Elucidating the Onset of Cross-Protective Immunity after Intranasal Vaccination with the Attenuated African Swine Fever Vaccine Candidate BA71ΔCD2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viruses
2.3. Animal Experiment
2.4. Quantitative PCR for the Detection of ASFV
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Multiplex Luminex Assay
2.7. RNA-Seq Library Preparation and Sequencing
2.8. Bioinformatic Analysis
2.9. Statistical Analyses
3. Results
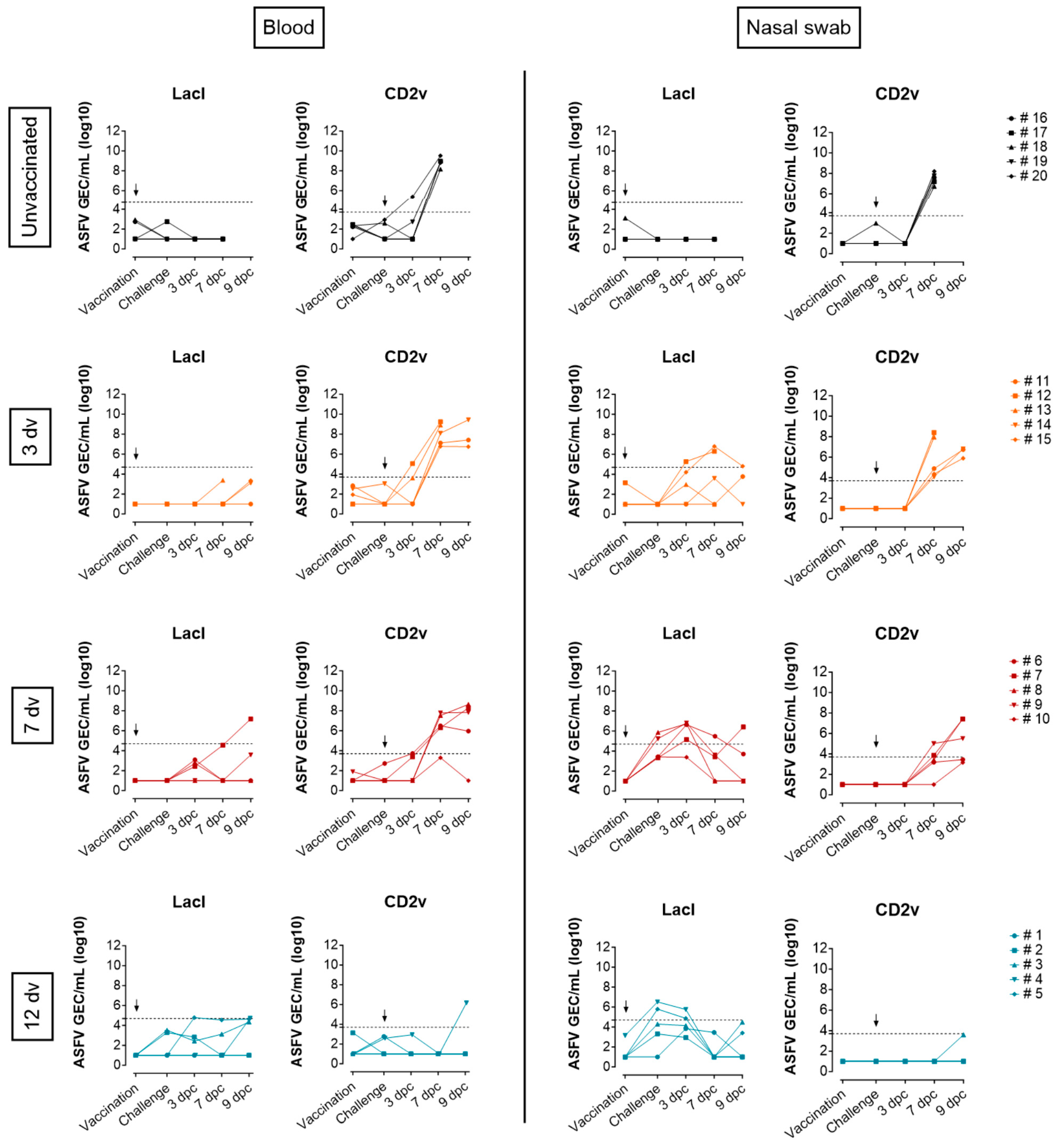
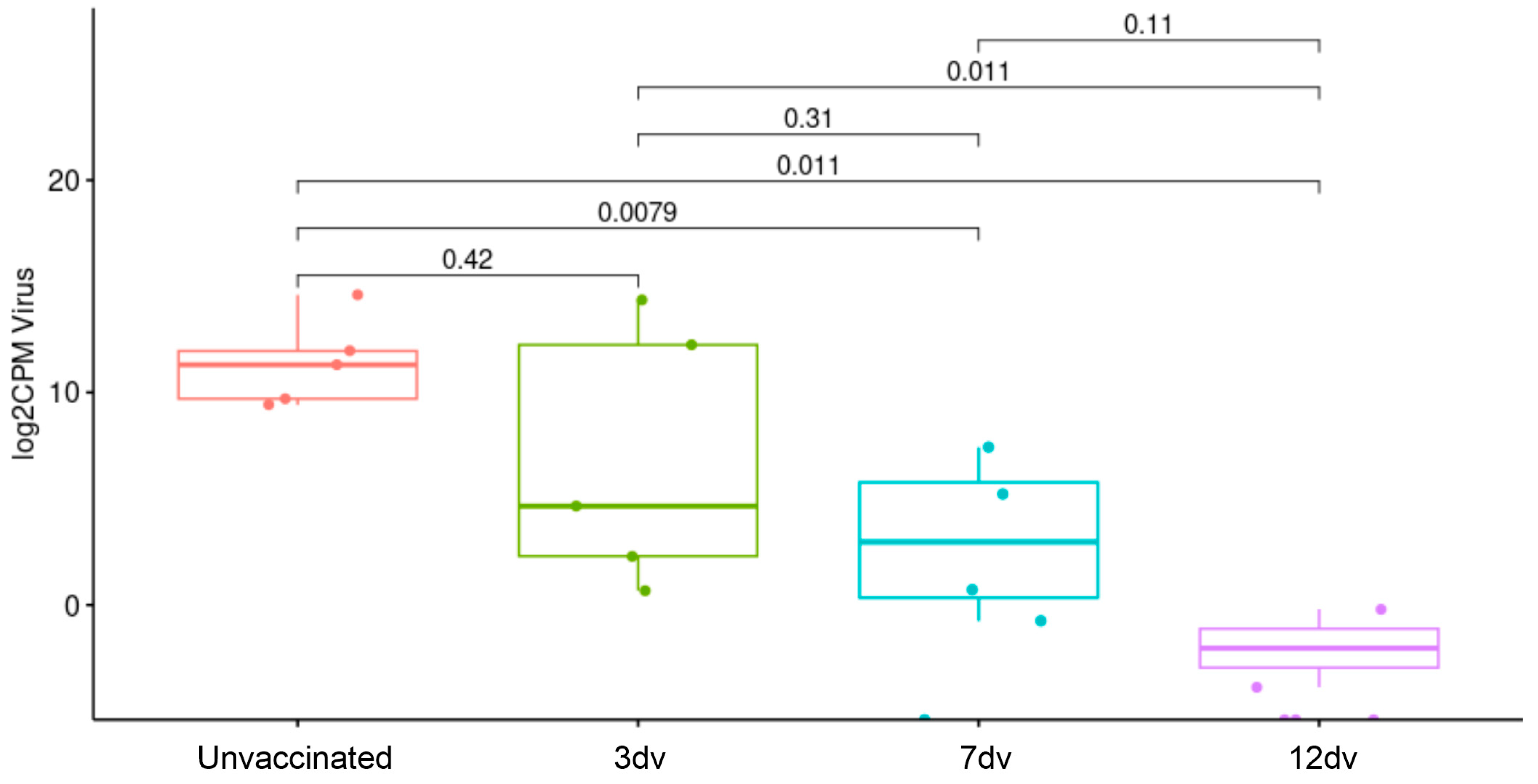
3.1. Onset of Cross-Protection Induced in Pigs Intranasally Vaccinated with BA71ΔCD2
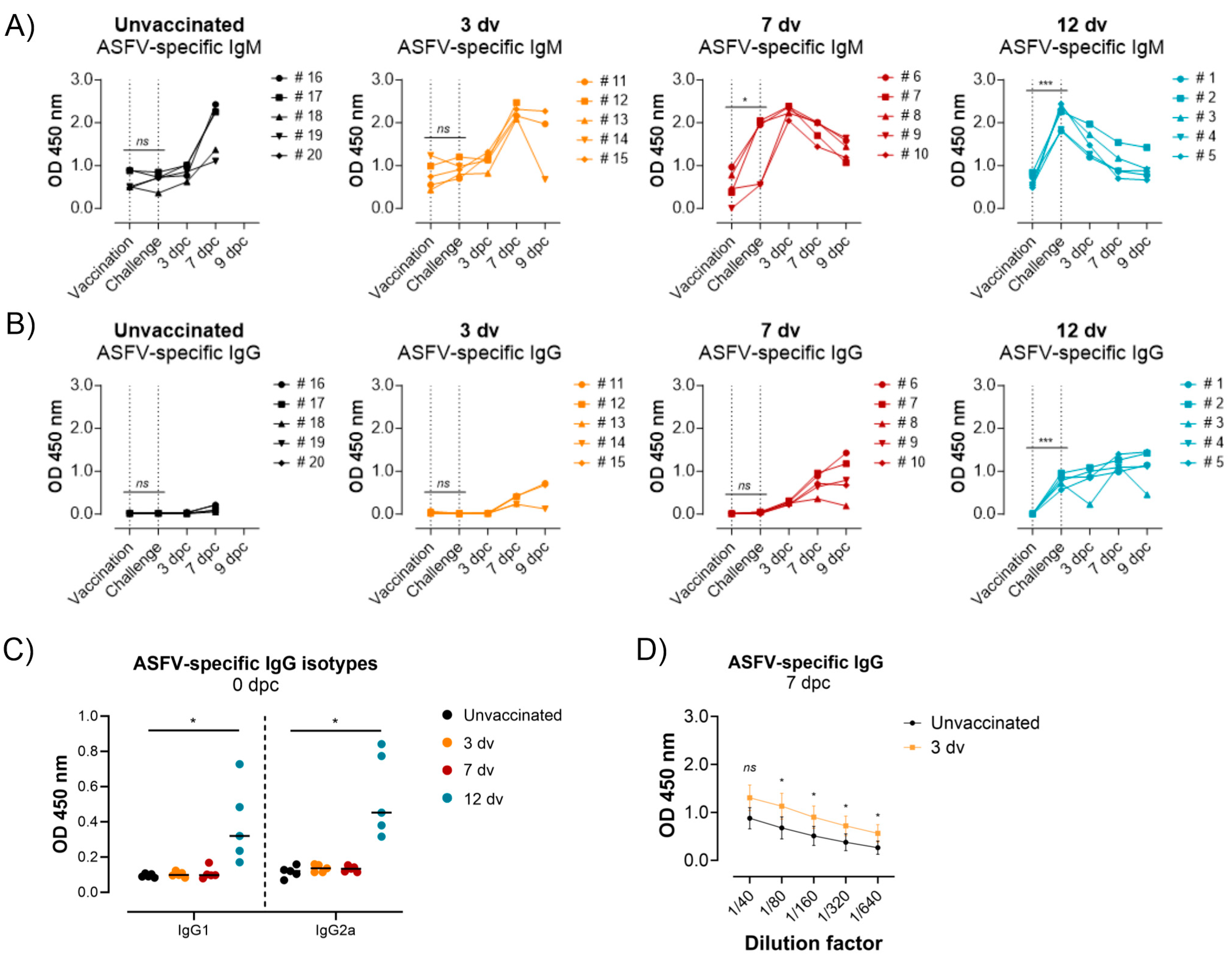
3.2. Presence of ASFV-Specific IgG Antibodies before Challenge Is Associated with Control of Infection
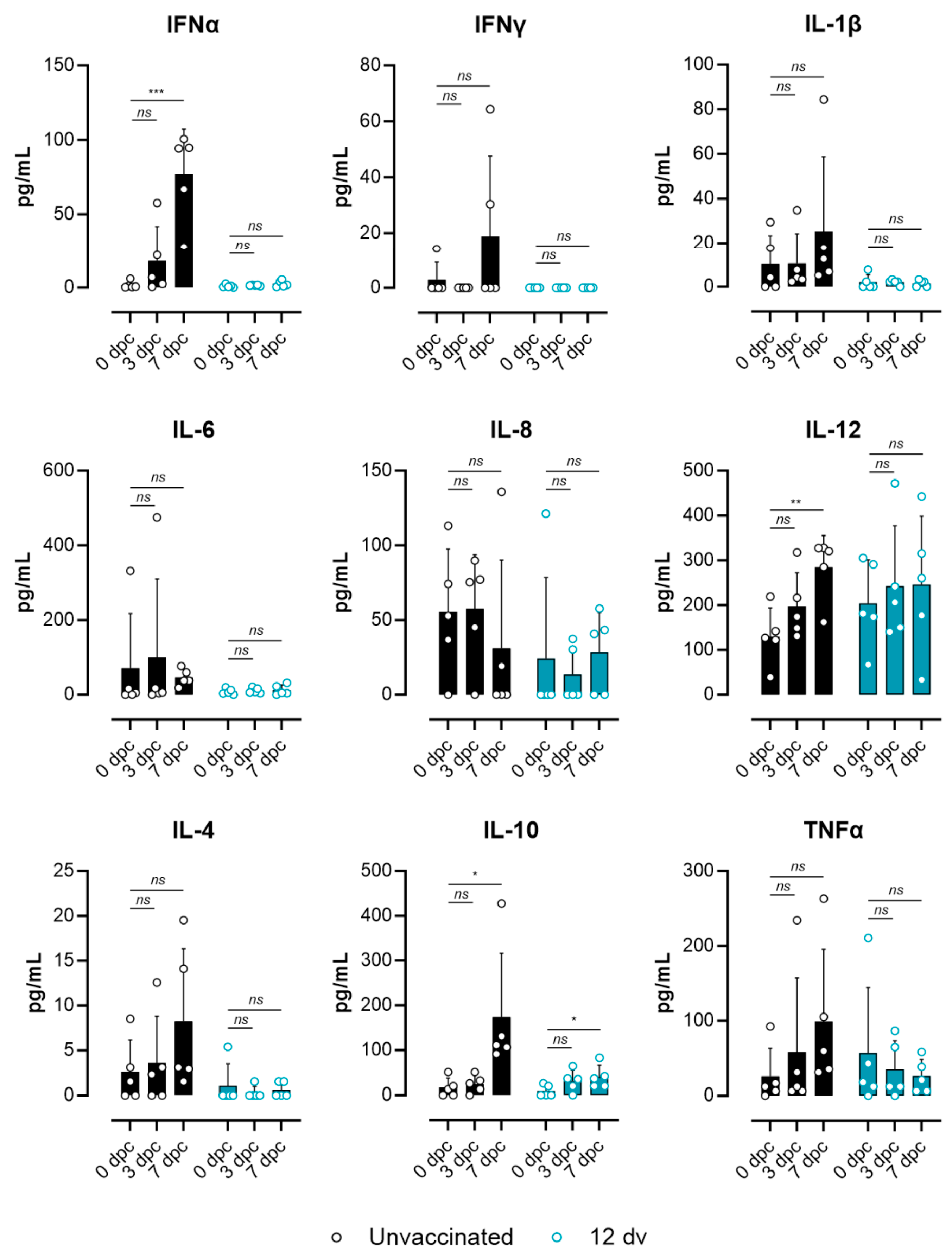
3.3. Lack of ASF-Associated Apoptosis and Immunopathology Markers in Pigs Vaccinated 12 Days Prior to the Challenge
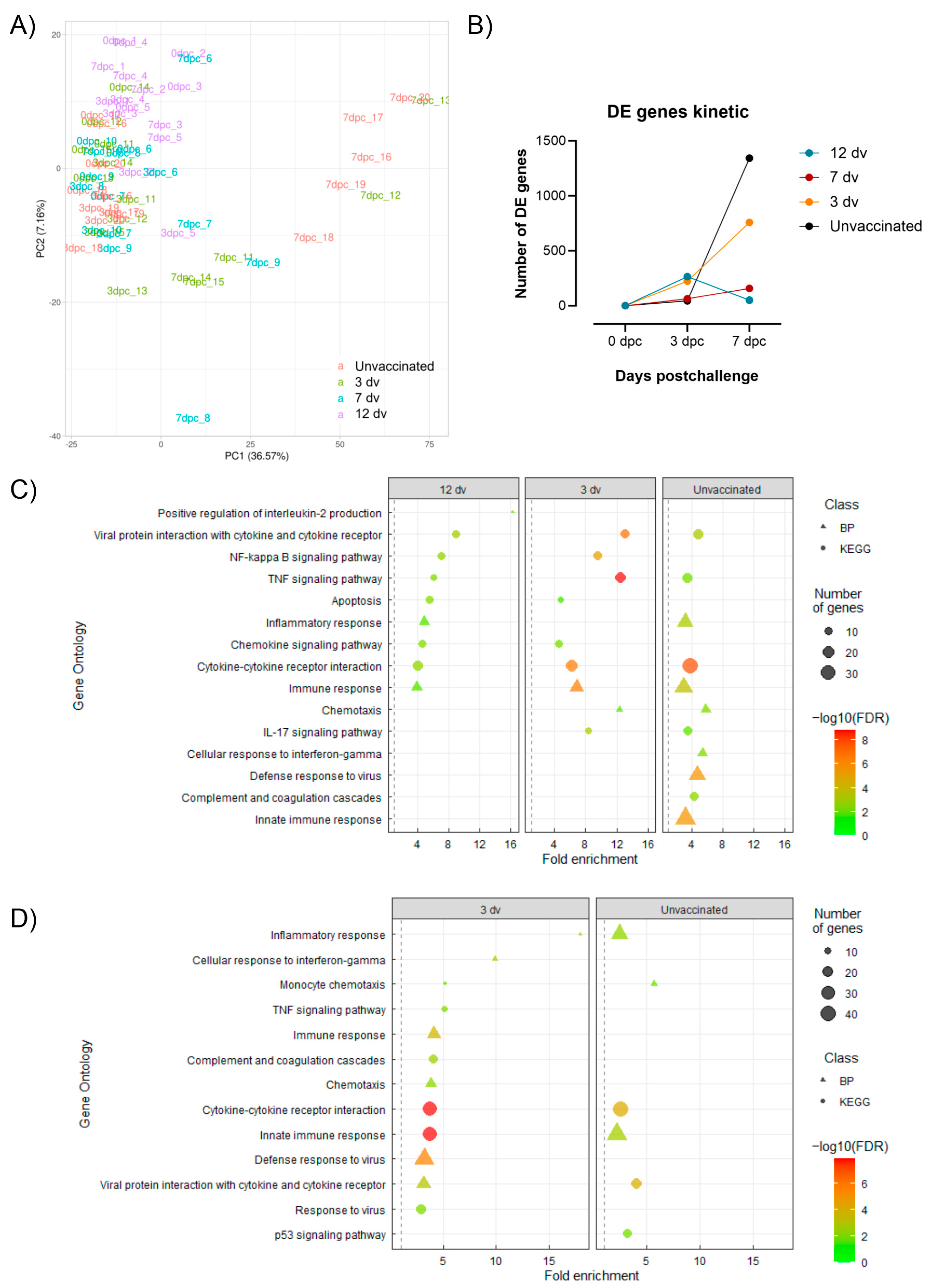
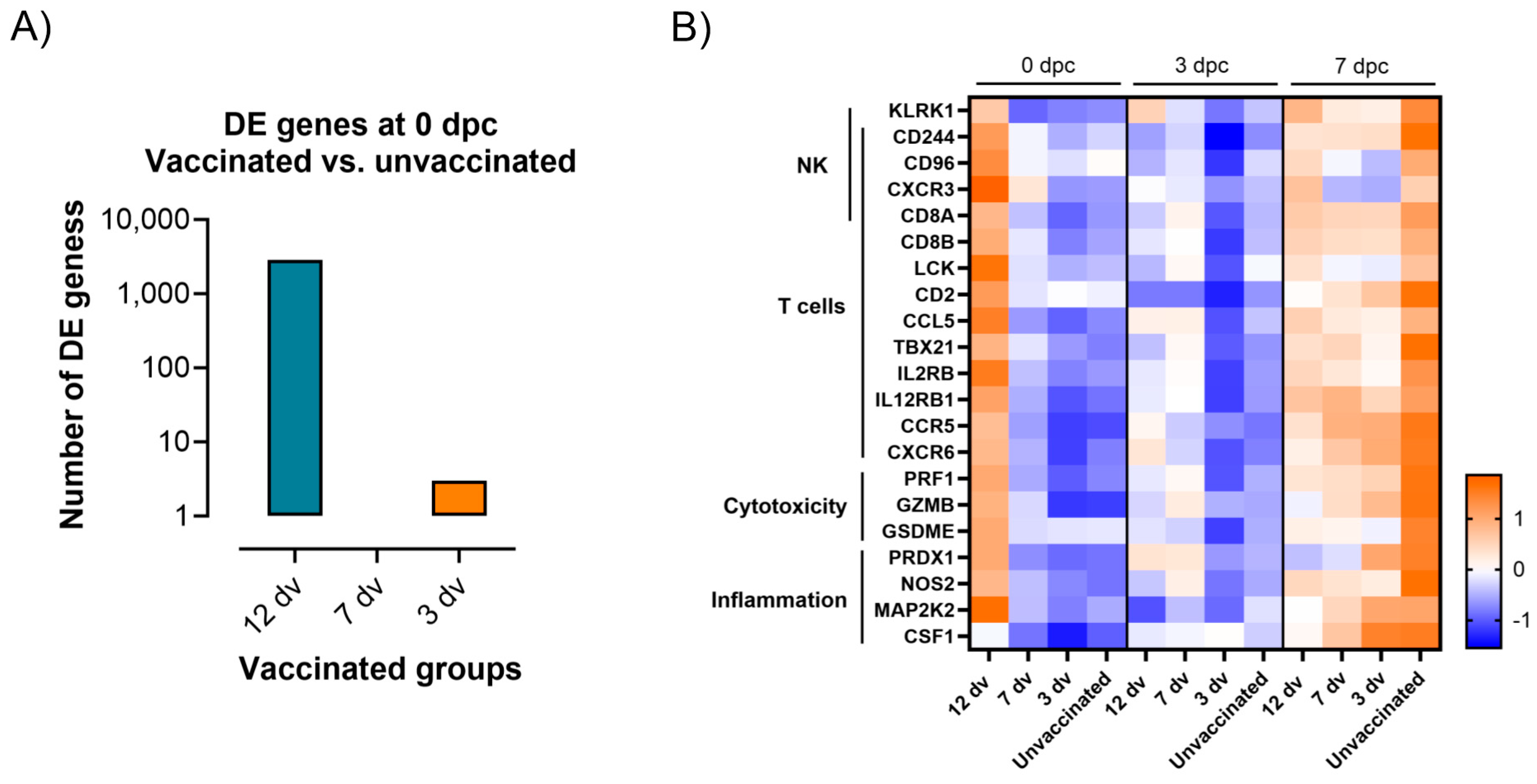
3.4. Control of Infection Is Associated with a Blood Cytotoxic Transcriptomic Signature before Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penrith, M.L.; Vosloo, W. Review of African Swine Fever: Transmission, Spread and Control. J. S. Afr. Vet. Assoc. 2009, 80, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African Swine Fever in Domestic Pigs and European Wild Boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Karalyan, Z.; Zakaryan, H.; Arzumanyan, H.; Sargsyan, K.; Voskanyan, H.; Hakobyan, L.; Abroyan, L.; Avetisyan, A.; Karalova, E. Pathology of Porcine Peripheral White Blood Cells during Infection with African Swine Fever Virus. BMC Vet. Res. 2012, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Pedrera, M.; Sánchez-Cordón, P.J. African Swine Fever Virus Infection and Cytokine Response In Vivo: An Update. Viruses 2023, 15, 233. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhang, Y.; Yang, J.; Wang, L.; Qi, Y.; Han, X.; Zhou, X.; Miao, F.; Chen, T.; et al. Cytokine Storm in Domestic Pigs Induced by Infection of Virulent African Swine Fever Virus. Front. Vet. Sci. 2020, 7, 601641. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Sánchez-Cordón, P.J.; Núñez, A.; Fernández de Marco, M.; Gómez-Villamandos, J.C. Proinflammatory Cytokines Induce Lymphocyte Apoptosis in Acute African Swine Fever Infection. J. Comp. Pathol. 2005, 132, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Podgórska, K. African Swine Fever: Transmission, Spread, and Control through Biosecurity and Disinfection, Including Polish Trends. Viruses 2023, 15, 2275. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African Swine Fever Vaccines: A Promising Work Still in Progress. Porcine Health Manag. 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, S.; Zhang, H.; Qin, Z.; Shan, H.; Cai, X. Vaccines for African Swine Fever: An Update. Front. Microbiol. 2023, 14, 1139494. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African Swine Fever Virus Vaccine Candidate ASFV-G-ΔI177L Efficiently Protects European and Native Pig Breeds against Circulating Vietnamese Field Strain. Transbound. Emerg. Dis. 2021, 69, e497–e504. [Google Scholar]
- Wang, Z.; Zhang, J.; Li, F.; Zhang, Z.; Chen, W.; Zhang, X.; Sun, E.; Zhu, Y.; Liu, R.; He, X.; et al. The Attenuated African Swine Fever Vaccine HLJ/18-7GD Provides Protection against Emerging Prevalent Genotype II Variants in China. Emerg. Microbes Infect. 2024, 13, 2300464. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly Lethal Genotype I and II Recombinant African Swine Fever Viruses Detected in Pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Camós, L.; Alonso, U.; Esteve-Codina, A.; Chang, C.-Y.; Martín-Mur, B.; Accensi, F.; Muñoz, M.; Navas, M.J.; Dabad, M.; Vidal, E.; et al. Cross-Protection against African Swine Fever Virus upon Intranasal Vaccination Is Associated with an Adaptive-Innate Immune Crosstalk. PLoS Pathog. 2022, 18, e1010931. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Krug, P.W.; Ramirez-Medina, E.; Valladares, A.; Rai, A.; Espinoza, N.; Gladue, D.P.; Borca, M.V. The Presence of Virus Neutralizing Antibodies Is Highly Associated with Protection against Virulent Challenge in Domestic Pigs Immunized with ASFV Live Attenuated Vaccine Candidates. Pathogens 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Argilaguet, J.M.; Pérez-Martín, E.; Nofrarías, M.; Gallardo, C.; Accensi, F.; Lacasta, A.; Mora, M.; Ballester, M.; Galindo-Cardiel, I.; López-Soria, S.; et al. DNA Vaccination Partially Protects against African Swine Fever Virus Lethal Challenge in the Absence of Antibodies. PLoS ONE 2012, 7, e40942. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Galindo-Cardiel, I.; Ballester, M.; Solanes, D.; Nofrarías, M.; López-Soria, S.; Argilaguet, J.M.; Lacasta, A.; Accensi, F.; Rodríguez, F.; Segalés, J. Standardization of Pathological Investigations in the Framework of Experimental ASFV Infections. Virus Res. 2013, 173, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Soler, A.; Nieto, R.; Carrascosa, A.L.; De Mia, G.M.; Bishop, R.P.; Martins, C.; Fasina, F.O.; Couacy-Hymman, E.; Heath, L.; et al. Comparative Evaluation of Novel African Swine Fever Virus (ASF) Antibody Detection Techniques Derived from Specific ASF Viral Genotypes with the OIE Internationally Prescribed Serological Tests. Vet. Microbiol. 2013, 162, 32–43. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; O’Donnell, V.; Alfano, M.; Velazquez Salinas, L.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Higgs, S.; Borca, M.V. Association of the Host Immune Response with Protection Using a Live Attenuated African Swine Fever Virus Model. Viruses 2016, 8, 291. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2016, 91, e01760-16. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, E.; Mehinagic, K.; Wüthrich, T.; Hilty, M.; Posthaus, H.; Summerfield, A.; Ruggli, N.; Benarafa, C. The Baseline Immunological and Hygienic Status of Pigs Impact Disease Severity of African Swine Fever. PLoS Pathog. 2022, 18, e1010522. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Blazek, K.; Byrne, A.J.; Perocheau, D.P.; Udalova, I.A. IRF5 Is a Specific Marker of Inflammatory Macrophages in Vivo. Mediat. Inflamm. 2013, 2013, 245804. [Google Scholar] [CrossRef] [PubMed]
- Paun, A.; Bankoti, R.; Joshi, T.; Pitha, P.M.; Stäger, S. Critical Role of IRF-5 in the Development of T Helper 1 Responses to Leishmania Donovani Infection. PLoS Pathog. 2011, 7, e1001246. [Google Scholar] [CrossRef]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African Swine Fever Viruses Emerged in Domestic Pigs in China and Caused Chronic Infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef]
- Graham, S.P.; Everett, H.E.; Haines, F.J.; Johns, H.L.; Sosan, O.A.; Salguero, F.J.; Clifford, D.J.; Steinbach, F.; Drew, T.W.; Crooke, H.R. Challenge of Pigs with Classical Swine Fever Viruses after C-Strain Vaccination Reveals Remarkably Rapid Protection and Insights into Early Immunity. PLoS ONE 2012, 7, e29310. [Google Scholar] [CrossRef]
- Bohórquez, J.A.; Wang, M.; Díaz, I.; Alberch, M.; Pérez-Simó, M.; Rosell, R.; Gladue, D.P.; Borca, M.V.; Ganges, L. The FlagT4G Vaccine Confers a Strong and Regulated Immunity and Early Virological Protection against Classical Swine Fever. Viruses 2022, 14, 1954. [Google Scholar] [CrossRef]
- Horsington, J.; Perez, C.B.; Maradei, E.; Novo, S.G.; Gonzales, J.L.; Singanallur, N.B.; Bonastre, P.; Vosloo, W. Protective Effects of High-Potency FMDV O1 Manisa Monovalent Vaccine in Cattle Challenged with FMDV O/SKR/2010 at 7 or 4 Days Post Vaccination. Vaccine 2017, 35, 5179–5185. [Google Scholar] [CrossRef]
- Cox, S.J.; Parida, S.; Voyce, C.; Reid, S.M.; Hamblin, P.A.; Hutchings, G.; Paton, D.J.; Barnett, P.V. Further Evaluation of Higher Potency Vaccines for Early Protection of Cattle against FMDV Direct Contact Challenge. Vaccine 2007, 25, 7687–7695. [Google Scholar] [CrossRef] [PubMed]
- Eblé, P.L.; Bouma, A.; de Bruin, M.G.M.; van Hemert-Kluitenberg, F.; van Oirschot, J.T.; Dekker, A. Vaccination of Pigs Two Weeks before Infection Significantly Reduces Transmission of Foot-and-Mouth Disease Virus. Vaccine 2004, 22, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively Transferred African Swine Fever Virus Antibodies Protect Swine against Lethal Infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; Mebus, C.A.; McVicar, J.W. African Swine Fever in Neonatal Pigs: Passively Acquired Protection from Colostrum or Serum of Recovered Pigs. Am. J. Vet. Res. 1984, 45, 1367–1372. [Google Scholar]
- Leitão, A.; Cartaxeiro, C.; Coelho, R.; Cruz, B.; Parkhouse, R.M.E.; Portugal, F.C.; Vigário, J.D.; Martins, C.L.V. The Non-Haemadsorbing African Swine Fever Virus Isolate ASFV/NH/P68 Provides a Model for Defining the Protective Anti-Virus Immune Response. J. Gen. Virol. 2001, 82, 513–523. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M.E. In Vivo Depletion of CD8+ T Lymphocytes Abrogates Protective Immunity to African Swine Fever Virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- Takamatsu, H.-H.; Denyer, M.S.; Lacasta, A.; Stirling, C.M.A.; Argilaguet, J.M.; Netherton, C.L.; Oura, C.A.L.; Martins, C.; Rodríguez, F. Cellular Immunity in ASFV Responses. Virus Res. 2013, 173, 110–121. [Google Scholar] [CrossRef]
- Webster, R.G.; Askonas, B.A. Cross-Protection and Cross-Reactive Cytotoxic T Cells Induced by Influenza Virus Vaccines in Mice. Eur. J. Immunol. 1980, 10, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Müllbacher, A.; Lobigs, M.; Alsharifi, M.; Regner, M. Cytotoxic T-Cell Immunity as a Target for Influenza Vaccines. Lancet Infect. Dis. 2006, 6, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Franzoni, G.; Netherton, C.L.; Hartmann, L.; Blome, S.; Blohm, U. Adaptive Cellular Immunity against African Swine Fever Virus Infections. Pathogens 2022, 11, 274. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Moraleda, D.; Muñoz-Basagoiti, J.; Tort-Miró, A.; Navas, M.J.; Muñoz, M.; Vidal, E.; Cobos, À.; Martín-Mur, B.; Meas, S.; Motuzova, V.; et al. Elucidating the Onset of Cross-Protective Immunity after Intranasal Vaccination with the Attenuated African Swine Fever Vaccine Candidate BA71ΔCD2. Vaccines 2024, 12, 517. https://doi.org/10.3390/vaccines12050517
Marín-Moraleda D, Muñoz-Basagoiti J, Tort-Miró A, Navas MJ, Muñoz M, Vidal E, Cobos À, Martín-Mur B, Meas S, Motuzova V, et al. Elucidating the Onset of Cross-Protective Immunity after Intranasal Vaccination with the Attenuated African Swine Fever Vaccine Candidate BA71ΔCD2. Vaccines. 2024; 12(5):517. https://doi.org/10.3390/vaccines12050517
Chicago/Turabian StyleMarín-Moraleda, David, Jordana Muñoz-Basagoiti, Aida Tort-Miró, María Jesús Navas, Marta Muñoz, Enric Vidal, Àlex Cobos, Beatriz Martín-Mur, Sochanwattey Meas, Veronika Motuzova, and et al. 2024. "Elucidating the Onset of Cross-Protective Immunity after Intranasal Vaccination with the Attenuated African Swine Fever Vaccine Candidate BA71ΔCD2" Vaccines 12, no. 5: 517. https://doi.org/10.3390/vaccines12050517
APA StyleMarín-Moraleda, D., Muñoz-Basagoiti, J., Tort-Miró, A., Navas, M. J., Muñoz, M., Vidal, E., Cobos, À., Martín-Mur, B., Meas, S., Motuzova, V., Chang, C.-Y., Gut, M., Accensi, F., Pina-Pedrero, S., Núñez, J. I., Esteve-Codina, A., Gavrilov, B., Rodriguez, F., Liu, L., & Argilaguet, J. (2024). Elucidating the Onset of Cross-Protective Immunity after Intranasal Vaccination with the Attenuated African Swine Fever Vaccine Candidate BA71ΔCD2. Vaccines, 12(5), 517. https://doi.org/10.3390/vaccines12050517








