to 67.73% (59.34 to 74.39) for ARI and 78.38% (67.93 to 85.41) for LRTD with three or more symptoms for RSVPreF3 and 52.42% (38.64 to 63.11) and 84.36% (59.88 to 93.91) for ARI and LRTD with three or more symptoms among subjects vaccinated with RSVpreF.
In fact, the reported VE was quite heterogeneous, as suggested by the analysis of 95% CI for I2 statistics, and several explanations could be suggested. Most notably, the observation time for RSVPreF3 included two full RSV seasons [48,80], while available data for RSVpreF were limited to the end of RSV season in the USA, representing a mid-season term [61], and estimates for mRNA-1345 represented an additional analysis collected 12 months after the vaccine, irrespective of the underlying seasonal epidemiology of RSV [85]. Nonetheless, a provisionary estimate for two end-season LRTD cases among RSVpreF vaccine recipients has recently been reported, including a preliminary VE equal to 77.8% (95.0% CI: 51.4, 91.1) [88]. Unfortunately, as the complete data are still not available, not only were such estimates not included in our systematic review and meta-analysis, but also, due the high heterogeneity in the overall length of the assessment, a pooled analysis for the across-season efficacy was not performed.
Another explanation is associated with the uneven sample size. In the study from Ison et al. [48], nearly half of the original vaccine group was randomized to receive a second dose of RSV vaccine and was therefore removed from the corresponding estimates, which were based on only 4991 vaccinated individuals (i.e., 40.02% of the original sample) and 10,031 placebos (i.e., 80.06% of the original sample). On the contrary, the RCT on the RSVpreF vaccine retained 58.24% and 58.54% of the vaccine and placebo groups, respectively, each one encompassing around 10,000 subjects, while the RCT on mRNA-1345 included more than 36,000 individuals, which is nearly the same number of cases as in the other two clinical trials combined. Finally, the overall epidemiology of RSV should be accurately considered. For example, the ratio of RSV-A vs. RSV-B-associated ARI in the placebo group was 0.267 in the first season of RCT on RSVpreF and 2.480 in mid-season two. On the contrary, the RSV A/B ratio in mRNA-1345 was the highest for the first series of cases, corresponding to the first RSV season, and the lowest in the supplementary analysis (1.645 vs. 1.325) (Table A7). As RSV cases reported from placebo groups were not influenced by characteristics of RSV vaccines, the aforementioned heterogeneities could be explained by underlying features of RSV epidemiology in the countries where the RCTs were performed.
4.2. Limits and Implications for Future Studies
Even though our study provides a pooled estimate of VE of the available formulates against RSV, being of potential significance for both public health and healthcare professionals, we acknowledge a number of limitations.
Firstly, we applied a strict search strategy on a relatively new topic (i.e., RSV vaccines) that guaranteed the good or very good quality of retrieved studies but resulted in a small number of collected studies. Notably, the very same shortcoming was identified in a similarly designed systematic review with meta-analysis focusing on maternal vaccination [65].
Second, even though most included studies were of appropriate or even of high quality, we gathered data on diverse vaccine strategies, and the variable vaccine type investigated in the retrieved RCTs reasonably introduced high heterogeneity in the results, potentially affecting the final results. Not coincidentally, estimates of the immunogenicity of the assessed vaccines were irregularly provided across the retrieved studies. A summary is provided in Table A8. In this regard, despite the differences in vaccine strategy and heterogeneity in the reporting strategies, all included vaccines were documented as highly immunogenic, at least during the first months after the delivery of the vaccine.
Thirdly, the collected studies were consistently heterogenous not only regarding the inquired vaccine but also in terms of timeframe, geographical settings, sample size, and reporting strategy. This specific topic should be accurately stressed. On the one hand, as summarized in Table A3 and Table A4, for some vaccines (i.e., RSVpreF, mRNA-1345, and Ad26.RSV.preF), VE was calculated regarding the incident ARI and LRTD with two and three or more symptoms [51,52,87,91], while for others (RSVPreF3) a case definition was employed as presenting two mild symptoms plus at least one severe symptom [48,80,81]. On the other hand, the very same nature of the included symptoms was in turn inconsistent across studies. For example, the NCT05035212 (also known as the RENOIR clinical trial) included a total of five symptoms (i.e., cough, wheezing, sputum, shortness of breath, tachypnea), two of which (i.e., cough and sputum) are usually associated with upper respiratory tract infections and are usually the first to appear during the RSV clinical syndrome, irrespective of its eventual severity [5,97,98,99,100]. Other studies (i.e., NCT03982199 [82], NCT02508194 [83], and NCT04886596 [48,80,81]) not only relied on a larger panel of signs and symptoms but also included in the case definition a series of systemic or lower respiratory tract findings. As a consequence, when dealing with LRTD defined by only two findings from a broader range of signs and symptoms, it is more likely to include only upper respiratory symptoms like sputum or cough, while a case definition with three or more findings may be associated with a more severe syndrome, as it would reasonably include signs and symptoms such tachypnea/breath shortness or wheezing. Not coincidentally, the Advisory Committee on Immunization Practices (ACIP) not only recommended both licensed RSV vaccines (RSVpreF and RSVPreF3) [43] but also considered LRTD with two symptoms from NCT04886596 [48,80,81] and LRTD with three or more symptoms from NCT05035212 [52] to be equal endpoints in their rationale.
Fourthly, as stressed by Melgar et al. [43], all available studies were deprived of sufficient statistical power to ascertain the efficacy of the included vaccines in averting RSV-related hospitalizations and deaths in older adults, particularly among adults aged 80 years or older. As previously outlined [20,101,102,103], RSV infections in older adults can result in quite severe and potentially lethal complications, particularly among individuals affected by cardiorespiratory comorbidities [43,104,105]. For example, in their report on the disease severity of RSV compared with COVID-19 and influenza, adults older than 60 years hospitalized because of RSV were more likely to require oxygen therapy, non-invasive ventilation, or admission to the intensive care unit than those hospitalized due to COVID-19 (adjusted odds ratio (aOR) 2.97 95% CI 2.07–4.27, aOR 2.25 95% CI 1.65–3.07, and aOR 1.49, 95% CI 1.13–1.97, respectively) or influenza (aOR 2.07 95% CI 1.37–3.11, aOR 1.99, 95% CI 1.36–2.90, and aOR 1.55, 95% CI 1.11–2.19, respectively) [106]. Similarly, a study from 25 hospitals in France on a total of 1168 adults and elderly individuals hospitalized for RSV infections from 1 January 2015 to 31 December 2019 reported a 25% rate of ICU admissions [107]. In this regard, another limit of the present study is related to the reported prevalence of comorbidities included in parent studies. In fact, there is considerable evidence that obesity, hypertension, chronic heart failure, COPD, and chronic respiratory failure are associated with an increased risk of ICU admission due to RSV infections [107]. Still, data on congestive heart disease, COPD, and asthma from the retrieved studies [48,51,52,82] hinted at a relatively lower rate of these comorbidities (6.65%, 1.60%, and 9.21%, respectively) compared to the general populations of the European Union and United States (i.e., the economic areas where RSV have been initially licensed and may be considered particularly suitable because of the high prevalence of older adults), particularly for COPD. For example, in 2019, an estimated worldwide COPD mean prevalence was identified as 13.1% (10.2–15.6%), with the following distribution by continents: Europe, 12.4% (8.8–16.0%); Africa, 13.9% (12.0–15.9%); America, 13.2% (10.5–15.9%); Asia, 13.5% (10.0–16.0%); and Oceania, 11.6% (9.8–13.1%) [108].
Fifthly, even though adults aged 80 years or older are likely to benefit the most from RSV vaccines [20,28,104], this age group was substantially under-represented in all of the assessed studies. Therefore, the potential role of RSV vaccines from a public health point of view should be carefully evaluated, particularly when tailoring potential strategies for age groups at higher risk for RSV complications [43].
Sixthly, our data should be reconciled with clinical features and diagnostic options of RSV infection. On the one hand, none of the included RCTs performed an active case search for incident infections but rather targeted symptomatic ones. On the other hand, even though RT-qPCR has been often considered the “gold standard” for the diagnosis of viral respiratory infections [109,110,111], the strategy for the collection of testing specimens could fail to achieve an appropriate diagnosis in accordance with the stage of the assessed infection [112,113]. More precisely, in severe cases associated with lower respiratory tract infections, RT-qPCR testing based on nasal swabs may result in false negative results, as the viral infection is more likely to be active in the lower regions of the respiratory tract [113]. In these cases, the addiction of sputum, paired serology, and oropharyngeal swabs would radically improve the diagnostic efficacy (+52%, + 44%, and +28%, respectively) [113], but none of the included RCTs actually implemented the aforementioned options. In other words, we cannot rule out that point estimates for VE, and the resulting pooling VE estimates, would been inflated by false negative RSV cases.
Finally, when addressing the potential use of RSV vaccines in real-world settings, not only VE but also the safety profile of interventions should be considered. In our study, we deliberately focused on VE. Even though all RCTs included in the study hinted at an acceptable safety profile [48,51,52,80,81,83,87,89,91], early reports from maternal vaccination [114,115,116] highlighted an increased risk for preterm birth, suggesting a previously unexpected health impact. The vaccines included in maternal studies were RSVpreF and RSVPreF3 in the very same formulate for adult vaccination, and therefore a continued focus on balancing the risks and potential benefits will be essential in tailoring future vaccination strategies.
5. Conclusions
RSV vaccination of older adults has been proven to be effective in preventing LRTD cases, particularly when considering more severe cases with three or more signs or symptoms. However, the limited statistical power of the included studies on hospitalizations and severe complications, including deaths, the lack of complete and fully comparable follow-up seasons for all of the retrieved studies, and the failure to adopt a uniform and appropriate case definition across studies, altogether stress the importance of future and improved reporting from ongoing RCTs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12050500/s1, Table S1: PRISMA checklist.
Author Contributions
Conceptualization, M.R., F.M. and P.M.; methodology, M.R., F.M. and R.G.; software, M.R. and S.C. validation, D.G., A.C., R.G. and P.G.G.; formal analysis, M.R., D.G., M.B. and R.G., investigation, S.C. and M.B.; resources, F.M.; data curation, M.R., D.G., R.G. and M.B. writing—original draft preparation, M.R., P.M. and A.C.; writing—review and editing, P.G.G., A.C., P.M., R.G. and M.B., visualization M.R.; supervision, M.R., A.C. and P.M. project administration, M.R., A.C. and P.M, funding acquisition, M.B. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
PECO worksheet [68,69].
Table A1.
PECO worksheet [68,69].
| Item | Definition |
|---|---|
| Population of interest | Adults aged 60 years or older having received at least one dose of any respiratory syncytial virus vaccine |
| Exposure | Exposed to RSV infection during the subsequent RSV season |
| Control/comparator | Adults aged 60 years or older not vaccinated against respiratory syncytial virus (placebo) |
| Outcome | Occurrence of lower respiratory tract disease and/or acute respiratory infection |

Table A2.
Search strategy and number of retrieved entries.
Table A2.
Search strategy and number of retrieved entries.
| Database | Keywords | No. of Entries |
|---|---|---|
| Pubmed | (“Vaccination”[MeSH Terms] OR (“vaccine”[Supplementary Concept] OR “vaccine”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR “vaccinable”[All Fields] OR “vaccinal”[All Fields] OR “vaccinate”[All Fields] OR “vaccinated”[All Fields] OR “vaccinates”[All Fields] OR “vaccinating”[All Fields] OR “vaccinations”[All Fields] OR “vaccination s”[All Fields] OR “vaccinator”[All Fields] OR “vaccinators”[All Fields] OR “vaccine s”[All Fields] OR “vaccined”[All Fields] OR “vaccines”[MeSH Terms] OR “vaccines”[All Fields] OR “vaccine”[All Fields] OR “vaccines”[All Fields] OR (“Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“immunization”[All Fields] AND “active”[All Fields]) OR “immunization active”[All Fields]) OR (“active immunisation”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“active”[All Fields] AND “immunization”[All Fields]) OR “active immunization”[All Fields]) OR (“active immunisations”[All Fields] OR “Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“active”[All Fields] AND “immunizations”[All Fields]) OR “active immunizations”[All Fields]) OR (“Vaccination”[MeSH Terms] OR “Vaccination”[All Fields] OR (“immunizations”[All Fields] AND “active”[All Fields]) OR “immunizations active”[All Fields]))) AND (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “virus”[All Fields]) OR “respiratory syncytial virus”[All Fields] OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“syncytial”[All Fields] AND “virus”[All Fields] AND “respiratory”[All Fields]) OR “syncytial virus respiratory”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“syncytial”[All Fields] AND “viruses”[All Fields] AND “respiratory”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“virus”[All Fields] AND “respiratory”[All Fields] AND “syncytial”[All Fields]) OR “virus respiratory syncytial”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“viruses”[All Fields] AND “respiratory”[All Fields] AND “syncytial”[All Fields]) OR “viruses respiratory syncytial”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“chimpanzee”[All Fields] AND “coryza”[All Fields] AND “agent”[All Fields]) OR “chimpanzee coryza agent”[All Fields]) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“chimpanzee”[All Fields] AND “coryza”[All Fields] AND “agents”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“coryza”[All Fields] AND “agent”[All Fields] AND “chimpanzee”[All Fields])) OR (“Respiratory Syncytial Viruses”[MeSH Terms] OR (“respiratory”[All Fields] AND “syncytial”[All Fields] AND “viruses”[All Fields]) OR “Respiratory Syncytial Viruses”[All Fields] OR (“coryza”[All Fields] AND “agents”[All Fields] AND “chimpanzee”[All Fields])))) Filter: aged: 65+ years, 80 and older: 80+ years | 432 |
| EMBASE | (“pneumovirus’/exp” OR “pneumovirus” OR “pneumovirus infection” OR “human respiratory syncytial virus” OR “respiratory syncytial virus infection”) AND (“immunization” OR “vaccination”) Filter: aged: 65+ years, 80 and older: 80+ years | 344 |
| SCOPUS | (vaccination* OR immunization*) AND (“respiratory syncytial virus” OR “RSV” OR “bronchiolitis”) AND (adult* OR elderly OR elder*) Filter: adults | 721 |
| medRxiv | (respiratory syncytial virus OR RSV) AND (vaccine OR vaccination OR immunization) | 599 |

Table A3.
Characteristics of vaccines against respiratory syncytial virus (RSV) that were included in the present systematic review with meta-analysis.
Table A3.
Characteristics of vaccines against respiratory syncytial virus (RSV) that were included in the present systematic review with meta-analysis.
| Vaccine | Characteristics | Approved | Clinical Trial | Reference |
|---|---|---|---|---|
| RSVPreF3 | Subunit A stabilized prefusion F protein (120 μg); monovalent; Adjuvant: AS01 Single dose | FDA, EMA | NCT04886596 | [48,80,81] |
| RSVpreF | Bivalent (RSV A and B) stabilized prefusion F protein subunit (60 μg + 60 μg); Not adjuvated Single dose | FDA, EMA | NCT04886596 | [52,61] |
| Ad26.RSV.preF | Adenovirus serotype 26 vector-based RSV vaccine encoding prefusion F protein Single dose Monovalent | Ongoing | NCT03982199 | [82] |
| mRNA-1345 | Single mRNA sequence encoding for a stabilized prefusion F glycoprotein (50 µg) Single dose Monovalent | Ongoing | [51,85] | |
| MEDI7510 | RSV soluble fusion protein F antigen plus glucopyranosyl lipid A in stable emulsion (GLA-SE) adjuvantSingle dose (120 μg) Monovalent | Discontinued | NCT02508194 | [83] |

Table A4.
Summary of case definitions.
Table A4.
Summary of case definitions.
| Clinical Trial (Vaccine) | |||||
|---|---|---|---|---|---|
| NCT03982199 (Ad26.RSV.preF) [82] | NCT02508194 (MEDI7510) [83] | NCT04886596 (RSVPreF3) [48,80,81] | NCT05035212 (RSVpreF) [52,61] | NCT04886596 (mRNA-1345) [51,85] | |
| Signs/Symptoms | |||||
| Cough | Any | Any | New or increased | New or increased | Any |
| Shortness of breath | Any | Alternative with dyspnea | - | New or increased | Any |
| Dyspnea | Alternative with decreased oxygen saturation | Alternative with shortness of breath | New or increased | - | - |
| Decreased oxygen saturation | Alternative with dyspnea | - | Any | - | Any |
| Oxygen requirement | - | - | Any | - | - |
| Wheezing | Any | Any | Any | New or increased | Any |
| Rales/Ronchi | Any | - | Alternative with crackles | - | Any |
| Crackles | - | - | Alternative with Rales/Ronchi | - | Any |
| Sputum production | Any | Any | New or increased | New or increased | Any |
| Tachypnea | Any | - | Any | Any | Any |
| Headache | Any | ||||
| Myalgias or Arthralgias | - | Any | Body aches | - | - |
| Malaise | Any | - | - | - | - |
| Fatigue | Any | Any | - | - | - |
| Fever (>37.8 °C) | Any | - | - | - | - |
| Feverishness | Any | Any | - | - | - |
| Pleuritic chest pain | - | - | - | - | Lasting ≥24 h |
| Radiologic evidence of pneumonia | - | - | - | - | Any |
| Case Definition | |||||
| Low Respiratory Tract Disease | Symptoms Shortness of breath Dyspnea or decreased oxygen saturation Cough Wheezing Rales and/or Rhonchi Sputum production Tachypnea Systemic symptoms Malaise Fatigue Fever 1 Feverishness 2 | Symptoms Dyspnea or shortness of breath Cough Wheezing Sputum production Systemic symptoms Myalgias or arthralgias Body aches Fatigue (tiredness) Headache Decreased appetite Feverishness | Symptoms Dyspnea (new or increased) Cough (new or increased) Sputum production (new or increased) Signs Wheezing (new or increased) Crackles and/or Rhonchi (new or increased) Tachypnea Decreased oxygen saturation Oxygen requirement | Shortness of breath (new or increased) Cough (new or increased) Wheezing (new or increased) Sputum production (new or increased) Tachypnea | Shortness of breath Cough Fever Wheezing Rales and/or Rhonchi Sputum production Tachypnea Hypoxemia Pleuritic chest pain ≥ 24 h Radiologic evidence of pneumonia |
1 = body temperature > 37.8 °C; 2 = feeling feverishness and having fever were considered 1 symptom.

Table A5.
Demographic characteristics of the primary studies on respiratory syncytial virus (RSV) vaccine.
Table A5.
Demographic characteristics of the primary studies on respiratory syncytial virus (RSV) vaccine.
| Study | Vaccinated (N.) | Placebo (N.) | Males | Age ≥70/75 years | White Ethnicity | |||
|---|---|---|---|---|---|---|---|---|
| Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | |||
| Ison et al. [48] | 12,470 | 12,503 | 5981 (47.96%) | 6074 (48.58%) | 5507 (44.16%) | 5521 (44.16%) | 9890 (79.31%) | 9936 (79.47%) |
| Papi et al. [81] | 12,466 | 12,494 | 5978 (47.95%) | 6067 (48.56%) | 5504 (44.15%) | 5519 (44.17%) | 9887 (79.31%) | 9932 (79.49%) |
| Feldman et al. [80] | 12,467 | 12,499 | 5979 (47.96%) | 6072 (48.58%) | 5504 (44.15%) | 5519 (44.16%) | 9987 (79.31%) | 9932 (79.46%) |
| Walsh et al. [52] | 17,215 | 17,069 | 8800 (51.12%) | 8601 (50.39%) | 6458 (37.51%) | 6389 (37.43%) | 13,475 (78.27%) | 13,360 (78.27%) |
| Wilson et al. [51] | 17,734 | 17,679 | 8974 (51.07%) | 8875 (50.67%) | 6453 (36.72%) | 6457 (36.86%) | 11,240 (63.97%) | 11,216 (64.03%) |
| Falsey et al. [82] | 2891 | 2891 | 1251 (43.27%) | 1197 (41.40%) | 765 (26.46%) | 759 (26.25%) | 2658 (91.94%) | 2690 (93.05%) |
| Falloon et al. [83] | 949 | 951 | 380 (41.90%) | 309 (34.45%) | 133 (14.66% | 135 (15.05%) | 771 (85.01%) | 773 (86.16%) |

Table A6.
Main comorbidities from primary studies on respiratory syncytial virus (RSV) vaccine. Note = COPD, chronic obstructive pulmonary disease.
Table A6.
Main comorbidities from primary studies on respiratory syncytial virus (RSV) vaccine. Note = COPD, chronic obstructive pulmonary disease.
| Study | Vaccinated (No.) | Placebo (No.) | COPD | Asthma | Congestive Hearth Disease | |||
|---|---|---|---|---|---|---|---|---|
| Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | Vaccinated (n./N., %) | Placebo (n./N., %) | |||
| Ison et al. [48] | 12,470 | 12,503 | 1131 (9.07%) | 1113 (8.90%) | 1193 (9.57%) | 1113 (8.90%) | 398 (3.19%) | 403 (3.22%) |
| Walsh et al. [52] | 17,215 | 17,069 | 960 (5.46%) | 978 (5.58%) | - | - | 53 (0.30 %) | 51 (0.29%) |
| Wilson et al. [51] | 17,734 | 17,679 | 1012 (5.88%) | 1080 (6.33%) | 1541 (8.95%) | 1508 (8.83%) | 293 (1.70%) | 307 (1.80%) |
| Falsey et al. [82] | 2891 | 2891 | 219 (7.58%) | 208 (7.19%) | 266 (9.20%) | 250 (8.65%) | 58 (2.01%) | 54 (1.87%) |

Table A7.
Occurrence of respiratory syncytial virus (RSV) A and RSV B infections in cases of acute respiratory infection (ARI) and lower respiratory tract disease with 3 or more signs/symptoms (LRTD 3+) among subjects having received a placebo during RCT in the primary season (1st) or during the follow-up (FU).
Table A7.
Occurrence of respiratory syncytial virus (RSV) A and RSV B infections in cases of acute respiratory infection (ARI) and lower respiratory tract disease with 3 or more signs/symptoms (LRTD 3+) among subjects having received a placebo during RCT in the primary season (1st) or during the follow-up (FU).
| Vaccine | Placebo (N.) | RSV A (n./N., %) | RSV B (n./N., %) | Ratio RSV A/B | ||||
|---|---|---|---|---|---|---|---|---|
| Season | ARI | LRTD 3+ | ARI | LRTD 3+ | ARI | LRTD 3+ | ||
| mRNA-1345 | 1st | 17,516 | 51 (0.29) | 10 (0.06) | 31 (0.18) | 7 (0.04) | 1.645 | 1.429 |
| FU | 18,045 | 106 (0.59) | 30 (0.17) | 80 (0.44) | 22 (0.12) | 1.325 | 1.364 | |
| RSVpreF | 1st | 17,069 | 12 (0.07) | 3 (0.02) | 45 (0.26) | 10 (0.06) | 0.267 | 0.300 |
| FU | 9992 | 62 (0.62) | 10 (0.10) | 25 (0.25) | 3 (0.03) | 2.480 | 3.333 | |
| RSVPreF3 | 1st | 12,494 | 32 (0.26) | 13 (0.10) | 61 (0.49) | 26 (0.21) | 0.525 | 0.500 |
| FU | 4991 | - | 34 (0.68) | - | 57 (1.14) | - | 0.596 | |

Table A8.
Summary of geometric mean increase (GMI) estimates for antibodies targeting respiratory syncytial virus (RSV) of group A and B from included studies. If the immunogenicity of reported vaccines was provided in a validation study, the latter was included in the reported values.
Table A8.
Summary of geometric mean increase (GMI) estimates for antibodies targeting respiratory syncytial virus (RSV) of group A and B from included studies. If the immunogenicity of reported vaccines was provided in a validation study, the latter was included in the reported values.
| Study | Vaccine | Day | GMI RSV A | GMI RSV B |
|---|---|---|---|---|
| Feldman et al. [80] | RSVPreF3 | 31 | 9.8 Range: 8.9–10.6 | 8.3 Range: 8.1–10.2 |
| Falsey et al. [82] | Ad26.RSV.preF | 15 | 12.1 | 9.4 |
| Papi et al. [81] | RSVPreF3 | 30 | 10.2 Range: 9.5–11 | 8.6 Range: 8.0–9.2 |
| Walsh et al. [52] (from Baber et al. [117]) | RSVpreF | 30 | 10.2 | 12.3 |
| Wilson et al. [51] (from Chen et al.) [118] | mRNA-1345 | 30 | Range: 12.1–16.6 | Range: 8.7–12.6 |
| Falloon et al. [83] | MEDI7510 | End of season | 4.6 Range: 4.3–4.9 | |
| Wilson et al. [85] | mRNA-1345 | 30 | 10.1 | 6.4 |
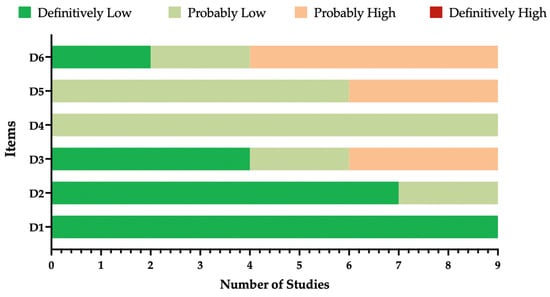
Figure A1.
Summary of the risk of bias (ROB) estimates for observational studies [74,84]. Analyses were performed according to the National Toxicology Program (NTP)’s Office of Health Assessment and Translation (OHAT) handbook and respective risk of bias (ROB) tool with all retrieved studies (n = 9).
Figure A1.
Summary of the risk of bias (ROB) estimates for observational studies [74,84]. Analyses were performed according to the National Toxicology Program (NTP)’s Office of Health Assessment and Translation (OHAT) handbook and respective risk of bias (ROB) tool with all retrieved studies (n = 9).
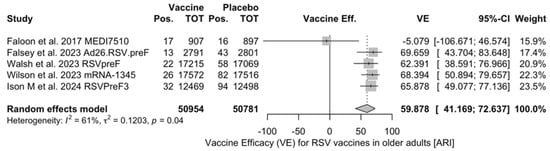
Figure A2.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with acute respiratory illness (ARI) (I2 60.6%, 95% CI 0.0 to 85.2; tau2 0.120, 95% CI 0.0 to 2.127; Q = 10.15, p = 0.038) [48,51,52,82,83].
Figure A2.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with acute respiratory illness (ARI) (I2 60.6%, 95% CI 0.0 to 85.2; tau2 0.120, 95% CI 0.0 to 2.127; Q = 10.15, p = 0.038) [48,51,52,82,83].
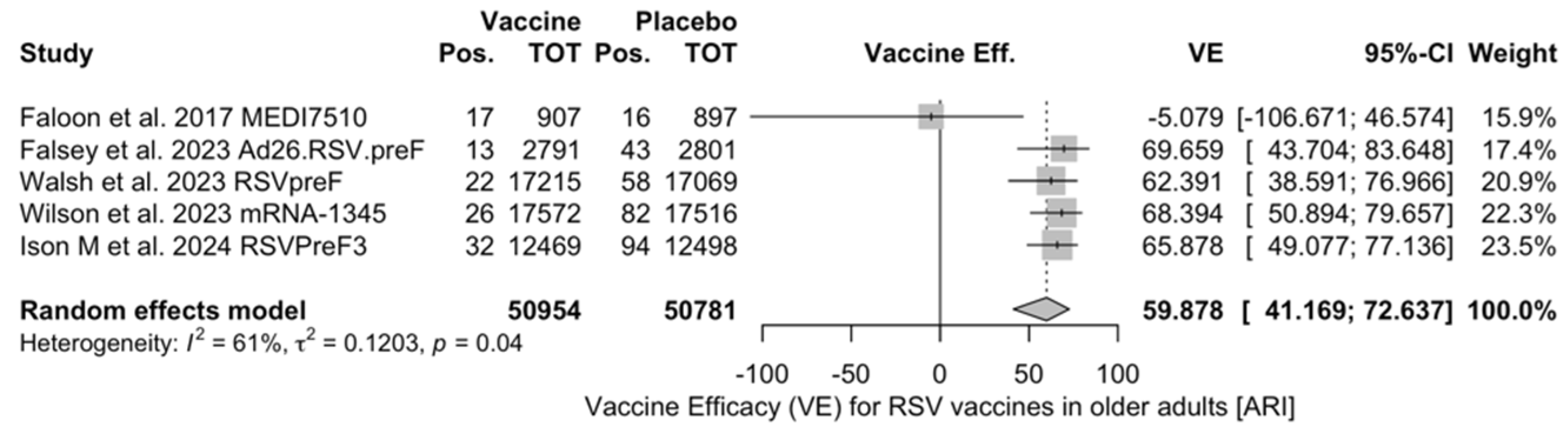
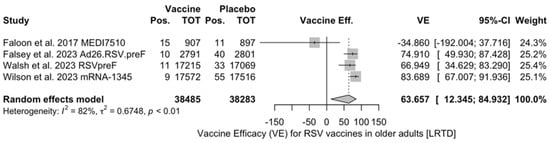
Figure A3.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease with 2 signs/symptoms (I2 82.5%, 95% CI 55.0 to 93.2; tau2 0.675, 95% CI 0.122 to 11.471; Q = 17.14, p < 0.001) [51,52,82,83].
Figure A3.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease with 2 signs/symptoms (I2 82.5%, 95% CI 55.0 to 93.2; tau2 0.675, 95% CI 0.122 to 11.471; Q = 17.14, p < 0.001) [51,52,82,83].
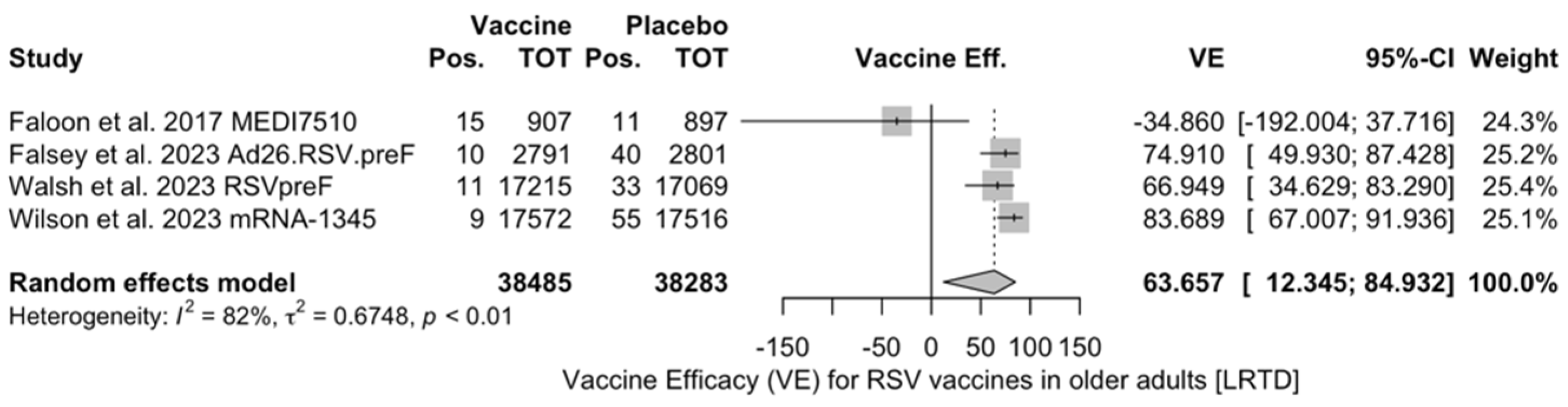
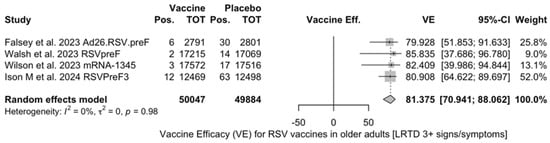
Figure A4.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract diseases with 3 signs/symptoms or more (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0; Q = 0.17, p = 0.982) [48,51,52,82].
Figure A4.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract diseases with 3 signs/symptoms or more (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0; Q = 0.17, p = 0.982) [48,51,52,82].
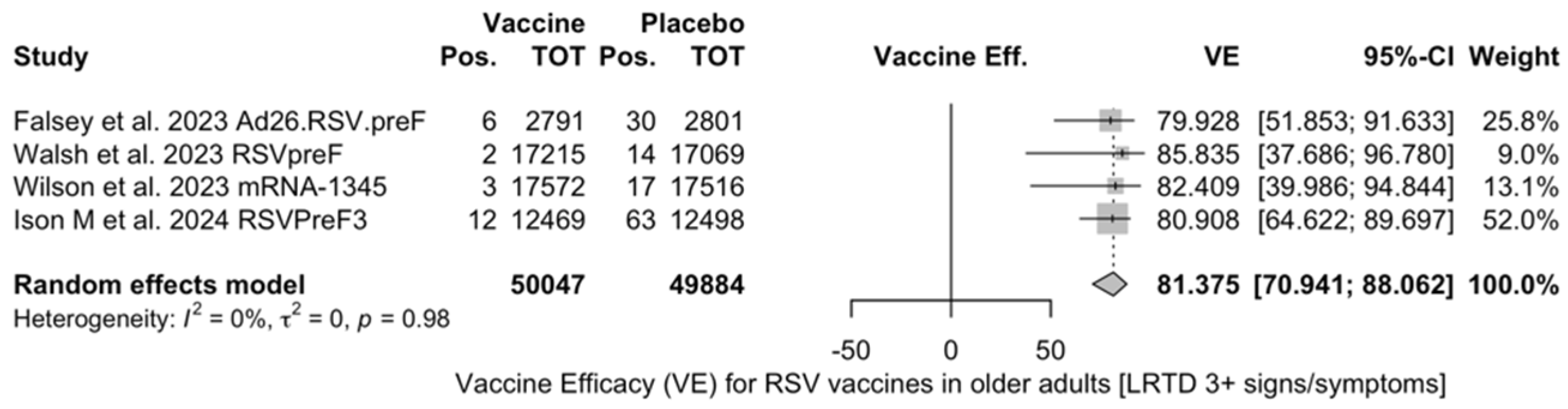
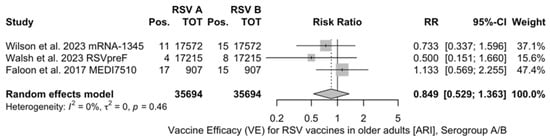
Figure A5.
Risk ratio for respiratory syncytial virus (RSV) acute respiratory infections from serogroup A vs. serogroup B in older adults (age ≥ 60 years) (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 2.523; Q = 1.56, p = 0.458) [51,52,83].
Figure A5.
Risk ratio for respiratory syncytial virus (RSV) acute respiratory infections from serogroup A vs. serogroup B in older adults (age ≥ 60 years) (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 2.523; Q = 1.56, p = 0.458) [51,52,83].
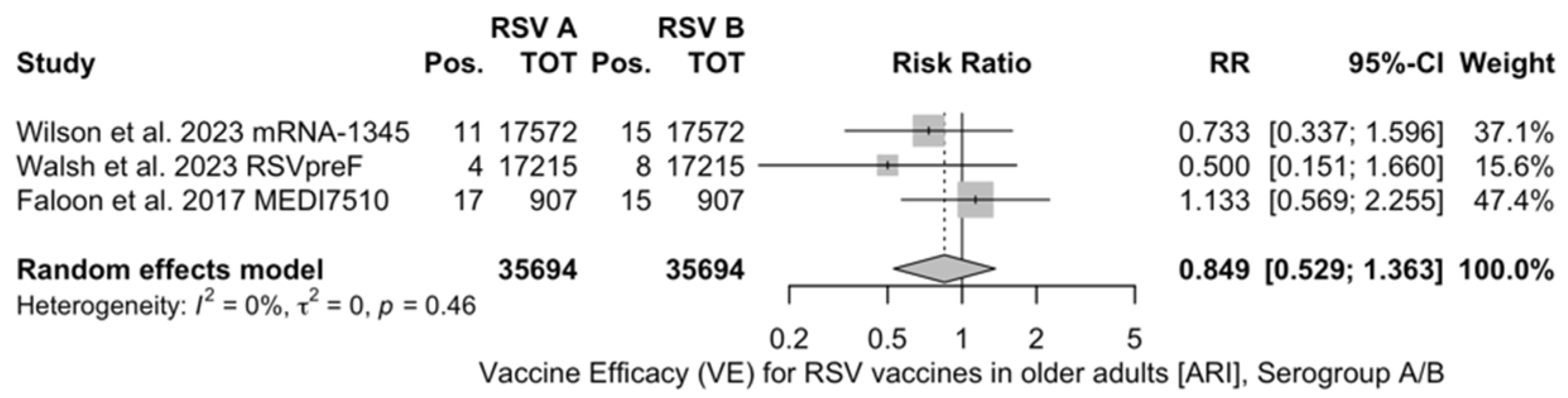
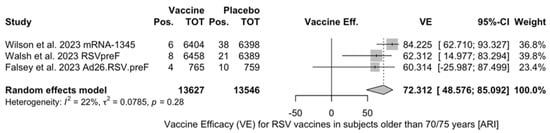
Figure A6.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection (ARI, I2 22.1%, 95% CI 0.0 to 91.9; tau2 0.079, 95% CI 0.0 to 10.349; Q = 2.57, p = 0.277) [51,52,82].
Figure A6.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection (ARI, I2 22.1%, 95% CI 0.0 to 91.9; tau2 0.079, 95% CI 0.0 to 10.349; Q = 2.57, p = 0.277) [51,52,82].
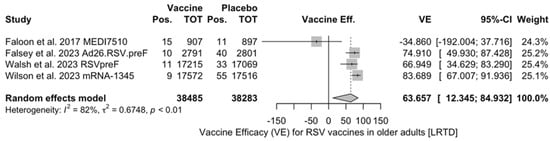
Figure A7.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with low respiratory tract disease (LRTD) with 2 or signs/symptoms (I2 82.5%, 95% CI 55.0 to 93.2; tau2 0.675, 95% CI 0.122 to 11.471; Q = 17.14, p < 0.001) [51,52,82,83].
Figure A7.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with low respiratory tract disease (LRTD) with 2 or signs/symptoms (I2 82.5%, 95% CI 55.0 to 93.2; tau2 0.675, 95% CI 0.122 to 11.471; Q = 17.14, p < 0.001) [51,52,82,83].
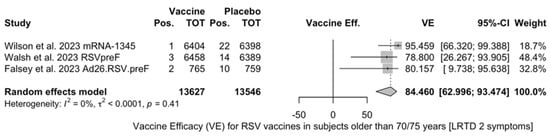
Figure A8.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 2 signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 29.114; Q = 1.79, p = 0.409) [51,52,82].
Figure A8.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 2 signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 29.114; Q = 1.79, p = 0.409) [51,52,82].
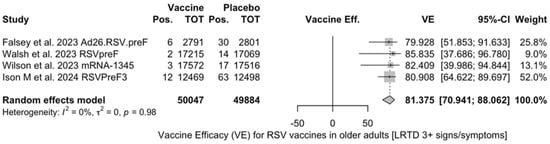
Figure A9.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0; Q = 0.17, p = 0.982) [48,51,52,82].
Figure A9.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0; Q = 0.17, p = 0.982) [48,51,52,82].
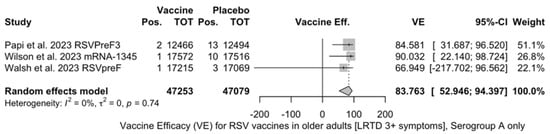
Figure A10.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup A with low respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 13.311; Q = 0.60, p = 0.741) [51,52,81].
Figure A10.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup A with low respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 13.311; Q = 0.60, p = 0.741) [51,52,81].
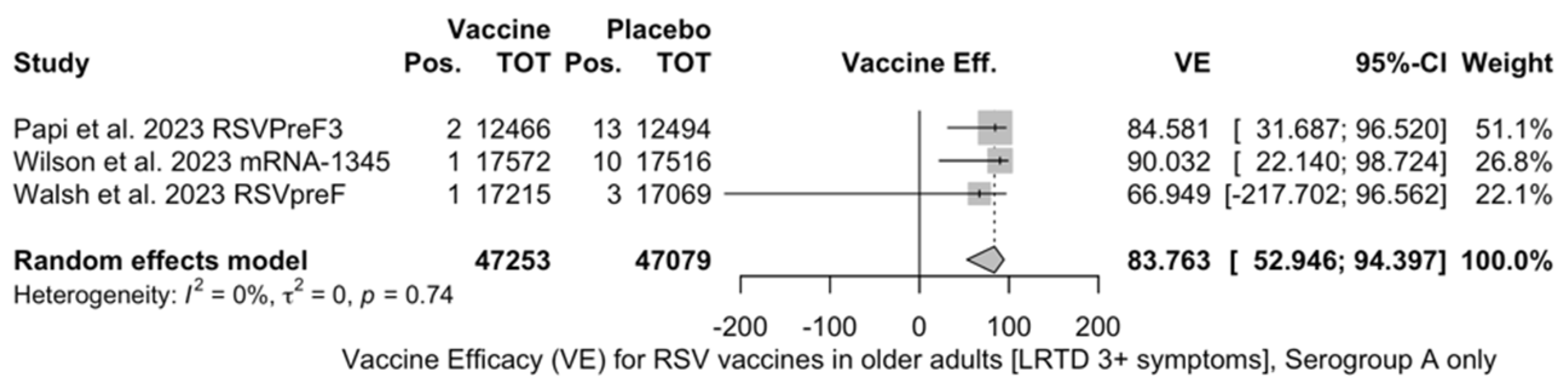
Figure A11.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup B with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 10.341; Q = 0.64, p = 0.727) [51,52,81].
Figure A11.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup B with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 10.341; Q = 0.64, p = 0.727) [51,52,81].
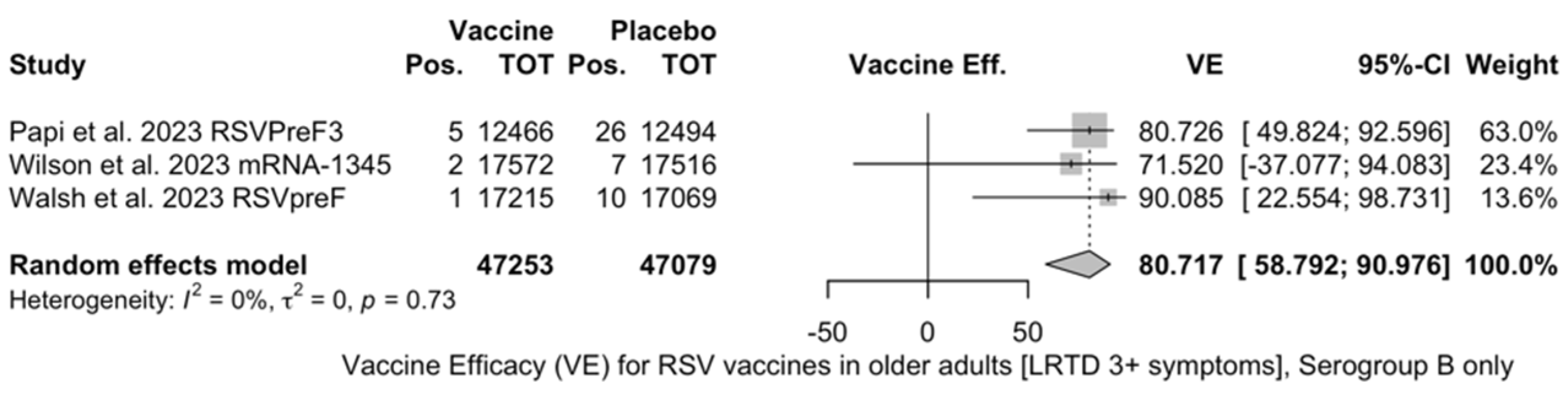
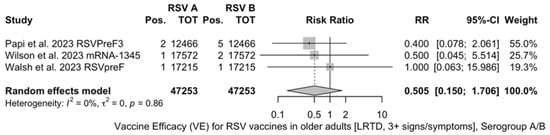
Figure A12.
Risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms in serogroup A vs. serogroup B (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 7.469; Q = 0.31, p = 0.856) [51,52,81].
Figure A12.
Risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms in serogroup A vs. serogroup B (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 7.469; Q = 0.31, p = 0.856) [51,52,81].
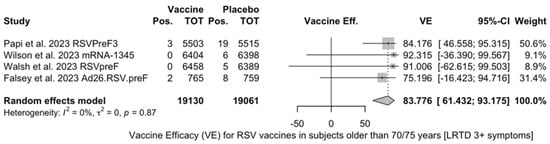
Figure A13.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0, 95% CI 0.0 to 2.732; Q = 0.71, p = 0.871) [51,52,81].
Figure A13.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms (I2 0.0%, 95% CI 0.0 to 84.7; tau2 0.0, 95% CI 0.0 to 2.732; Q = 0.71, p = 0.871) [51,52,81].
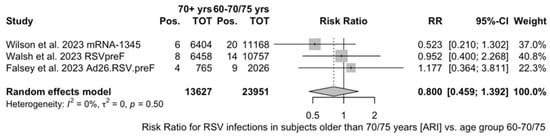
Figure A14.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0.0%; 95% CI 0.0 to 89.6; tau2 = 0, 95% CI 0.000; 6.721, Q 1.40, p = 0.496) with acute respiratory infection (ARI) [51,52,82].
Figure A14.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0.0%; 95% CI 0.0 to 89.6; tau2 = 0, 95% CI 0.000; 6.721, Q 1.40, p = 0.496) with acute respiratory infection (ARI) [51,52,82].
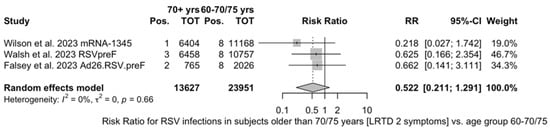
Figure A15.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0 0.0%, 95% CI 0.0 to 89.6%; tau2 = 0.0, 95% CI 0.000 to 14.509; Q 0.84, p = 0.657) with lower respiratory tract disease (LRTD) with 2 signs/symptoms [51,52,82].
Figure A15.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0 0.0%, 95% CI 0.0 to 89.6%; tau2 = 0.0, 95% CI 0.000 to 14.509; Q 0.84, p = 0.657) with lower respiratory tract disease (LRTD) with 2 signs/symptoms [51,52,82].
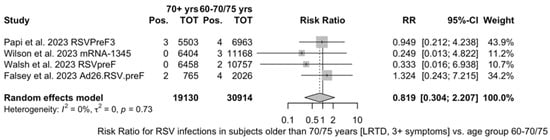
Figure A16.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0.0%, 95% CI 0.0 to 84.7, tau2 = 0.0, 95% CI 0.000 to 7.504; Q 1.30, p = 0.729) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms [51,52,81,82].
Figure A16.
Risk ratio for breakthrough respiratory syncytial virus (RSV) infections in vaccinated individuals older than 70 years (I2 0.0%, 95% CI 0.0 to 84.7, tau2 = 0.0, 95% CI 0.000 to 7.504; Q 1.30, p = 0.729) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms [51,52,81,82].
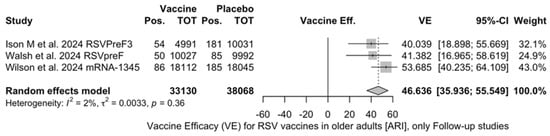
Figure A17.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with acute respiratory illness (ARI) in follow-up studies (I2 1.8%, 95% CI 0.0 to 89.8; tau2 0.003, 95% CI 0.0 to 0.788; Q = 2.04, p = 0.361) [48,51,52].
Figure A17.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with acute respiratory illness (ARI) in follow-up studies (I2 1.8%, 95% CI 0.0 to 89.8; tau2 0.003, 95% CI 0.0 to 0.788; Q = 2.04, p = 0.361) [48,51,52].
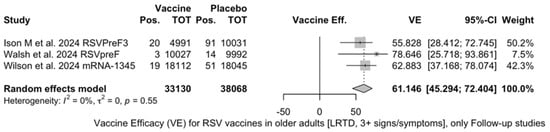
Figure A18.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.003, 95% CI 0.0 to 5.411; Q = 1.19, p = 0.553) [48,61,85].
Figure A18.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) with lower respiratory tract disease (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.003, 95% CI 0.0 to 5.411; Q = 1.19, p = 0.553) [48,61,85].
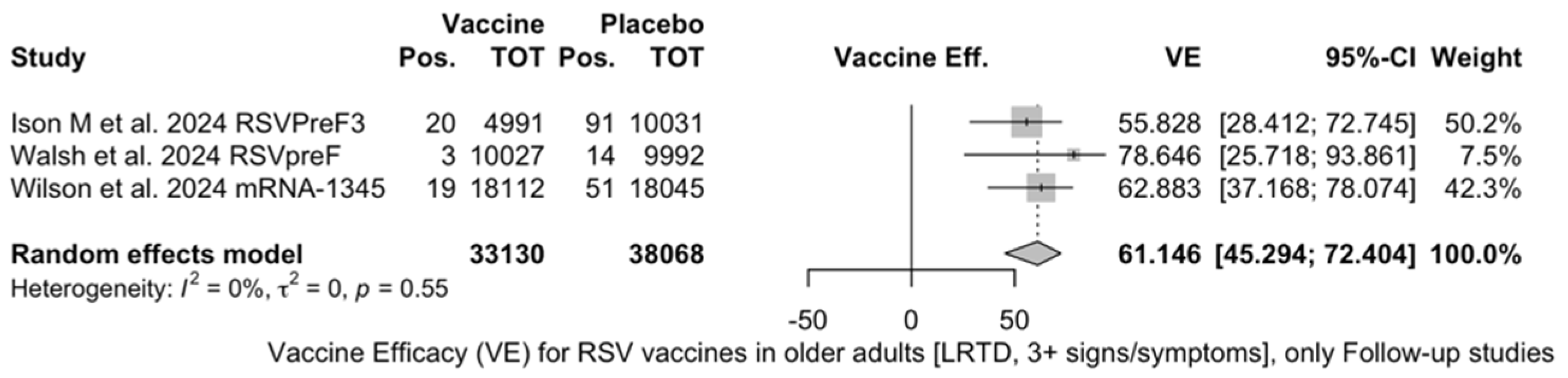
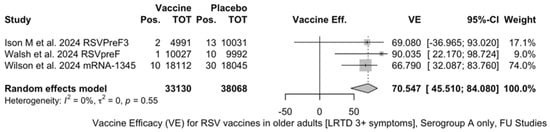
Figure A19.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup A with lower respiratory tract diseases (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 17.178; Q = 1.18, p = 0.554) [48,61,85].
Figure A19.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup A with lower respiratory tract diseases (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 17.178; Q = 1.18, p = 0.554) [48,61,85].
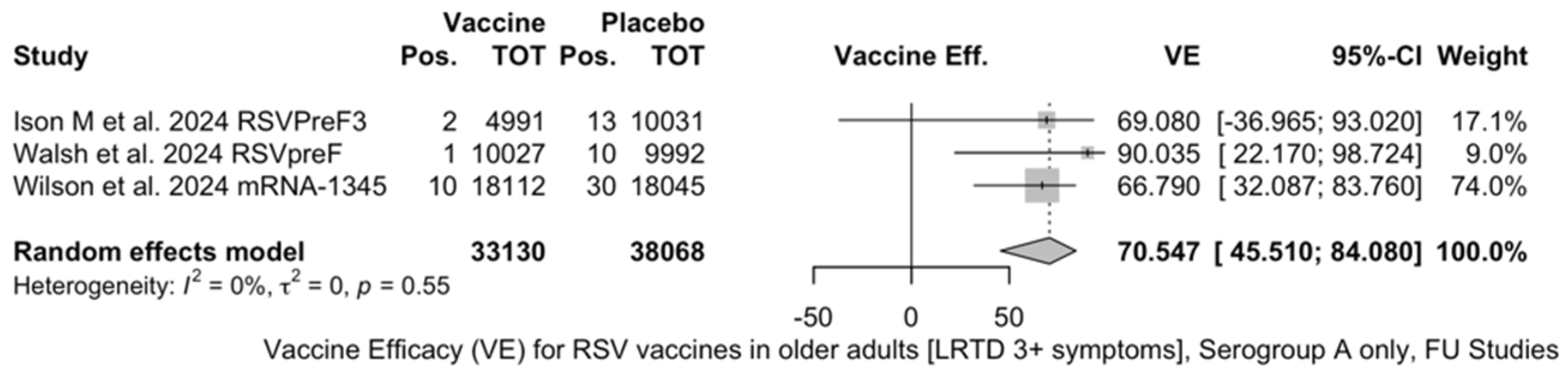
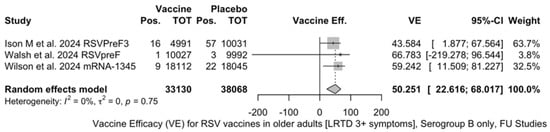
Figure A20.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup B with lower respiratory tract disease (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 2.270; Q = 0.57, p = 0.750) [51,52,82].
Figure A20.
Vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 60 years) only in serogroup B with lower respiratory tract disease (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0.0, 95% CI 0.0 to 2.270; Q = 0.57, p = 0.750) [51,52,82].
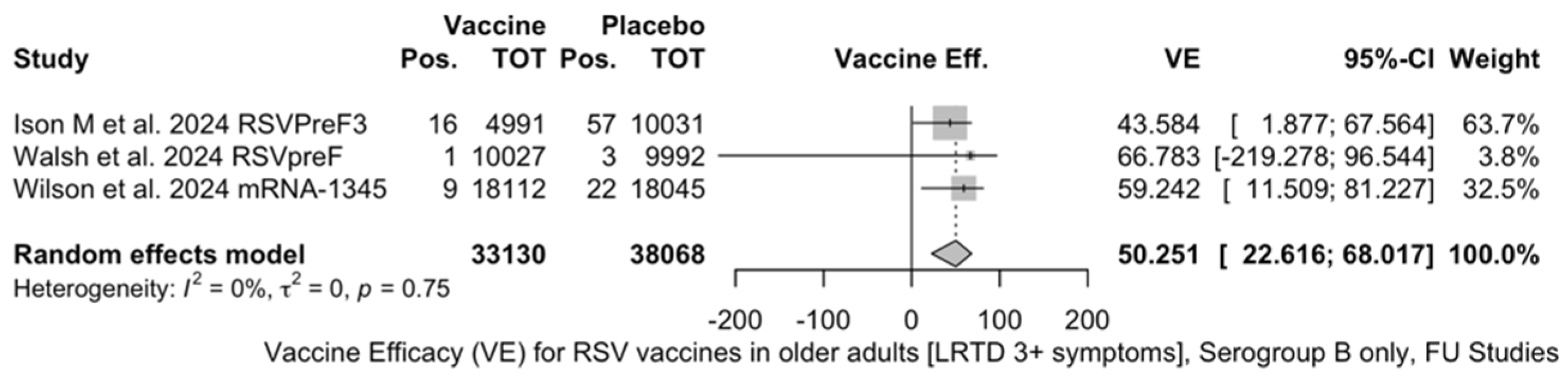
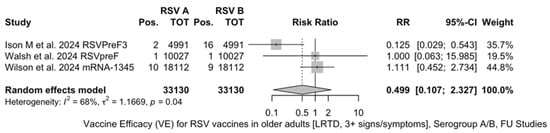
Figure A21.
Risk ratio for respiratory syncytial virus (RSV) infections in older adults (age ≥ 60 years) in serogroup A vs. serogroup B with lower respiratory tract diseases (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 1.167, 95% CI 0.0 to 59.266; Q = 6.29, p = 0.043) [48,61,85].
Figure A21.
Risk ratio for respiratory syncytial virus (RSV) infections in older adults (age ≥ 60 years) in serogroup A vs. serogroup B with lower respiratory tract diseases (LRTD) with 3 signs/symptoms or more in follow-up studies (I2 0.0%, 95% CI 0.0 to 89.6; tau2 1.167, 95% CI 0.0 to 59.266; Q = 6.29, p = 0.043) [48,61,85].
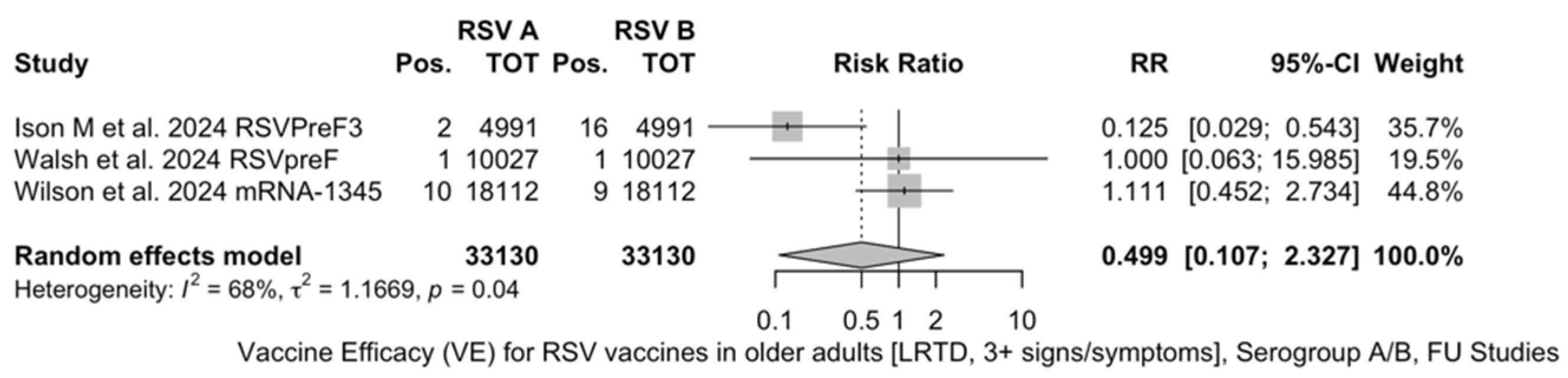
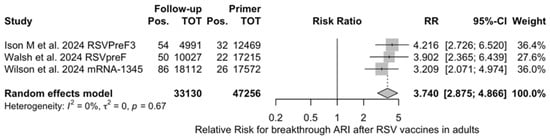
Figure A22.
Risk ratio for breakthrough acute respiratory infection (ARI) after the delivery of respiratory syncytial virus (RSV) vaccine (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0, 95% CI 0.0 to 0.731; Q = 0.79, p = 0.675) in follow up studies [48,61,85].
Figure A22.
Risk ratio for breakthrough acute respiratory infection (ARI) after the delivery of respiratory syncytial virus (RSV) vaccine (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0, 95% CI 0.0 to 0.731; Q = 0.79, p = 0.675) in follow up studies [48,61,85].
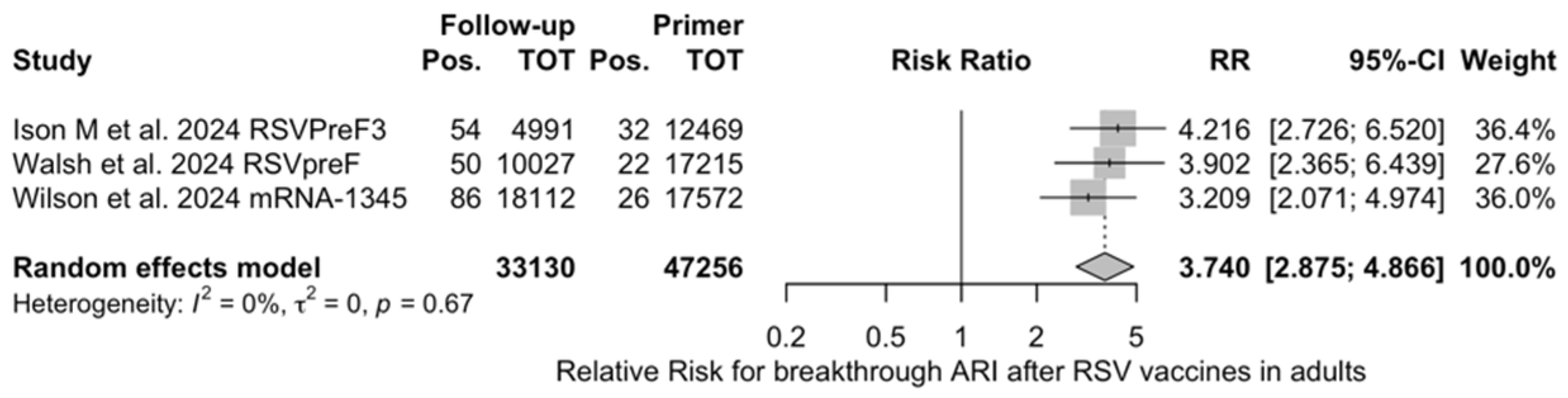
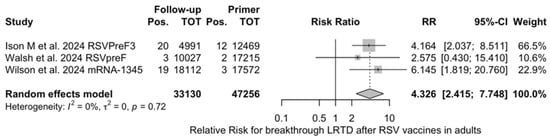
Figure A23.
Risk ratio for breakthrough lower respiratory tract disease (LRTD) with 3 or more signs/symptoms after the delivery of respiratory syncytial virus (RSV) vaccine (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0, 95% CI 0.0 to 6.872; Q = 0.65, p = 0.721) in follow up studies [48,61,85].
Figure A23.
Risk ratio for breakthrough lower respiratory tract disease (LRTD) with 3 or more signs/symptoms after the delivery of respiratory syncytial virus (RSV) vaccine (I2 0.0%, 95% CI 0.0 to 89.6; tau2 0, 95% CI 0.0 to 6.872; Q = 0.65, p = 0.721) in follow up studies [48,61,85].
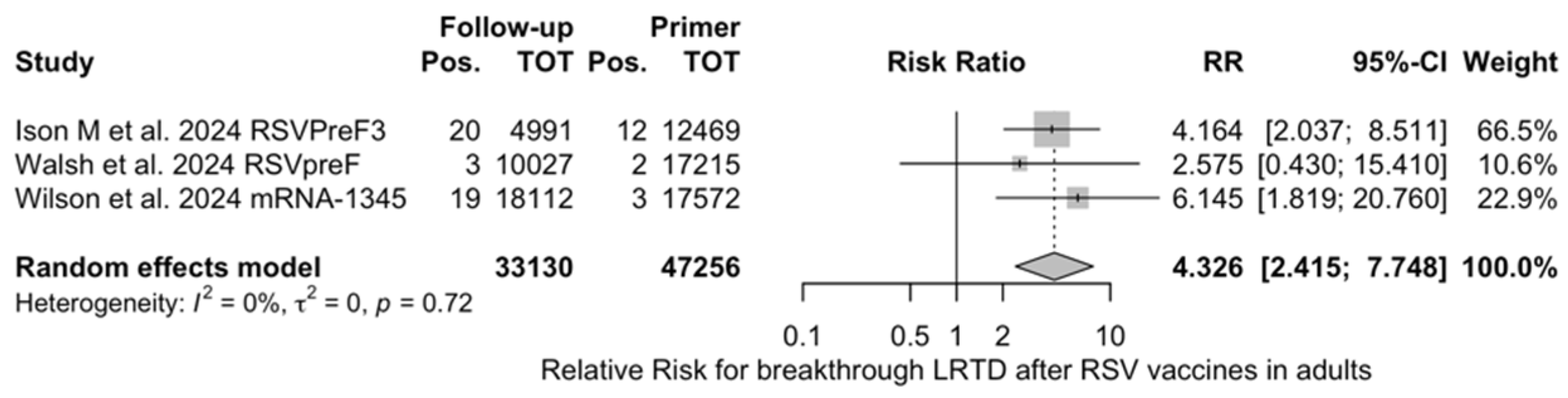
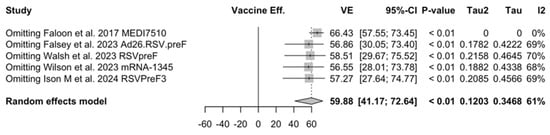
Figure A24.
Sensitivity analysis, acute respiratory illnesses [48,51,52,82,83].
Figure A24.
Sensitivity analysis, acute respiratory illnesses [48,51,52,82,83].
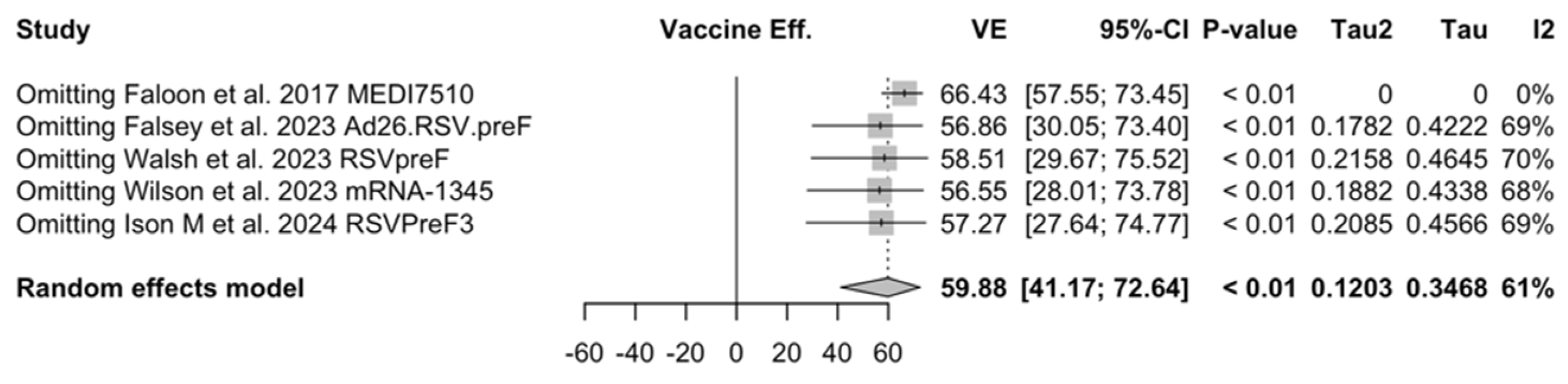
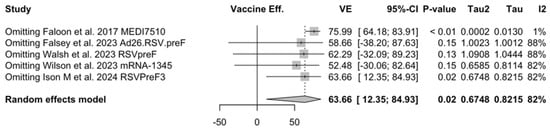
Figure A25.
Sensitivity analysis, lower respiratory tract disease with 2 signs/symptoms [48,51,52,82,83].
Figure A25.
Sensitivity analysis, lower respiratory tract disease with 2 signs/symptoms [48,51,52,82,83].
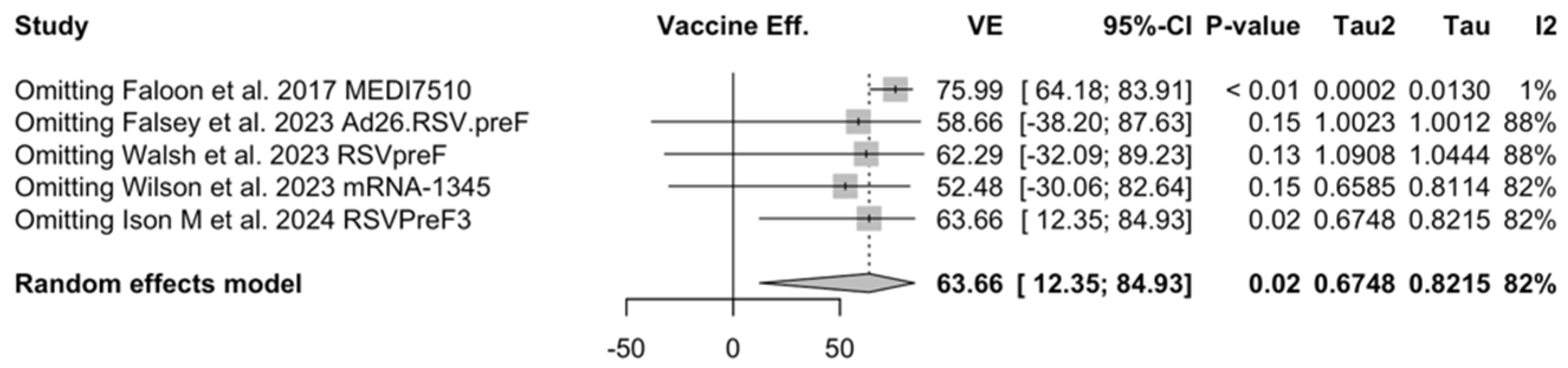
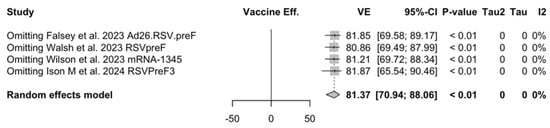
Figure A26.
Sensitivity analysis, lower respiratory tract disease with 3 or more signs/symptoms [48,51,52,82,83].
Figure A26.
Sensitivity analysis, lower respiratory tract disease with 3 or more signs/symptoms [48,51,52,82,83].
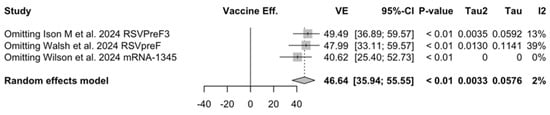
Figure A27.
Sensitivity analysis, acute respiratory illnesses in the follow-up [48,61,85].
Figure A27.
Sensitivity analysis, acute respiratory illnesses in the follow-up [48,61,85].
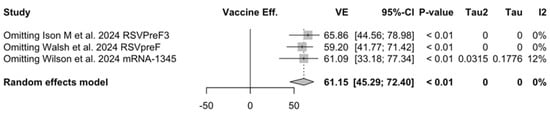
Figure A28.
Sensitivity analysis, lower respiratory tract disease with 3 or more signs/symptoms associated with respiratory syncytial virus infection in follow-up studies [48,61,85].
Figure A28.
Sensitivity analysis, lower respiratory tract disease with 3 or more signs/symptoms associated with respiratory syncytial virus infection in follow-up studies [48,61,85].
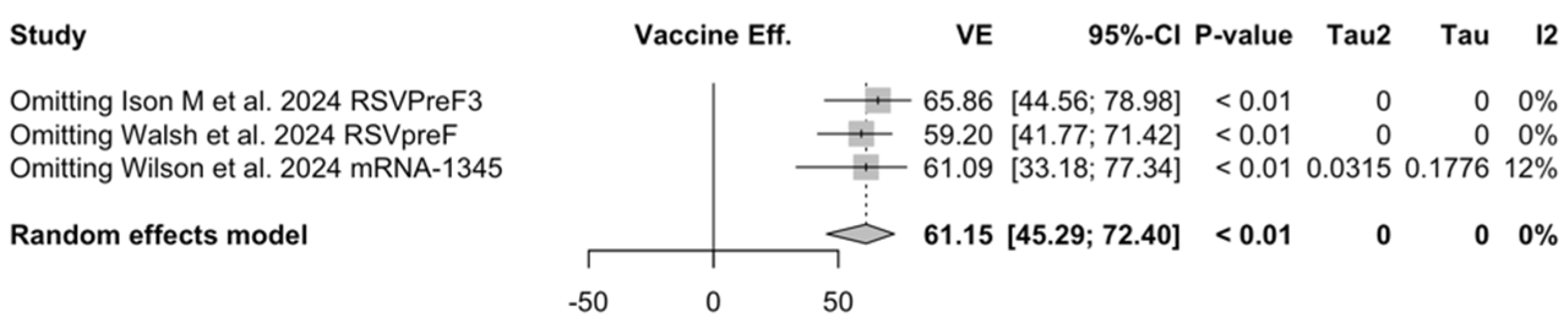
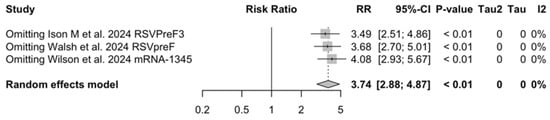
Figure A29.
Sensitivity analysis, acute respiratory illness associated with respiratory syncytial virus infection in follow-up studies [48,61,85].
Figure A29.
Sensitivity analysis, acute respiratory illness associated with respiratory syncytial virus infection in follow-up studies [48,61,85].
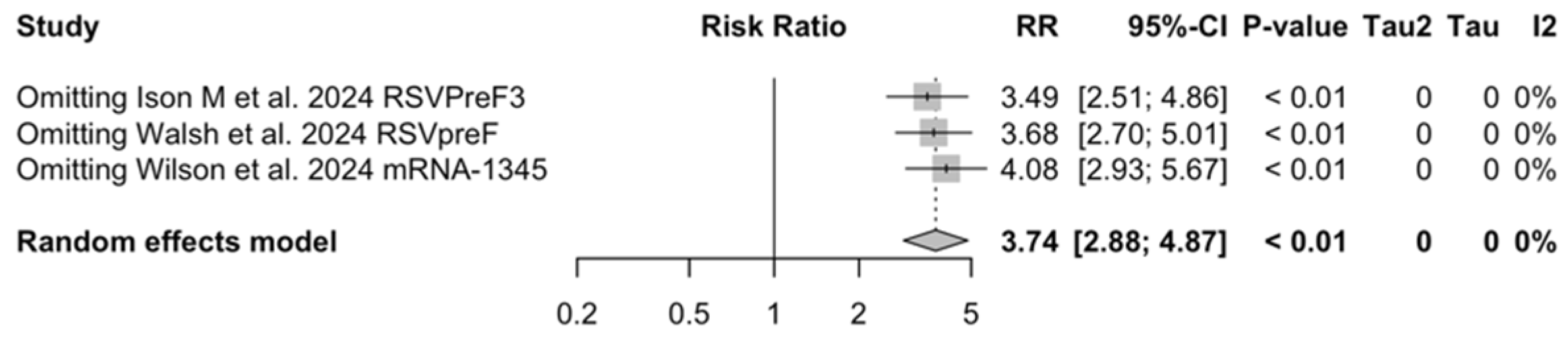
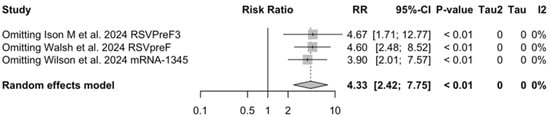
Figure A30.
Sensitivity analysis, breakthrough infections with lower respiratory tract infection (LRTD) with 3 or more signs/symptoms associated with respiratory syncytial virus infection in follow-up studies [48,61,85].
Figure A30.
Sensitivity analysis, breakthrough infections with lower respiratory tract infection (LRTD) with 3 or more signs/symptoms associated with respiratory syncytial virus infection in follow-up studies [48,61,85].

Figure A31.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms [51,52,81,82].
Figure A31.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease (LRTD) with 3 or more signs/symptoms [51,52,81,82].
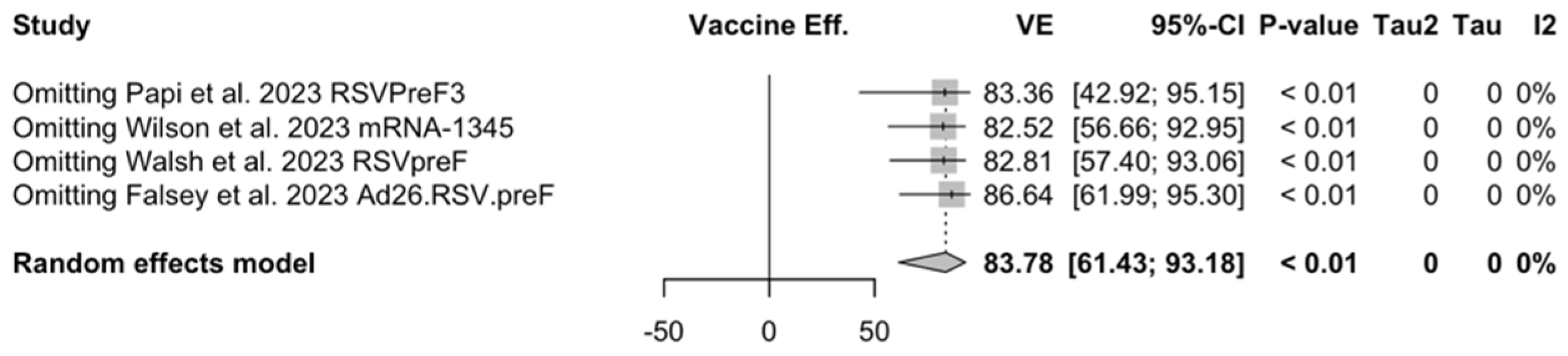
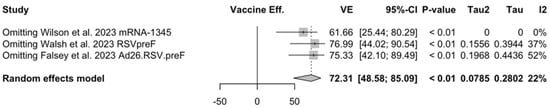
Figure A32.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection [51,52,81,82].
Figure A32.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection [51,52,81,82].
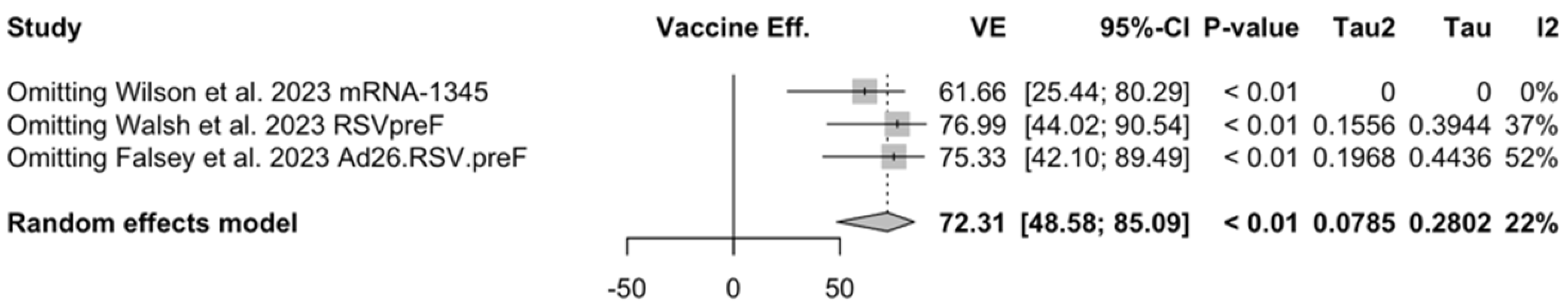
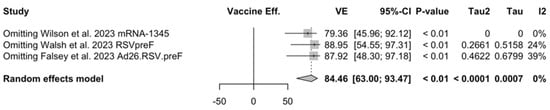
Figure A33.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 2 signs/symptoms [51,52,81,82].
Figure A33.
Sensitivity analysis on vaccine efficacy (VE) for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 2 signs/symptoms [51,52,81,82].
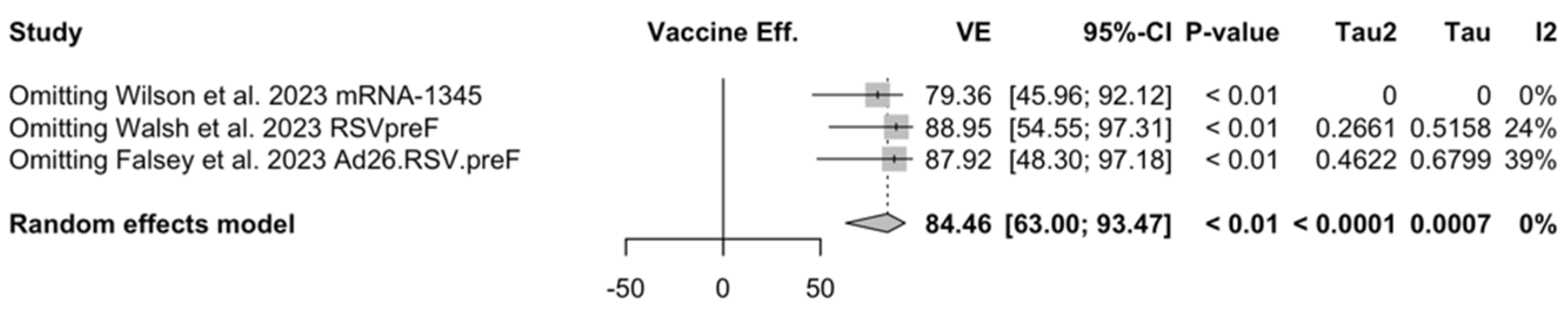
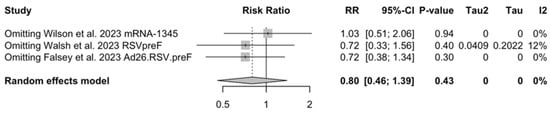
Figure A34.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection [51,52,81,82].
Figure A34.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with acute respiratory infection [51,52,81,82].
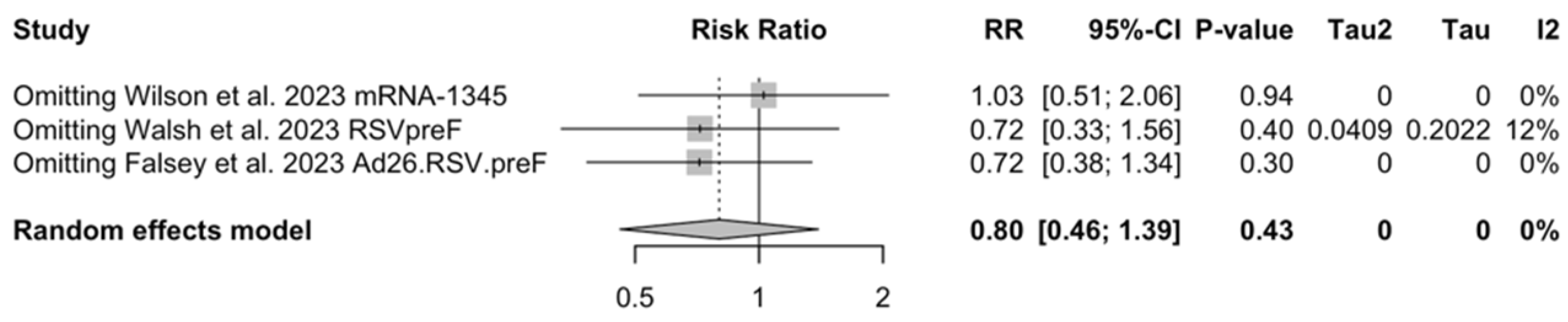
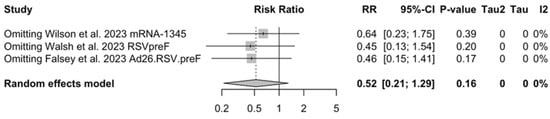
Figure A35.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 2 signs/symptoms [51,52,81,82].
Figure A35.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 2 signs/symptoms [51,52,81,82].
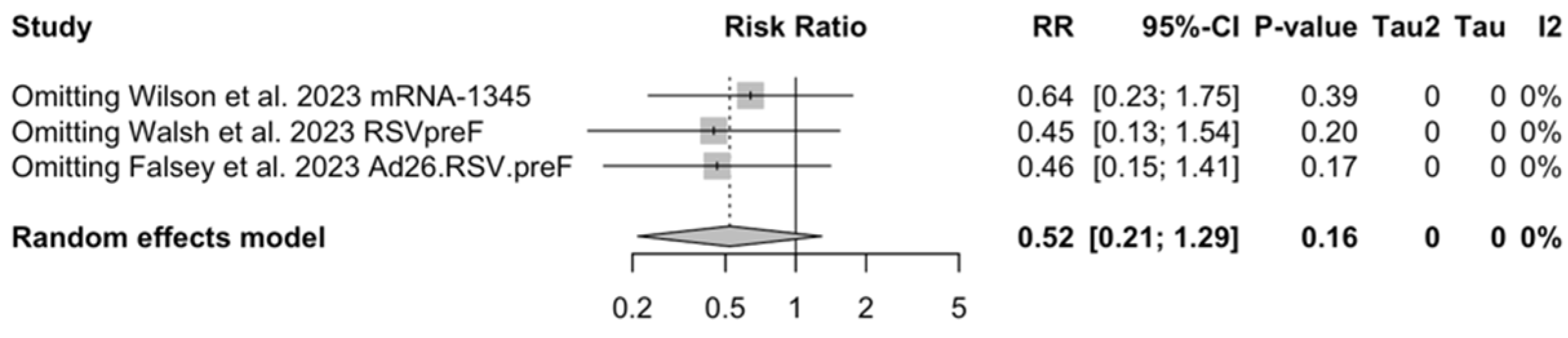
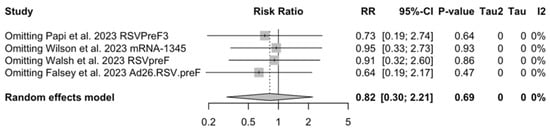
Figure A36.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 3 or more signs/symptoms [51,52,81,82].
Figure A36.
Sensitivity analysis on risk ratio for respiratory syncytial virus (RSV) vaccines in older adults (age ≥ 70/75 years) with lower respiratory tract disease with 3 or more signs/symptoms [51,52,81,82].
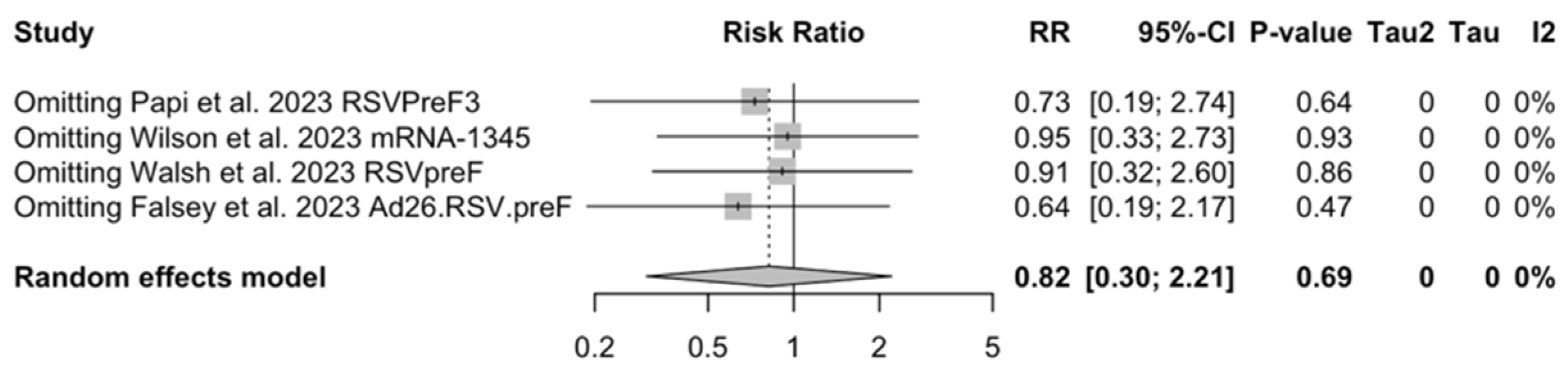
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccines Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Nair DNB, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar]
- Luo, W.; Liu, Q.; Zhou, Y.; Ran, Y.; Liu, Z.; Hou, W.; Pei, S.; Lai, S. Spatiotemporal Variations of “Triple-Demic” Outbreaks of Respiratory Infections in the United States in the Post-COVID-19 Era. BMC Public Health 2023, 23, 2452. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.A.; Jain, B.; Raifman, J. Revamping Public Health Systems: Lessons Learned from the Tripledemic. Am. J. Prev. Med. 2023, 66, 185–188. [Google Scholar] [CrossRef]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Regassa, B.T.; Gebrewold, L.A.; Mekuria, W.T.; Kassa, N.A. Molecular Epidemiology of Respiratory Syncytial Virus in Children with Acute Respiratory Illnesses in Africa: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04001. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Y.; Chmaisse, A.; Boutros, C.; Chamseddine, S.; Fayad, D.; Zaraket, H.; Dbaibo, G. The Burden of Respiratory Syncytial Virus (RSV) Infection in the Middle East and North Africa (MENA) Region across Age Groups: A Systematic Review. Vaccine 2021, 39, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A Phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety and Immunogenicity of an mRNA-Based RSV Prefusion F Protein Vaccine in Healthy Younger and Older Adults. Hum. Vaccines Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Raybould, J.E.; Sastry, S.; de la Cruz, O. Respiratory Viruses in Transplant Recipients: More than Just a Cold. Clinical Syndromes and Infection Prevention Principles. Int. J. Infect. Dis. 2017, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Chiara Vittucci, A.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus-Associated Hospitalizations among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–661. [Google Scholar]
- Leader, S.; Kohlhase, K.; Pearlman, M.H.; Williams, J.V.; Engle, W.A. Recent Trends in Severe Respiratory Syncytial Virus (RSV) among US Infants, 1997 to 2000. J. Pediatr. 2003, 143, S127–S132. [Google Scholar] [CrossRef]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus–Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef]
- Kenmoe, S.; Nair, H. The Disease Burden of Respiratory Syncytial Virus in Older Adults. Curr. Opin. Infect. Dis. 2024, 37, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F. Respiratory Syncytial Virus, Influenza and SARS-CoV-2 in Homeless People from Urban Shelters: A Systematic Review and Meta-Analysis (2023). Epidemiologia 2024, 5, 41–79. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of Severe RSV Disease among Immunocompromised Children and Adults: A 10 Year Retrospective Study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Christaki, E.; Marques, T.M.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; Maikanti, P.; et al. Severity of RSV Infection in Southern European Elderly Patients during Two Consecutive Winter Seasons (2017–2018). J. Med. Virol. 2021, 93, 5152–5157. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Fernandes, J.; de Pouvourville, G.; Sosnowiez, K.; Elong, A.; Guilmet, C.; Omichessan, H.; Bureau, I.; Fagnani, F.; Emery, C.; et al. Respiratory Syncytial Virus-Related Hospital Stays in Adults in France from 2012 to 2021: A National Hospital Database Study. J. Clin. Virol. 2024, 171, 105635. [Google Scholar] [CrossRef]
- Polkowska-Kramek, A.; Begier, E.; Bruyndonckx, R.; Liang, C.; Beese, C.; Brestrich, G.; Tran, T.M.P.; Nuttens, C.; Casas, M.; Bayer, L.J.; et al. Estimated Incidence of Hospitalizations and Deaths Attributable to Respiratory Syncytial Virus Infections among Adults in Germany between 2015 and 2019. Infect. Dis. Ther. 2024, 13, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, M.P.; D’Agostino, H.; Dauer, K.; Stiegler, M.; Zimmerman, R.K.; Balasubramani, G.K. Estimating the Burden of Adult Hospitalized RSV Infection Including Special Populations. Vaccine 2022, 40, 4121–4127. [Google Scholar] [CrossRef]
- Narejos Pérez, S.; Ramón Torrell, J.M.; Põder, A.; Leroux-Roels, I.; Pérez-Breva, L.; Steenackers, K.; Vandermeulen, C.; Meisalu, S.; McNally, D.; Bowen, J.S.; et al. Respiratory Syncytial Virus Disease Burden in Community-Dwelling and Long-Term Care Facility Older Adults in Europe and the United States: A Prospective Study. Open Forum Infect. Dis. 2023, 10, ofad111. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory Syncytial Virus Disease Burden in Adults Aged 60 Years and Older in High-Income Countries: A Systematic Literature Review and Meta-Analysis. Influenza Other Respir. Viruses 2022, 17, e13031. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.P.; Tapia, L.I.; Catalán, P.; De la Maza, V.; Mejías, A. Intravenous Palivizumab in Respiratory Syncytial Virus Infection after Hematopoietic Stem Cell Transplant in Children. Pediatr. Blood Cancer 2017, 64, e26667. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Mahoney, M.V.; McCoy, C.; Atsawarungruangkit, A.; Gold, H.S.; Levine, J.D.; Wong, M.T.; LaSalvia, M.T.; Alonso, C.D. Clinical Characteristics and Treatment Outcomes among Respiratory Syncytial Virus (RSV)-Infected Hematologic Malignancy and Hematopoietic Stem Cell Transplant Recipients Receiving Palivizumab. Leuk. Lymphoma 2019, 60, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Cutland, C.L.; Downs, S.; Jones, S.; Van Niekerk, N.; Simoes, E.A.F.; Nunes, M.C. Burden of Respiratory Syncytial Virus Infection in South African Human Immunodeficiency Virus (HIV)-Infected and HIV-Uninfected Pregnant and Postpartum Women: A Longitudinal Cohort Study. Clin. Infect. Dis. 2018, 66, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, L.; Zhang, Y.; Zhang, X.; Zheng, M.; Kyaw, M.H. Burden of Respiratory Syncytial Virus Infections in China: Systematic Review and Meta-Analysis. J. Glob. Health 2015, 5, 020417. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare Costs within a Year of Respiratory Syncytial Virus among Medicaid Infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory Syncytial Virus Hospitalization Outcomes and Costs of Full-Term and Preterm Infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Am. J. Transplant. 2023, 23, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Morbidity and Mortality Weekly Report Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar]
- Papazisis, G.; Topalidou, X.; Gioula, G.; González, P.A.; Bueno, S.M.; Kalergis, A.M. Respiratory Syncytial Virus Vaccines: Analysis of Pre-Marketing Clinical Trials for Immunogenicity in the Population over 50 Years of Age. Vaccines 2024, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Bastian, A.R.; Feldman, R.A.; Omoruyi, E.; de Paepe, E.; Hendriks, J.; van Zeeburg, H.; Godeaux, O.; Langedijk, J.P.M.; Schuitemaker, H.; et al. Phase 1 Safety and Immunogenicity Study of a Respiratory Syncytial Virus Vaccine With an Adenovirus 26 Vector Encoding Prefusion F (Ad26.RSV.preF) in Adults Aged ≥60 Years. J. Infect. Dis. 2020, 222, 979–988. [Google Scholar] [PubMed]
- Cicconi, P.; Jones, C.; Sarkar, E.; Silva-Reyes, L.; Klenerman, P.; de Lara, C.; Hutchings, C.; Moris, P.; Janssens, M.; Fissette, L.A.; et al. First-in-Human Randomized Study to Assess the Safety and Immunogenicity of an Investigational Respiratory Syncytial Virus (RSV) Vaccine Based on Chimpanzee-Adenovirus-155 Viral Vector-Expressing RSV Fusion, Nucleocapsid, and Antitermination Viral Proteins in Healthy Adults. Clin. Infect. Dis. 2020, 70, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and Immunogenicity of an MRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults. J. Infect. Dis. 2024, jiae081. [Google Scholar] [CrossRef] [PubMed]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal Parainfluenza Virus Type 5 (PIV5)-Vectored RSV Vaccine Is Safe and Immunogenic in Healthy Adults in a Phase 1 Clinical Study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G.; Papi, A.; Athan, E.; Feldman, R.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2024, ciae010. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Kabir, G.; Schultz, S.; Silbernagl, G.; Schmidt, D.; Jenkins, V.A.; Weidenthaler, H.; Stroukova, D.; Martin, B.K.; De Moerlooze, L. Reduced Respiratory Syncytial Virus Load, Symptoms, and Infections: A Human Challenge Trial of MVA-BN-RSV Vaccine. J. Infect. Dis. 2023, 228, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.A.; Phung, E.; Crank, M.C.; Morabito, K.M.; Villafana, T.; Dubovsky, F.; Falloon, J.; Esser, M.T.; Lin, B.C.; Chen, G.L.; et al. A Prefusion-Stabilized RSV F Subunit Vaccine Elicits B Cell Responses with Greater Breadth and Potency than a Postfusion F Vaccine. Sci. Transl. Med. 2022, 14, eade0424. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an MRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Saeland, E.; Thoma, A.; van den Hoogen, W.; Tettero, L.; Drijver, J.; Vaneman, C.; van Polanen, Y.; Ritschel, T.; Bastian, A.R.; et al. RSV A2-Based Prefusion F Vaccine Candidates Induce RSV A and RSV B Cross Binding and Neutralizing Antibodies and Provide Protection against RSV A and RSV B Challenge in Preclinical Models. Vaccines 2023, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.; Cao, X.; Railkar, R.A.; Sachs, J.R.; Spellman, D.S.; Luk, J.; Shaw, C.A.; Cejas, P.J.; Citron, M.P.; Al-Ibrahim, M.; et al. Evaluation of a Stabilized RSV Pre-Fusion F MRNA Vaccine: Preclinical Studies and Phase 1 Clinical Testing in Healthy Adults. Vaccine 2023, 41, 6488–6501. [Google Scholar] [CrossRef] [PubMed]
- Samy, N.; Reichhardt, D.; Schmidt, D.; Chen, L.M.; Silbernagl, G.; Vidojkovic, S.; Meyer, T.P.; Jordan, E.; Adams, T.; Weidenthaler, H.; et al. Safety and Immunogenicity of Novel Modified Vaccinia Ankara-Vectored RSV Vaccine: A Randomized Phase I Clinical Trial. Vaccine 2020, 38, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.S.V.; Virta, M.; Williams, K.; Seppa, I.; Hartvickson, R.; Greenland, M.; Omoruyi, E.; Bastian, A.R.; Haazen, W.; Salisch, N.; et al. Phase 1/2a Safety and Immunogenicity of an Adenovirus 26 Vector Respiratory Syncytial Virus (RSV) Vaccine Encoding Prefusion F in Adults 18–50 Years and RSV-Seropositive Children 12–24 Months. J. Infect. Dis. 2023, 227, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Athan, E.; Baber, J.; Quan, K.; Scott, R.J.; Jaques, A.; Jiang, Q.; Li, W.; Cooper, D.; Cutler, M.W.; Kalinina, E.V.; et al. Safety and Immunogenicity of Bivalent RSVpreF Vaccine Coadministered with Seasonal Inactivated Influenza Vaccine in Older Adults. Clin. Infect. Dis. 2023, ciad707. [Google Scholar] [CrossRef] [PubMed]
- Díez-Domingo, J.; Sáez-Llorens, X.; Rodriguez-Weber, M.A.; Epalza, C.; Chatterjee, A.; Chiu, C.H.; Lin, C.Y.; Berry, A.A.; Martinón-Torres, F.; Baquero-Artigao, F.; et al. Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus (RSV) Vaccine in Healthy RSV-Seropositive Children 12-23 Months of Age. J. Infect. Dis. 2023, 227, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Green, C.A.; Scarselli, E.; Voysey, M.; Capone, S.; Vitelli, A.; Nicosia, A.; Cortese, R.; Thompson, A.J.; Sande, C.S.; De Lara, C.; et al. Safety and Immunogenicity of Novel Respiratory Syncytial Virus (RSV) Vaccines Based on the RSV Viral Proteins F, N and M2-1 Encoded by Simian Adenovirus (PanAd3-RSV) and MVA (MVA-RSV); Protocol for an Open-Label, Dose-Escalation, Single-Centre, Phase 1 Clinical Trial in Healthy Adults. BMJ Open 2015, 5, e008748. [Google Scholar] [CrossRef] [PubMed]
- Malkin, E.; Yogev, R.; Abughali, N.; Sliman, J.; Wang, C.K.; Zuo, F.; Yang, C.F.; Eickhoff, M.; Esser, M.T.; Tang, R.S.; et al. Safety and Immunogenicity of a Live Attenuated RSV Vaccine in Healthy RSV-Seronegative Children 5 to 24 Months of Age. PLoS ONE 2013, 8, e77104. [Google Scholar] [CrossRef]
- Walsh, E.; Falsey, A.; Patton, M.; Stacey, H.; Eiras, D.P.; Jiang, Q.; Woodside, J.; Mikati, T.; Kalinina, E.; Cooper, D.; et al. Efficacy of a Bivalent RSVpreF Vaccine in Older Adults Beyond a First RSV Season. In Proceedings of the 8th ReSViNET Conference, Mumbai, India, 13–16 February 2024; Respiratory Syncytial Virus Foundation: Mumbai, India, 2024; pp. 99–100. [Google Scholar]
- Karron, R.A.; Luongo, C.; Woods, S.; Oliva, J.; Collins, P.L.; Buchholz, U.J.; Council-Dibitetto, C.; Gatto, M.; Ghasri, T.; Gormley, A.; et al. Evaluation of the Live-Attenuated Intranasal Respiratory Syncytial Virus (RSV) Vaccine RSV/6120/ΔNS2/1030s in RSV-Seronegative Young Children. J. Infect. Dis. 2024, 229, 346–354. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel Antigens for RSV Vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Tang, S.F.; Shi, Y. Efficacy and Safety of Respiratory Syncytial Virus Vaccination during Pregnancy to Prevent Lower Respiratory Tract Illness in Newborns and Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2023, 11, 1260740. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Mintzker, Y.; Blum, D.; Adler, L. Replacing PICO in Non-Interventional Studies. BMJ Evid.-Based Med. 2023, 28, 284. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Von Hippel, P.T. The Heterogeneity Statistic I2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Krumpal, I. Determinants of Social Desirability Bias in Sensitive Surveys: A Literature Review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). OHAT Risk of Bias Rating Tool for Human and Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2015. [Google Scholar]
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.G.; Antonelli-Incalzi, R.; Steenackers, K.; Lee, D.G.; Papi, A.; Ison, M.G.; Fissette, L.; David, M.P.; Marechal, C.; Van Der Wielen, M.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults with Underlying Medical Conditions. Clin. Infect. Dis. 2024, 78, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.H.; et al. Efficacy and Safety of an Ad26.RSV.PreF–RSV PreF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Falloon, J.; Yu, J.; Esser, M.T.; Villafana, T.; Yu, L.; Dubovsky, F.; Takas, T.; Levin, M.J.; Falsey, A.R. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J. Infect. Dis. 2017, 216, 1362–1370. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration; Office of Health Assessment and Translation (OHAT), Division of the National Toxicology Program, National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2019. [Google Scholar]
- Wilson, E.; Goswami, J.; Doreski, P.A.; Marc, G.P.; Jimenez, G.; Priddy, F.; Lin, N.; Le Cam, N.; Karen Slobod, K.; Stoszek, S.K.; et al. Efficacy and Safety of MRNA-1345, an RSV Vaccine, in Older Adults: Results through ≥6 Months of Follow-up and Evaluation of Correlate of Protection Against RSV. In Proceedings of the 8th ReSViNET Conference, Mumbai, India, 13–16 February 2024; Respiratory Syncytial Virus Society: Mumbai, India, 2024; pp. 87–88. [Google Scholar]
- Falloon, J.; Talbot, H.K.; Curtis, C.; Ervin, J.; Krieger, D.; Dubovsky, F.; Takas, T.; Yu, J.; Yu, L.; Lambert, S.L. Dose Selection for an Adjuvanted Respiratory Syncytial Virus F Protein Vaccine for Older Adults Based on Humoral and Cellular Immune Responses. Clin. Vaccine Immunol. 2017, 24, e00157-17. [Google Scholar] [CrossRef]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. Pfizer Announces Positive Top-Line Data for Full Season Two Efficacy of ABRYSVO® for RSV in Older Adults. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-full-season-two (accessed on 28 March 2024).
- Falloon, J.; Ji, F.; Curtis, C.; Bart, S.; Sheldon, E.; Krieger, D.; Dubovsky, F.; Lambert, S.; Takas, T.; Villafana, T.; et al. A Phase 1a, First-in-Human, Randomized Study of a Respiratory Syncytial Virus F Protein Vaccine with and without a Toll-like Receptor-4 Agonist and Stable Emulsion Adjuvant. Vaccine 2016, 34, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Baral, R.; Higgins, D.; Khan, S.; Kochar, S.; Li, Y.; Ortiz, J.R.; Cherian, T.; Feikin, D.; Jit, M.; et al. Value Profile for Respiratory Syncytial Virus Vaccines and Monoclonal Antibodies. Vaccine 2023, 41, S7–S40. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Scott, D.A.; Gurtman, A.; Zareba, A.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Jiang, Q.; et al. Phase 1/2 Randomized Study of the Immunogenicity, Safety, and Tolerability of a Respiratory Syncytial Virus Prefusion F Vaccine in Adults with Concomitant Inactivated Influenza Vaccine. J. Infect. Dis. 2022, 225, 2056–2066. [Google Scholar] [CrossRef]
- Comeaux, C.A.; Bart, S.; Bastian, A.R.; Klyashtornyy, V.; De Paepe, E.; Omoruyi, E.; Van Der Fits, L.; Van Heesbeen, R.; Heijnen, E.; Callendret, B.; et al. Safety, Immunogenicity, and Regimen Selection of Ad26.RSV.PreF-Based Vaccine Combinations: A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2a Study. J. Infect. Dis. 2024, 229, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; De Paepe, E.; Devincenzo, J.; Gymnopoulou, E.; Menten, J.; Murray, B.; Rosemary Bastian, A.; Vandebosch, A.; Haazen, W.; Noulin, N.; et al. Prevention of Respiratory Syncytial Virus Infection in Healthy Adults by a Single Immunization of Ad26.RSV.PreF in a Human Challenge Study. J. Infect. Dis. 2022, 226, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.D.; et al. Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding after Vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population Based Cohort Study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Cizmeci, D.; Yuan, D.; Mehta, N.; Tolboom, J.; De Paepe, E.; van Heesbeen, R.; Sadoff, J.; Comeaux, C.A.; Heijnen, E.; et al. Vaccine-Induced Antibody Fc-Effector Functions in Humans Immunized with a Combination Ad26.RSV.PreF/RSV PreF Protein Vaccine. J. Virol. 2023, 97, e00771-23. [Google Scholar] [CrossRef] [PubMed]
- Widagdo, W.; Bastian, A.R.; Jastorff, A.M.; Scheys, I.; De Paepe, E.; Comeaux, C.A.; Ligtenberg, N.; Callendret, B.; Heijnen, E. Concomitant Administration of Ad26.RSV.PreF/RSV PreF Protein Vaccine and High-Dose Influenza Vaccine in Adults 65 Years and Older: A Noninferiority Trial. J. Infect. Dis. 2023, jiad594. [Google Scholar] [CrossRef]
- Chu, H.Y.; Chin, J.; Pollard, J.; Zerr, D.M.; Englund, J.A. Clinical Outcomes in Outpatient Respiratory Syncytial Virus Infection in Immunocompromised Children. Influenza Other Respir. Viruses 2016, 10, 205–210. [Google Scholar] [CrossRef]
- Yoon, J.G.; Noh, J.Y.; Choi, W.S.; Park, J.J.; Suh, Y.B.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Clinical Characteristics and Disease Burden of Respiratory Syncytial Virus Infection among Hospitalized Adults. Sci. Rep. 2020, 10, 12106. [Google Scholar] [CrossRef]
- Reeves, R.M.; van Wijhe, M.; Lehtonen, T.; Stona, L.; Teirlinck, A.C.; Vazquez Fernandez, L.; Li, Y.; Osei-Yeboah, R.; Fischer, T.K.; Heikkinen, T.; et al. A Systematic Review of European Clinical Practice Guidelines for Respiratory Syncytial Virus Prophylaxis. J. Infect. Dis. 2022, 226, S110–S116. [Google Scholar] [CrossRef]
- Loubet, P.; Lenzi, N.; Valette, M.; Foulongne, V.; Krivine, A.; Houhou, N.; Lagathu, G.; Rogez, S.; Alain, S.; Duval, X.; et al. Clinical Characteristics and Outcome of Respiratory Syncytial Virus Infection among Adults Hospitalized with Influenza-like Illness in France. Clin. Microbiol. Infect. 2017, 23, 253–259. [Google Scholar] [CrossRef]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef]
- Malosh, R.E.; Martin, E.T.; Callear, A.P.; Petrie, J.G.; Lauring, A.S.; Lamerato, L.; Fry, A.M.; Ferdinands, J.; Flannery, B.; Monto, A.S. Respiratory Syncytial Virus Hospitalization in Middle-Aged and Older Adults. J. Clin. Virol. 2017, 96, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.; Ravanfar, B.; Rutter, H.; Allen, J.; Falsey, A.; Pirçon, J.Y.; Gruselle, O.; et al. Burden of Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults in Europe (RESCEU): An International Prospective Cohort Study. Eur. Respir. J. 2021, 57, 2002688. [Google Scholar] [CrossRef]
- Ellis, S.E.; Coffey, C.S.; Mitchel, E.F.; Dittus, R.S.; Griffin, M.R. Influenza-and Respiratory Syncytial Virus-Associated Morbidity and Mortality in the Nursing Home Population. J. Am. Geriatr. Soc. 2003, 51, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Atamna, A.; Babich, T.; Froimovici, D.; Yahav, D.; Sorek, N.; Ben-Zvi, H.; Leibovici, L.; Bishara, J.; Avni, T. Morbidity and Mortality of Respiratory Syncytial Virus Infection in Hospitalized Adults: Comparison with Seasonal Influenza. Int. J. Infect. Dis. 2021, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Surie, D.; Yuengling, K.A.; Decuir, J.; Zhu, Y.; Gaglani, M.; Ginde, A.A.; Keipp Talbot, H.; Casey, J.D.; Mohr, N.M.; Ghamande, S.; et al. Disease Severity of Respiratory Syncytial Virus Compared with COVID-19 and Influenza among Hospitalized Adults Aged ≥60 Years—IVY Network, 20 U.S. States, February 2022–May 2023. Morb. Mortal. Wkly. Rev. 2023, 72, 1083–1088. [Google Scholar] [CrossRef]
- Celante, H.; Oubaya, N.; Fourati, S.; Beaune, S.; Khellaf, M.; Casalino, E.; Ricard, J.D.; Vieillard-Baron, A.; Heming, N.; Mekontso Dessap, A.; et al. Prognosis of Hospitalised Adult Patients with Respiratory Syncytial Virus Infection: A Multicentre Retrospective Cohort Study. Clin. Microbiol. Infect. 2023, 29, e1–e943. [Google Scholar] [CrossRef]
- Blanco, I.; Diego, I.; Bueno, P.; Casas-Maldonado, F.; Miravitlles, M. Geographic Distribution of COPD Prevalence in the World Displayed by Geographic Information System Maps. Eur. Respir. J. 2019, 54, 1900610. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Ferraro, P.; Gualerzi, G.; Ranzieri, S.; Henry, B.M.; Said, Y.B.; Pyatigorskaya, N.V.; Nevolina, E.; Wu, J.; Bragazzi, N.L.; et al. Point-of-Care Diagnostic Tests for Detecting SARS-CoV-2 Antibodies: A Systematic Review and Meta-Analysis of Real-World Data. J. Clin. Med. 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Zaniboni, A.; Satta, E.; Ranzieri, S.; Marchesi, F. Potential Use of Exhaled Breath Condensate for Diagnosis OfSARS-CoV-2 Infections: A Systematic Review AndMeta-Analysis. Diagnostics 2022, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Vila, J.; Hansen, G.; Manissero, D.; Pareja, J.; Rao, S.N.; Visseaux, B. Systematic Review on the Association between Respiratory Virus Real-Time PCR Cycle Threshold Values and Clinical Presentation or Outcomes. J. Antimicrob. Chemother. 2021, 76, III33–III49. [Google Scholar] [CrossRef] [PubMed]
- Wishaupt, J.O.; van der Ploeg, T.; Smeets, L.C.; de Groot, R.; Versteegh, F.G.A.; Hartwig, N.G. Pitfalls in Interpretation of CT-Values of RT-PCR in Children with Acute Respiratory Tract Infections. J. Clin. Virol. 2017, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-Analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Maternal RSV Vaccine: Further Analysis Is Urged on Preterm Births. BMJ 2023, 381, 1021. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Concerns over Informed Consent for Pregnant Women in Pfizer’s RSV Vaccine Trial. BMJ 2023, 383, 2620. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J. Maternal RSV Vaccine—Weighing Benefits and Risk. N. Engl. J. Med. 2024, 390, 1050–1051. [Google Scholar] [CrossRef]
- Baber, J.; Arya, M.; Moodley, Y.; Jaques, A.; Jiang, Q.; Swanson, K.A.; Cooper, D.; Maddur, M.S.; Loschko, J.; Gurtman, A.; et al. A Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine with and without Adjuvant in Healthy Older Adults. J. Infect. Dis. 2022, 226, 2054–2063. [Google Scholar] [CrossRef]
- Chen, G.L.; Mithani, R.; Kapoor, A.; Lu, S.; El Asmar, L.; Panozzo, C.A.; Shaw, C.A.; Stoszek, S.K.; August, A. 234. Safety and Immunogenicity of MRNA-1345, an MRNA-Based RSV Vaccine in Younger and Older Adult Cohorts: Results from a Phase 1, Randomized Clinical Trial. Open Forum Infect. Dis. 2022, 9, S164–S165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).