Vaccines for the Elderly and Vaccination Programs in Europe and the United States
Abstract
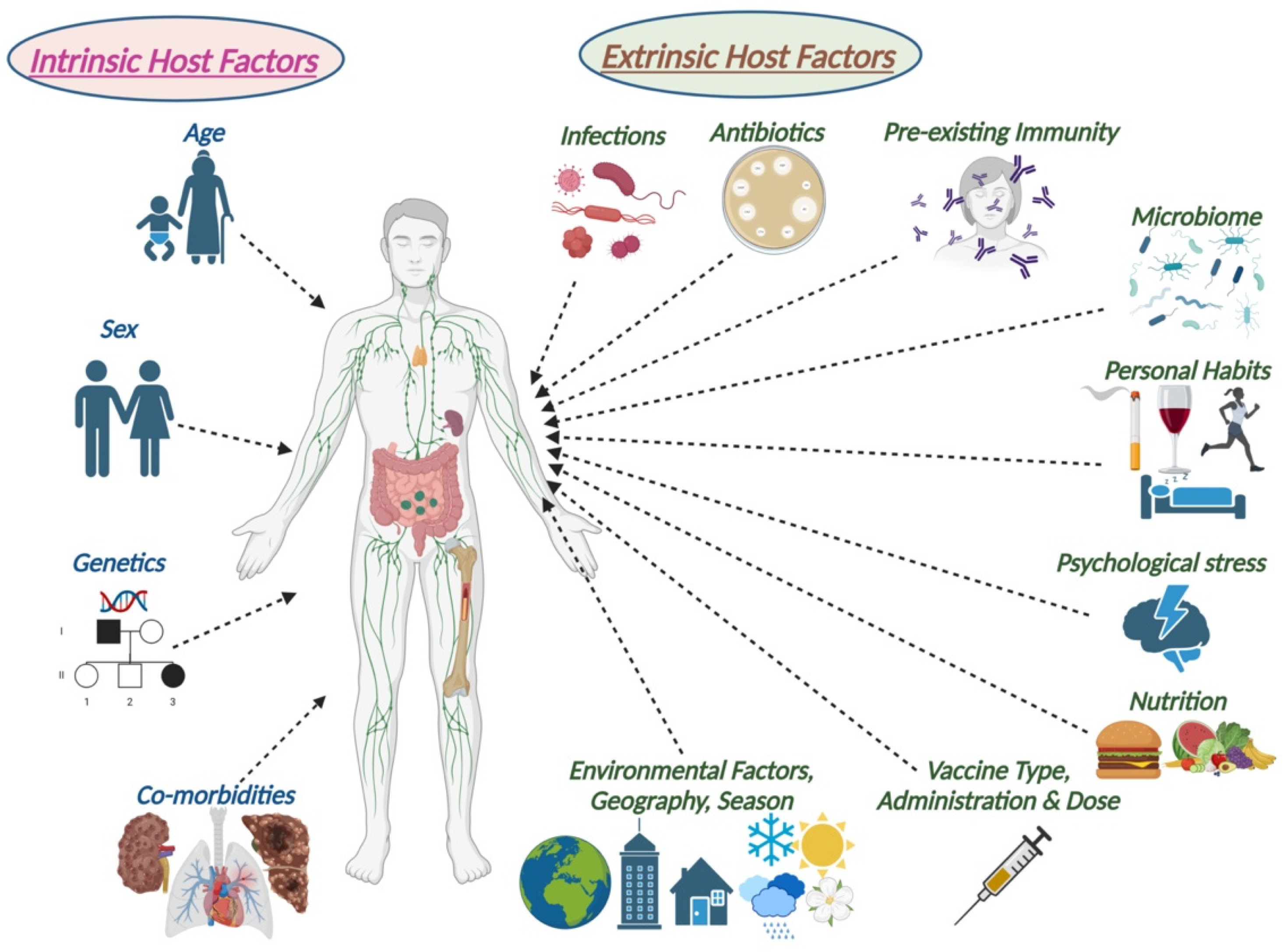
1. Introduction
1.1. The Aging of the Global Population
1.2. The Aging of the Immune System
1.3. The Need for Vaccine-Induced Immunity in the Elderly
2. Methods
3. Results
3.1. Herpes Zoster Vaccines and the Elderly
3.1.1. Epidemiology and Clinical Manifestations of Varicella and Herpes Zoster
3.1.2. Immunology of VZV Latency
3.1.3. Vaccines against VZV reactivation and Herpes Zoster
3.1.4. Efficacy of ZLV in the Elderly
3.1.5. Improving VZV Vaccination—The Use of RZV
3.2. Influenza Vaccines and the Elderly
3.2.1. Epidemiology and Clinical Manifestations of Influenza
3.2.2. Immunology of Influenza and the Elderly
3.2.3. High-Dose vs. Standard-Dose Influenza Vaccines
3.2.4. Adjuvanted vs. Standard-Dose Influenza Vaccines
3.2.5. Recombinant Hemagglutinin Vaccine
3.2.6. Which Augmented Vaccine for the Elderly?
3.3. RSV Vaccines and the Elderly
3.3.1. Epidemiology and Clinical Manifestations of RSV Infection
3.3.2. Immunology of RSV Infection and Vaccine Design
3.3.3. RSV Vaccines and Recommendations for the Elderly
3.4. COVID-19 Vaccines and the Elderly
3.4.1. Epidemiology and Clinical Manifestations of SARS-CoV-2 Infection
3.4.2. Immunology of SARS-CoV-2 Infection
3.4.3. The Effect of Age on COVID-19 Vaccine Responsiveness—Pivotal Clinical Trials
3.4.4. Vaccination and Duration of Immunity—Real-World Data and the Role of Boosters
3.4.5. Putting It All Together
3.4.6. Safety and COVID-19 Vaccination
3.4.7. COVID-19 Vaccination Recommendations for Older Adults
3.5. Pneumococcal Vaccines and the Elderly
3.5.1. Epidemiology and Clinical Manifestations of Pneumococcal Disease
3.5.2. Available Vaccines for Pneumococcal Disease
3.5.3. PPV—Protecting against Invasive or Non-Bacteremic Disease?
4. Discussion
5. Conclusions
- Infectious diseases are among the most common causes of death in the elderly, especially in those with comorbidities and weakened immune systems.
- Vaccination of the elderly reduces the risk of severe infections and of related hospitalizations and complications, as well as mortality rates associated with vaccine-preventable diseases.
- By reducing the incidence of infectious diseases, vaccination of the elderly potentially reduces the need for antibiotics use, indirectly affecting bacterial resistance, while reducing treatment and other related economic costs.
- Currently available vaccines are still unable to provide long-term protection. Despite reduced immunogenicity, vaccination of older adults may still provide significant benefits in terms of reducing the risk of severe illness, hospitalizations, and complications.
- Vaccines that induce long-lasting immune responses by strengthening cellular and mucosal immunity are essential for the elderly.
- There are significant differences between immunization policies, especially between European countries, but also between Europe and the US, in terms of recipient age, number of doses, and vaccination schedule and implementation (mandatory or recommended).
- A consensus-based strategy in Europe could help to fill the gaps in immunization policy in the elderly, particularly regarding RSV and pneumococcal vaccination.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACIP | (CDC’s) Advisory Committee on Immunization Practice |
| ARDS | Acute respiratory distress syndrome |
| BCG | Bacillus Calmette–Guérin vaccine |
| CDC | Centers for Disease Control and Prevention |
| DIC | Disseminated intravascular coagulation |
| ECDC | European Centre for Disease Prevention and Control |
| EEA | European Economic Area |
| EU | European Union |
| HA | Hemagglutinin |
| HZ | Herpes zoster (shingles) |
| ICU | Intensive care unit |
| IPD | Invasive pneumococcal disease |
| MMR | Measles, mumps, rubella |
| MMRV | MMR with varicella |
| NA | Neuraminidase |
| PP | Pneumococcal pneumonia |
| PCVs | Pneumococcal conjugate vaccines |
| PPVs | Pneumococcal polysaccharide vaccines |
| PHN | Post-herpetic neuralgia |
| RBD | Receptor-binding domain |
| RSV | Respiratory syncytial virus |
| RSV-ARI | RSV-related acute respiratory illness |
| RSV-LRTD | RSV-related lower respiratory tract disease |
| RZV | Recombinant zoster vaccine |
| ZLV | Zoster live vaccine |
| VZV | Varicella zoster virus |
| WHO | World Health Organization |
References
- GBD 2021 Demographics Collaborators. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Federation of European Academies of Medicine (FEAM). Immunisation for Old Adults in Europe: Scientific and Social Strategies. Feam Report. 2022. Available online: https://www.feam.eu/wp-content/uploads/Adult-Vaccination-Report-Design-V12-23-March-2022.pdf (accessed on 21 March 2024).
- Cunningham, A.L.; McIntyre, P.; Subbarao, K.; Booy, R.; Levin, M.J. Vaccines for older adults. BMJ 2021, 372, n188. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Caterino, J.M.; Kline, D.M.; Leininger, R.; Southerland, L.T.; Carpenter, C.R.; Baugh, C.W.; Pallin, D.J.; Hunold, K.M.; Stevenson, K.B. Nonspecific Symptoms Lack Diagnostic Accuracy for Infection in Older Patients in the Emergency Department. J. Am. Geriatr. Soc. 2019, 67, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Toubi, E.; Porat, B.S.; Shoenfeld, Y. Autoimmunity in the Elderly: Insights from Basic Science and Clinics—A Mini-Review. Gerontology 2017, 63, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J.; Netea, M.G. Overcoming immune dysfunction in the elderly: Trained immunity as a novel approach. Int. Immunol. 2020, 32, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M. The effect of ageing of the immune system on vaccination responses. Hum. Vaccin. Immunother. 2013, 9, 1364–1367. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef]
- Tran Van Hoi, E.; De Glas, N.A.; Portielje, J.E.A.; Van Heemst, D.; Van Den Bos, F.; Jochems, S.P.; Mooijaart, S.P. Biomarkers of the ageing immune system and their association with frailty—A systematic review. Exp. Gerontol. 2023, 176, 112163. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R.L.; Klein, S.L. Sex Differences in Respiratory Viral Pathogenesis and Treatments. Annu Rev. Virol. 2021, 8, 393–414. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Soegiarto, G.; Purnomosari, D. Challenges in the Vaccination of the Elderly and Strategies for Improvement. Pathophysiology 2023, 30, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Hamson, E.; Forbes, C.; Wittkopf, P.; Pandey, A.; Mendes, D.; Kowalik, J.; Czudek, C.; Mugwagwa, T. Impact of pandemics and disruptions to vaccination on infectious diseases epidemiology past and present. Hum. Vaccin. Immunother. 2023, 19, 2219577. [Google Scholar] [CrossRef] [PubMed]
- Ates Bulut, E.; Badak, S.O.; Aksoy, H.; Fadiloglu, A.; Isik, A.T. The Awareness and Attitude of Physicians to Older Adult Routine Vaccination Scheme. Clin. Interv. Aging 2022, 17, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A.; Gershon, M.D. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin. Microbiol. Rev. 2013, 26, 728–743. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly. Epidemiol. Rec. 2014, 89, 265–288. [Google Scholar]
- Kennedy, P.G.E.; Gershon, A.A. Clinical Features of Varicella-Zoster Virus Infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Grinfeld, E.; Gow, J.W. Latent varicella-zoster virus is located predominantly in neurons in human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 1998, 95, 4658–4662. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Gershon, A.; Gershon, M.; Cohen, J.I.; Arvin, A.; Zerboni, L.; Zhu, H.; Gray, W.; Messaoudi, I.; Traina-Dorge, V. Current In Vivo Models of Varicella-Zoster Virus Neurotropism. Viruses 2019, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.A.; Patel, B.C. Herpes Zoster. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441824/ (accessed on 21 March 2024). [PubMed]
- Schmader, K. Herpes Zoster. Clin. Geriatr Med. 2016, 32, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Opstelten, W.; Eekhof, J.; Neven, A.K.; Verheij, T. Treatment of herpes zoster. Can. Fam. Physician. 2008, 54, 373–377. [Google Scholar] [PubMed]
- John, A.R.; Canaday, D.H. Herpes Zoster in the Older Adult. Infect Dis. Clin. N. Am. 2017, 31, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Hall, A.J. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bardach, A.E.; Palermo, C.; Alconada, T.; Sandoval, M.; Balan, D.J.; Guevara, J.N.; Gómez, J.; Ciapponi, A. Herpes zoster epidemiology in Latin America: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255877. [Google Scholar] [CrossRef] [PubMed]
- Gruver, C.; Guthmiller, K.B. Postherpetic Neuralgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493198/ (accessed on 21 March 2024).
- Delaney, A.; Colvin, L.A.; Fallon, M.T.; Dalziel, R.G.; Mitchell, R.; Fleetwood-Walker, S.M. Postherpetic neuralgia: From preclinical models to the clinic. Neurotherapeutics 2009, 6, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Forbes, H.J.; Thomas, S.L.; Langan, S.M. The Epidemiology and Prevention of Herpes Zoster. Curr. Derm. Rep. 2012, 1, 39–47. [Google Scholar] [CrossRef]
- Kawai, K.; Gebremeskel, B.G.; Acosta, C.J. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open 2014, 4, e004833. [Google Scholar] [CrossRef]
- Marin, M.; Leung, J.; Anderson, T.C.; Lopez, A.S. Monitoring Varicella Vaccine Impact on Varicella Incidence in the United States: Surveillance Challenges and Changing Epidemiology, 1995–2019. J. Infect Dis. 2022, 226 (Suppl. S4), S392–S399. [Google Scholar] [CrossRef] [PubMed]
- Herman, L.; Levin, M.J.; Rehm, S. Shedding Light on Shingles: The Power of Prevention. Am. J. Med. 2016, 129, 1137. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (2015). Varicella Vaccination in the European Union. Stockholm: ECDC. Available online: http://ecdc.europa.eu/en/publications/Publications/Varicella-Guidance-2015.pdf (accessed on 30 March 2024).
- Carrillo-Santisteve, P.; Lopalco, P.L. Varicella vaccination: A laboured take-off. Clin. Microbiol. Inf. 2014, 20, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hope-Simpson, R.E. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc. R Soc. Med. 1965, 58, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Anthony LCunningham Myron JLevin Thomas, C. Heineman. Chapter 4—Herpes Zoster Vaccines: What’s New? In Vaccinations; Poland, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–73. [Google Scholar] [CrossRef]
- Oxman, M.N. Zoster vaccine: Current status and future prospects. Clin. Infect Dis. 2010, 51, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology 1980, 30, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Park, S.K.; Kumar, M.; Lee, C.H.; Shin, O.S. Insights into the role of immunosenescence during varicella zoster virus infection (shingles) in the aging cell model. Oncotarget 2015, 6, 35324–35343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asada, H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: The SHEZ study. Vaccine 2019, 37, 6776–6781. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, R.; Ortega-Sanchez, I.R.; Seward, J.F.; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2008, 57, 1–30. [Google Scholar]
- Brisson, M.; Gay, N.J.; Edmunds, W.J.; Andrews, N.J. Exposure to varicella boosts immunity to herpes-zoster: Implications for mass vaccination against chickenpox. Vaccine 2002, 20, 2500–2507. [Google Scholar] [CrossRef]
- Harpaz, R.; van Hoek, A.J. Point-Counterpoint: The Hope-Simpson Hypothesis and Its Implications Regarding an Effect of Routine Varicella Vaccination on Herpes Zoster Incidence. J. Infect Dis. 2018, 218 (Suppl. S2), S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Dooling, K.; Marin, M.; Anderson, T.C.; Harpaz, R. The Impact of Universal Varicella Vaccination on Herpes Zoster Incidence in the United States: Comparison of Birth Cohorts Preceding and Following Varicella Vaccination Program Launch. J. Infect. Dis. 2022, 226 (Suppl. S4), S470–S477. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, G.; Bolognesi, N.; Sandri, F.; Florescu, C.; Stefanati, A. Varicella zoster virus vaccines: An update. Immunotargets Ther. 2019, 8, 15–28. [Google Scholar] [CrossRef] [PubMed]
- CDC Guidelines RZV. Available online: https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/recommendations.html (accessed on 27 March 2024).
- Harbecke, R.; Cohen, J.I.; Oxman, M.N. Herpes Zoster Vaccines. J. Infect Dis. 2021, 224 (Suppl. S2), S429–S442. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Burny, W.; Hervé, C.; Hyung Kim, J.; Levin, M.J.; Zahaf, T.; Cunningham, A.L.; Didierlaurent, A.M. Association Between Immunogenicity and Reactogenicity: A Post Hoc Analysis of 2 Phase 3 Studies with the Adjuvanted Recombinant Zoster Vaccine. J. Infect Dis. 2022, 226, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- FDA. Zostavax Package Insert. Available online: https://www.fda.gov/media/82524/download?unique_id=aYVJ_1661040000084 (accessed on 27 March 2024).
- Levin, M.J.; Oxman, M.N.; Zhang, J.H.; Johnson, G.R.; Stanley, H.; Hayward, A.R.; Caulfield, M.J.; Irwin, M.R.; Smith, J.G.; Clair, J.; et al. Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect Dis. 2008, 197, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Pang, L.; Johnson, M.J.; Caldas, Y.; Cho, A.; Tovar-Salazar, A.; Canniff, J.; Schmader, K.E.; Popmihajlov, Z.; Levin, M.J. The Effect of Age on the Immunogenicity of the Live Attenuated Zoster Vaccine Is Predicted by Baseline Regulatory T Cells and Varicella-Zoster Virus-Specific T Cell Immunity. J. Virol. 2019, 93, e00305-19. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Smith, N.; Harpaz, R.; Bialek, S.R.; Sy, L.S.; Jacobsen, S.J. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011, 305, 160–166. [Google Scholar] [CrossRef]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Marks, M.A.; Hansen, J.; Lewis, E.; Aukes, L.; Saddier, P. Effectiveness of the live zoster vaccine during the 10 years following vaccination: Real world cohort study using electronic health records. BMJ 2023, 383, e076321. [Google Scholar] [CrossRef]
- Schmader, K.E.; Oxman, M.N.; Levin, M.J.; Johnson, G.; Zhang, J.H.; Betts, R.; Morrison, V.A.; Gelb, L.; Guatelli, J.C.; Harbecke, R.; et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin. Infect Dis. 2012, 55, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.J.; Murray, M.; Zerbe, G.O.; White, C.J.; Hayward, A.R. Immune responses of elderly persons 4 years after receiving a live attenuated varicella vaccine. J. Infect Dis. 1994, 170, 522–526. [Google Scholar] [CrossRef]
- Levin, M.J.; Barber, D.; Goldblatt, E.; Jones, M.; LaFleur, B.; Chan, C.; Stinson, D.; Zerbe, G.O.; Hayward, A.R. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: Duration of booster effect. J. Infect Dis. 1998, 178 (Suppl. S1), S109–S112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morrison, V.A.; Johnson, G.R.; Schmader, K.E.; Levin, M.J.; Zhang, J.H.; Looney, D.J.; Betts, R.; Gelb, L.; Guatelli, J.C.; Harbecke, R.; et al. Long-term persistence of zoster vaccine efficacy. Clin. Infect Dis. 2015, 60, 900–909. [Google Scholar] [CrossRef]
- Levin, M.J.; Schmader, K.E.; Pang, L.; Williams-Diaz, A.; Zerbe, G.; Canniff, J.; Johnson, M.J.; Caldas, Y.; Cho, A.; Lang, N.; et al. Cellular and Humoral Responses to a Second Dose of Herpes Zoster Vaccine Administered 10 Years After the First Dose Among Older Adults. J. Infect Dis. 2016, 213, 14–22. [Google Scholar] [CrossRef]
- Chlibek, R.; Bayas, J.M.; Collins, H.; de la Pinta, M.L.; Ledent, E.; Mols, J.F.; Heineman, T.C. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults >=50 years of age. J. Infect Dis. 2013, 208, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.; et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef]
- Boutry, C.; Hastie, A.; Diez-Domingo, J.; Tinoco, J.C.; Yu, C.J.; Andrews, C.; Beytout, J.; Caso, C.; Cheng, H.S.; Cheong, H.J.; et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-Term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase 3 Clinical Trials ZOE-50 and ZOE-70. Clin. Infect Dis. 2022, 74, 1459–1467. [Google Scholar] [CrossRef]
- Sun, Y.; Jackson, K.; Dalmon, C.A.; Shapiro, B.L.; Nie, S.; Wong, C.; Arnold, B.F.; Porco, T.C.; Acharya, N.R. Effectiveness of the recombinant zoster vaccine among Kaiser Permanente Hawaii enrollees aged 50 and older: A retrospective cohort study. Vaccine 2021, 39, 3974–3982. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Wu, X.; Forshee, R.; Lu, Y.; Sung, H.M.; Agger, P.E.; Chillarige, Y.; Link-Gelles, R.; Lufkin, B.; Wernecke, M.; et al. Recombinant Zoster Vaccine (Shingrix): Real-World Effectiveness in the First 2 Years Post-Licensure. Clin. Infect Dis. 2021, 73, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kim, E.; Kong, C.L.; Arnold, B.F.; Porco, T.C.; Acharya, N.R. Effectiveness of the Recombinant Zoster Vaccine in Adults Aged 50 and Older in the United States: A Claims-Based Cohort Study. Clin. Infect Dis. 2021, 73, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Factsheet about Seasonal Influenza. 2024. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet (accessed on 25 March 2024).
- Agor, J.K.; Özaltın, O.Y. Models for predicting the evolution of influenza to inform vaccine strain selection. Hum. Vaccin. Immunother. 2018, 14, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Boktor, S.W.; Hafner, J.W. Influenza. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Near, A.; Tse, J.; Young-Xu, Y.; Hong, D.; Reyes, C. Pin24 Incidence and costs of influenza-related hospitalizations by comorbidity in the United States. Value Health 2020, 23 (Suppl. S1), S172–S173. [Google Scholar] [CrossRef]
- Langer, J.; Welch, V.L.; Moran, M.M.; Cane, A.; Lopez, S.M.C.; Srivastava, A.; Enstone, A.L.; Sears, A.; Markus, K.J.; Heuser, M.; et al. High Clinical Burden of Influenza Disease in Adults Aged ≥ 65 Years: Can We Do Better? A Systematic Literature Review. Adv. Ther. 2023, 40, 1601–1627. [Google Scholar] [CrossRef] [PubMed]
- Lafond, K.E.; Porter, R.M.; Whaley, M.J.; Suizan, Z.; Ran, Z.; Aleem, M.A.; Thapa, B.; Sar, B.; Proschle, V.S.; Peng, Z.; et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Med. 2021, 18, e1003550. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 25 March 2024).
- McElhaney, J.E.; Verschoor, C.P.; Andrew, M.K.; Haynes, L.; Kuchel, G.A.; Pawelec, G. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing 2020, 17, 10. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Flu & People 65 Years and Older. 2021. Available online: https://www.cdc.gov/flu/highrisk/65over.htm (accessed on 23 March 2024).
- WHO Influenza Vaccine Announcement. Available online: https://www.who.int/news/item/23-02-2024-recommendations-announced-for-influenza-vaccine-composition-for-the-2024-2025-northern-hemisphere-influenza-season (accessed on 26 March 2024).
- Ellebedy, A.H.; Webby, R.J. Influenza vaccines. Vaccine 2009, 27 (Suppl. S4), D65–D68. [Google Scholar] [CrossRef]
- Eichelberger, M.C.; Wan, H. Influenza neuraminidase as a vaccine antigen. Curr. Top. Microbiol. Immunol. 2015, 386, 275–299. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Montomoli, E. Influenza immunology evaluation and correlates of protection: A focus on vaccines. Expert Rev. Vaccines 2016, 15, 967–976. [Google Scholar] [CrossRef]
- Dunning, A.J.; DiazGranados, C.A.; Voloshen, T.; Hu, B.; Landolfi, V.A.; Talbot, H.K. Correlates of Protection against Influenza in the Elderly: Results from an Influenza Vaccine Efficacy Trial. Clin. Vaccine Immunol. 2016, 23, 228–235. [Google Scholar] [CrossRef]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef]
- Hobson, D.; Curry, R.L.; Beare, A.S.; Ward-Gardner, A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. Epidemiology Infect. 1972, 70, 767–777. [Google Scholar] [CrossRef]
- Keilich, S.R.; Bartley, J.M.; Haynes, L. Diminished immune responses with aging predispose older adults to common and uncommon influenza complications. Cell Immunol. 2019, 345, 103992. [Google Scholar] [CrossRef]
- Bernstein, E.; Kaye, D.; Abrutyn, E.; Gross, P.; Dorfman, M.; Murasko, D.M. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999, 17, 82–94. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun. Ageing 2020, 17, 37. [Google Scholar] [CrossRef]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Uemura, K.; Ono, S.; Michihata, N.; Yamana, H.; Yasunaga, H. Duration of influenza vaccine effectiveness in the elderly in Japan: A retrospective cohort study using large-scale population-based registry data. Vaccine 2023, 41, 3092–3098. [Google Scholar] [CrossRef]
- Dalcin, D.; Kwong, J.C. High-dose influenza vaccination. CMAJ 2019, 191, E313. [Google Scholar] [CrossRef]
- FDA. Approval Letter. Available online: https://www.fda.gov/media/132239/download?attachment (accessed on 26 March 2024).
- Chen, W.H.; Cross, A.S.; Edelman, R.; Sztein, M.B.; Blackwelder, W.C.; Pasetti, M.F. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine 2011, 29, 2865–2873. [Google Scholar] [CrossRef]
- Keitel, W.A.; Atmar, R.L.; Cate, T.R.; Petersen, N.J.; Greenberg, S.B.; Ruben, F.; Couch, R.B. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch. Intern. Med. 2006, 166, 1121–1127. [Google Scholar] [CrossRef]
- Noh, J.Y.; Jang, Y.S.; Lee, S.N.; Choi, M.J.; Yoon, J.G.; Yu, D.H.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Randomized, single-blind, active-controlled phase I clinical trial to evaluate the immunogenicity and safety of GC3114 (high-dose, quadrivalent influenza vaccine) in healthy adults. Vaccine 2019, 37, 5171–5176. [Google Scholar] [CrossRef]
- Johansen, N.D.; Modin, D.; Skaarup, K.G.; Nealon, J.; Samson, S.; Dufournet, M.; Loiacono, M.M.; Harris, R.C.; Larsen, C.S.; Jensen, A.M.R.; et al. Effectiveness of high-dose versus standard-dose quadrivalent influenza vaccine against recurrent hospitalizations and mortality in relation to influenza circulation: A post-hoc analysis of the DANFLU-1 randomized clinical trial. Clin. Microbiol. Infect. 2024. [Google Scholar] [CrossRef]
- Chaves, S.S.; Naeger, S.; Lounaci, K.; Zuo, Y.; Loiacono, M.M.; Pilard, Q.; Nealon, J.; Genin, M.; Mahe, C. High-Dose Influenza Vaccine Is Associated With Reduced Mortality Among Older Adults With Breakthrough Influenza Even When There Is Poor Vaccine-Strain Match. Clin. Infect Dis. 2023, 77, 1032–1042. [Google Scholar] [CrossRef]
- Robertson, C.A.; DiazGranados, C.A.; Decker, M.D.; Chit, A.; Mercer, M.; Greenberg, D.P. Fluzone® High-Dose Influenza Vaccine. Expert Rev. Vaccines. 2016, 15, 1495–1505. [Google Scholar] [CrossRef]
- Doyle, J.D.; Beacham, L.; Martin, E.T.; Talbot, H.K.; Monto, A.; Gaglani, M.; Middleton, D.B.; Silveira, F.P.; Zimmerman, R.K.; Alyanak, E.; et al. Relative and Absolute Effectiveness of High-Dose and Standard-Dose Influenza Vaccine Against Influenza-Related Hospitalization Among Older Adults-United States, 2015–2017. Clin. Infect Dis. 2021, 72, 995–1003. [Google Scholar] [CrossRef]
- Kaka, A.S.; Filice, G.A.; Myllenbeck, S.; Nichol, K.L. Comparison of Side Effects of the 2015–2016 High-Dose, Inactivated, Trivalent Influenza Vaccine and Standard Dose, Inactivated, Trivalent Influenza Vaccine in Adults ≥65 Years. Open Forum Infect. Dis. 2017, 4, ofx001. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Minutello, M.; Senatore, F.; Cecchinelli, G.; Bianchi, M.; Andreani, T.; Podda, A.; Crovari, P. Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. Vaccine 1999, 17, 99–104. [Google Scholar] [CrossRef]
- Khurana, S.; Verma, N.; Yewdell, J.W.; Hilbert, A.K.; Castellino, F.; Lattanzi, M.; Del Giudice, G.; Rappuoli, R.; Golding, H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 2011, 3, 85ra48. [Google Scholar] [CrossRef]
- Martin, J.T. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals 1997, 25, 209–213. [Google Scholar] [CrossRef]
- De Donato, S.; Granoff, D.; Minutello, M.; Lecchi, G.; Faccini, M.; Agnello, M.; Senatore, F.; Verweij, P.; Fritzell, B.; Podda, A. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine 1999, 17, 3094–3101. [Google Scholar] [CrossRef]
- Gasparini, R.; Pozzi, T.; Montomoli, E.; Fragapane, E.; Senatore, F.; Minutello, M.; Podda, A. Increased immunogenicity of the MF59-adjuvanted influenza vaccine compared to a conventional subunit vaccine in elderly subjects. Eur. J. Epidemiol. 2001, 17, 135–140. [Google Scholar] [CrossRef]
- Podda, A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine 2001, 19, 2673–2680. [Google Scholar] [CrossRef]
- Squarcione, S.; Sgricia, S.; Biasio, L.R.; Perinetti, E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 2003, 21, 1268–1274. [Google Scholar] [CrossRef]
- Domnich, A.; Arata, L.; Amicizia, D.; Puig-Barberà, J.; Gasparini, R.; Panatto, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Keitel, W.A.; Treanor, J.J.; El Sahly, H.M.; Gilbert, A.; Meyer, A.L.; Patriarca, P.A.; Cox, M.M. Comparative immunogenicity of recombinant influenza hemagglutinin (rHA) and trivalent inactivated vaccine (TIV) among persons > or =65 years old. Vaccine 2009, 28, 379–385. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M.J.; PSC12 Study Team. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Patricia Nowalk, M.; Dauer, K.; Clarke, L.; Raviotta, J.M.; Balasubramani, G.K. Vaccine effectiveness of recombinant and standard dose influenza vaccines against influenza related hospitalization using a retrospective test-negative design. Vaccine 2023, 41, 5134–5140. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Dauer, K.; Clarke, L.; Nowalk, M.P.; Raviotta, J.M.; Balasubramani, G.K. Vaccine effectiveness of recombinant and standard dose influenza vaccines against outpatient illness during 2018–2019 and 2019–2020 calculated using a retrospective test-negative design. Hum. Vaccin Immunother. 2023, 19, 2177461. [Google Scholar] [CrossRef]
- Cowling, B.J.; Perera, R.A.P.M.; Valkenburg, S.A.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C.; et al. Comparative Immunogenicity of Several Enhanced Influenza Vaccine Options for Older Adults: A Randomized, Controlled Trial. Clin. Infect Dis. 2020, 71, 1704–1714. [Google Scholar] [CrossRef]
- Domnich, A.; de Waure, C. Comparative effectiveness of adjuvanted versus high-dose seasonal influenza vaccines for older adults: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022, 122, 855–863. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin. Infect. Dis. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023–2024 Influenza Season. MMWR Recomm Rep. 2023, 72, 1–28. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Seasonal Influenza Vaccination Recommendations and Coverage Rates in EU/EEA Member States—An Overview of Vaccination Recommendations for 2021–22 and Coverage Rates for the 2018–19 to 2020–21 Influenza Seasons; ECDC: Stockholm, Sweden, 2023. [Google Scholar]
- Ogra, P.L. Respiratory syncytial virus: The virus, the disease and the immune response. Paediatr. Respir. Rev. 2004, 5, S119–S126. [Google Scholar] [CrossRef]
- Rosas-Salazar, C.; Chirkova, T.; Gebretsadik, T.; Chappell, J.D.; Peebles RSJr Dupont, W.D.; Jadhao, S.J.; Gergen, P.J.; Anderson, L.J.; Hartert, T.V. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): A population-based, prospective birth cohort study. Lancet 2023, 401, 1669–1680. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus--a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Foley, D.A.; Minney-Smith, C.A.; Lee, W.H.; Oakes, D.B.; Hazelton, B.; Ford, T.J.; Wadia, U.; Sikazwe, C.; Moore, H.C.; Nicol, M.P.; et al. Respiratory Syncytial Virus Reinfections in Children in Western Australia. Viruses 2023, 15, 2417. [Google Scholar] [CrossRef]
- Tin Tin Htar, M.; Yerramalla, M.S.; Moïsi, J.C.; Swerdlow, D.L. The burden of respiratory syncytial virus in adults: A systematic review and meta-analysis. Epidemiol. Infect. 2020, 148, e48. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Park, S.H.; Kim, M.A.; Kim, H.J.; Park, J.S.; Lee, M.Y.; Lee, C.W.; Dauti, S.; Choi, W.I. Risk of mortality associated with respiratory syncytial virus and influenza infection in adults. BMC Infect. Dis. 2017, 17, 785. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000, 13, 371–384. [Google Scholar] [CrossRef]
- Njue, A.; Nuabor, W.; Lyall, M.; Margulis, A.; Mauskopf, J.; Curcio, D.; Kurosky, S.; Gessner, B.D.; Begier, E. Systematic Literature Review of Risk Factors for Poor Outcomes Among Adults with Respiratory Syncytial Virus Infection in High-Income Countries. Open Forum Infect. Dis. 2023, 10, ofad513. [Google Scholar] [CrossRef]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 2004, 189, 233–238. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005, 22, 577–587. [Google Scholar] [CrossRef]
- Kenmoe, S.; Nair, H. The disease burden of respiratory syncytial virus in older adults. Curr. Opin. Infect. Dis. 2024, 37, 129–136. [Google Scholar] [CrossRef]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222 (Suppl. S7), S577–S583. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). RSV in Older Adults and Adults with Chronic Medical Conditions. 2024. Available online: https://www.cdc.gov/rsv/high-risk/older-adults.html (accessed on 26 March 2024).
- Redondo, E.; Rivero-Calle, I.; Mascarós, E.; Ocaña, D.; Jimeno, I.; Gil, Á.; Linares, M.; Onieva-García, M.Á.; González-Romo, F.; Yuste, J.; et al. Respiratory Syncytial Virus Vaccination Recommendations for Adults Aged 60 Years and Older: The NeumoExperts Prevention Group Position Paper. Arch. Bronconeumol. 2024, 60, 161–170. [Google Scholar] [CrossRef]
- Giersing, B.K.; Vekemans, J.; Nava, S.; Kaslow, D.C.; Moorthy, V.; WHO Product Development for Vaccines Advisory Committee. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8–10th June 2016. Vaccine 2019, 37, 7315–7327. [Google Scholar] [CrossRef]
- Habibi, M.S.; Chiu, C. Controlled human infection with RSV: The opportunities of experimental challenge. Vaccine 2017, 35, 489–495. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Rosenberg, H.F. Respiratory syncytial virus infection: Immune response, immunopathogenesis, and treatment. Clin. Microbiol. Rev. 1999, 12, 298–309. [Google Scholar] [CrossRef]
- Mills, J., 5th; Van Kirk, J.E.; Wright, P.F.; Chanock, R.M. Experimental respiratory syncytial virus infection of adults. Possible mechanisms of resistance to infection and illness. J. Immunol. 1971, 107, 123–130. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Relationship of serum antibody to risk of respiratory syncytial virus infection in elderly adults. J. Infect. Dis. 1998, 177, 463–466. [Google Scholar] [CrossRef]
- Shehata, L.; Wieland-Alter, W.F.; Maurer, D.P.; Chen, E.; Connor, R.I.; Wright, P.F.; Walker, L.M. Systematic comparison of respiratory syncytial virus-induced memory B cell responses in two anatomical compartments. Nat. Commun. 2019, 10, 1126. [Google Scholar] [CrossRef]
- Habibi, M.S.; Jozwik, A.; Makris, S.; Dunning, J.; Paras, A.; DeVincenzo, J.P.; de Haan, C.A.M.; Wrammert, J.; Openshaw, P.J.M.; Chiu, C. Impaired Antibody-mediated Protection and Defective IgA B-Cell Memory in Experimental Infection of Adults with Respiratory Syncytial Virus. Am. J. Respir. Crit. Care Med. 2015, 191, 1040–1049. [Google Scholar] [CrossRef]
- Morens, D.M.; Taubenberger, J.K.; Fauci, A.S. Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host Microbe 2023, 31, 146–157. [Google Scholar] [CrossRef]
- Yang, K.; Varga, S.M. Mucosal vaccines against respiratory syncytial virus. Curr. Opin. Virol. 2014, 6, 78–84. [Google Scholar] [CrossRef]
- Zohar, T.; Hsiao, J.C.; Mehta, N.; Das, J.; Devadhasan, A.; Karpinski, W.; Callahan, C.; Citron, M.P.; DiStefano, D.J.; Touch, S.; et al. Upper and lower respiratory tract correlates of protection against respiratory syncytial virus following vaccination of nonhuman primates. Cell Host Microbe 2022, 30, 41–52. [Google Scholar] [CrossRef]
- Venkatesan, P. First RSV vaccine approvals. Lancet Microbe 2023, 4, e577. [Google Scholar] [CrossRef]
- Feldman, R.G.; Antonelli-Incalzi, R.; Steenackers, K.; Lee, D.G.; Papi, A.; Ison, M.G.; Fissette, L.; David, M.P.; Maréchal, C.; Van der Wielen, M.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine Is Efficacious in Older Adults with Underlying Medical Conditions. Clin. Infect Dis. 2024, 78, 202–209. [Google Scholar] [CrossRef]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb. Mortal. Wkly Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC) Vaccine Scheduler, RSV: Recommended Vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=53&SelectedCountryIdByDisease=-1 (accessed on 19 March 2024).
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- WHO. COVID Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 19 March 2024).
- Anastassopoulou, C.; Gkizarioti, Z.; Patrinos, G.P.; Tsakris, A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020, 14, 40. [Google Scholar] [CrossRef]
- Oordt-Speets, A.; Spinardi, J.; Mendoza, C.; Yang, J.; Morales, G.; McLaughlin, J.M.; Kyaw, M.H. Effectiveness of COVID-19 Vaccination on Transmission: A Systematic Review. COVID 2023, 3, 1516–1527. [Google Scholar] [CrossRef]
- Soheili, M.; Khateri, S.; Moradpour, F.; Mohammadzedeh, P.; Zareie, M.; Mortazavi, S.M.M.; Manifar, S.; Kohan, H.G.; Moradi, Y. The efficacy and effectiveness of COVID-19 vaccines around the world: A mini-review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Hatziantoniou, S.; Boufidou, F.; Patrinos, G.P.; Tsakris, A. The Role of Oral Antivirals for COVID-19 Treatment in Shaping the Pandemic Landscape. J. Pers. Med. 2022, 12, 439. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/speeches/item/who-director-general-s-opening-remarks-at-the-media-briefing (accessed on 5 May 2023).
- Boufidou, F.; Medić, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int. J. Mol. Sci. 2023, 24, 12962. [Google Scholar] [CrossRef]
- Chen, C.L.; Teng, C.K.; Chen, W.C.; Liang, S.J.; Tu, C.Y.; Shih, H.M.; Cheng, W.J.; Lin, Y.C.; Hsueh, P.R. Clinical characteristics and treatment outcomes among the hospitalized elderly patients with COVID-19 during the late pandemic phase in central Taiwan. J. Microbiol. Immunol. Infect. 2024, 57, 257–268. [Google Scholar] [CrossRef]
- Saito, Z.; Uchiyama, S.; Nishioka, S.; Tamura, K.; Tamura, N.; Kuwano, K. Predictors of in-hospital mortality in elderly unvaccinated patients during SARS-CoV-2 Alpha variants epidemic. Infect. Prev. Pract. 2024, 6, 100341. [Google Scholar] [CrossRef]
- Sornette, D.; Mearns, E.; Schatz, M.; Wu, K.; Darcet, D. Interpreting, analysing and modelling COVID-19 mortality data. Nonlinear Dyn. 2020, 101, 1751–1776. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Baca, S.; Saydah, S.; Boehmer, T.K.; Adjei, S.; Gray, S.; Harris, A.M. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years—United States, March 2020–November 2021. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 713–717. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and older people. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef]
- Takheaw, N.; Liwsrisakun, C.; Chaiwong, W.; Laopajon, W.; Pata, S.; Inchai, J.; Duangjit, P.; Pothirat, C.; Bumroongkit, C.; Deesomchok, A.; et al. Correlation Analysis of Anti-SARS-CoV-2 RBD IgG and Neutralizing Antibody against SARS-CoV-2 Omicron Variants after Vaccination. Diagnostics 2022, 12, 1315. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Xue, J.H.; Wang, Y.J.; Li, W.; Li, Q.L.; Xu, Q.Y.; Niu, J.J.; Liu, L.L. Anti-Receptor-Binding Domain Immunoglobulin G Antibody as a Predictor of Seropositivity for Anti-SARS-CoV-2 Neutralizing Antibody. Arch. Pathol. Lab. Med. 2022, 146, 814–821. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- EMA Report Moderna. Available online: https://www.ema.europa.eu/en/documents/assessment-report/spikevax-previously-covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 26 March 2024).
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Kotloff, K.L.; Gay, C.L.; Áñez, G.; Adelglass, J.M.; Hernández, A.Q.B.; Harper, W.L.; Duncanson, D.M.; McArthur, M.A.; Florescu, D.F.; et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. N. Engl. J. Med. 2022, 386, 531–543. [Google Scholar] [CrossRef]
- Turley, C.B.; Tables, L.; Fuller, T.; Sanders, L.J.; Scott, H.; Moodley, A.; Woodward Davis, A.; Leav, B.; Miller, J.; Schoemaker, K.; et al. Modifiers of COVID-19 vaccine efficacy: Results from four COVID-19 prevention network efficacy trials. Vaccine 2023, 41, 4899–4906. [Google Scholar] [CrossRef]
- Nehme, J.; Borghesan, M.; Mackedenski, S.; Bird, T.G.; Demaria, M. Cellular senescence as a potential mediator of COVID-19 severity in the elderly. Aging Cell 2020, 19, e13237. [Google Scholar] [CrossRef]
- Ciabattini, A.; Garagnani, P.; Santoro, F.; Rappuoli, R.; Franceschi, C.; Medaglini, D. Shelter from the cytokine storm: Pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin. Immunopathol. 2020, 42, 619–634. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Antoni, D.; Manoussopoulos, Y.; Stefanou, P.; Argyropoulou, S.; Vrioni, G.; Tsakris, A. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS ONE 2022, 17, e0266958. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Addo, I.Y.; Dadzie, F.A.; Okeke, S.R.; Boadi, C.; Boadu, E.F. Duration of immunity following full vaccination against SARS-CoV-2: A systematic review. Arch. Public Health 2022, 80, 200. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Efficacy of COVID-19 vaccine booster doses in older people. Eur. Geriatr. Med. 2022, 13, 275–278. [Google Scholar] [CrossRef]
- Yang, X.H.; Bao, W.J.; Zhang, H.; Fu, S.K.; Jin, H.M. The Efficacy of SARS-CoV-2 Vaccination in the Elderly: A Systemic Review and Meta-analysis. J. Gen. Intern. Med. 2023, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Li, F.; Li, Y.; Li, Y.; Peng, P.; Li, S.; He, L.; Liu, T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 965971. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Qin, M.; Gao, Y.; Luo, N.; Xie, W.; Zou, Y.; Wang, J.; Ma, X. A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front. Immunol. 2023, 14, 1113156. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, L.; Tian, T.; Li, W.; Pan, Y.; Wang, Y. Efficacy and Safety of COVID-19 Vaccination in Older Adults: A Systematic Review and Meta-Analysis. Vaccines 2022, 11, 33. [Google Scholar] [CrossRef]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, Y.; Liu, S.; Shu, Q.; Yang, X.; Chu, K.; Qiao, Y.; Hu, Y.; Wang, K.; Pan, H. Safety and immunogenicity of a modified Omicron-adapted inactivated vaccine in healthy adults: A randomized, double-blind, active-controlled Phase III clinical trial. Front. Immunol. 2023, 14, 1241153. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Maltezou, H.C.; Tsakris, A.; Poland, G.A.; Anastassopoulou, C. Anaphylactic reactions to mRNA COVID-19 vaccines: A call for further study. Vaccine 2021, 39, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Hatziantoniou, S.; Vlachopoulos, C.; Spanakis, N.; Tsioufis, C.; Tsakris, A.; Lazaros, G. Temporal relationship of myocarditis and pericarditis following COVID-19 vaccination: A pragmatic approach. Int. J. Cardiol. 2022, 358, 136–139. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Boufidou, F.; Hatziantoniou, S.; Vasileiou, K.; Spanakis, N.; Tsakris, A. Adverse events of acute nephrotoxicity reported to EudraVigilance and VAERS after COVID-19 vaccination. Vaccine 2023, 41, 7176–7182. [Google Scholar] [CrossRef]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Front. Med. 2021, 8, 700014. [Google Scholar] [CrossRef]
- Jeong, S.; Hong, S.; Oh, T.; Woo, S.H.; Lee, W.J.; Kim, D.; Jeong, W.J. Analysis of older adults visiting the emergency department with fever as a suspected COVID-19 vaccine-related adverse reaction: A retrospective multicenter study. J. Infect. Chemother. 2022, 28, 1159–1164. [Google Scholar] [CrossRef]
- Khalid, M.B.; Frischmeyer-Guerrerio, P.A. The conundrum of COVID-19 mRNA vaccine-induced anaphylaxis. J. Allergy Clin. Immunol. Glob. 2023, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.; Prasad, V. COVID-19 vaccine induced myocarditis in young males: A systematic review. Eur. J. Clin. Investig. 2023, 53, e13947. [Google Scholar] [CrossRef] [PubMed]
- CDC. Available online: https://www.cdc.gov/media/releases/2024/s-0228-covid.html (accessed on 29 March 2024).
- European Comission, EU Vaccines Strategy. Available online: https://commission.europa.eu/strategy-and-policy/coronavirus-response/public-health/eu-vaccines-strategy_en#documents (accessed on 29 March 2024).
- European Centre for Disease Prevention and Control. Interim COVID-19 Vaccination Coverage in the EU/EEA during the 2023–24 Season Campaigns; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- European Centre for Disease Prevention and Control. Factsheet about Pneumococcal Disease. Available online: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed on 25 March 2024).
- Paton, J.C.; Trappetti, C. Streptococcus pneumoniae Capsular Polysaccharide. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, is the 100th Serotype and Has a Large cps Fragment from an Oral Streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Kuttel, M.M.; Ravenscroft, N.; Thompson, A.; Prasad, A.K.; Gangolli, S.; Tan, C.; Cooper, D.; Watson, W.; Liberator, P.; et al. Streptococcus pneumoniae serotype 15B polysaccharide conjugate elicits a cross-functional immune response against serotype 15C but not 15A. Vaccine 2022, 40, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E. Pneumococcal Pneumonia and Invasive Pneumococcal Disease in Those 65 and Older: Rates of Detection, Risk Factors, Vaccine Effectiveness, Hospitalisation and Mortality. Geriatrics 2021, 6, 13. [Google Scholar] [CrossRef]
- Earle, K.; Williams, S. Burden of pneumococcal disease in adults aged 65 years and older: An Australian perspective. Pneumonia 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease—Annual epidemiological Report for 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_IPD.pdf (accessed on 29 March 2024).
- Zarabi, N.; Aldvén, M.; Sjölander, S.; Fues Wahl, H.; Bencina, G.; Johnson, K.D.; Silfverdal, S.A. Clinical and economic burden of pneumococcal disease among adults in Sweden: A population-based register study. PLoS ONE 2023, 18, e0287581. [Google Scholar] [CrossRef]
- Grant, L.R.; Meche, A.; McGrath, L.; Miles, A.; Alfred, T.; Yan, Q.; Chilson, E. Risk of Pneumococcal Disease in US Adults by Age and Risk Profile. Open Forum. Infect. Dis. 2023, 10, ofad192. [Google Scholar] [CrossRef]
- Pelton, S.I.; Shea, K.M.; Weycker, D.; Farkouh, R.A.; Strutton, D.R.; Edelsberg, J. Rethinking risk for pneumococcal disease in adults: The role of risk stacking. Open Forum. Infect. Dis. 2015, 2, ofv020. [Google Scholar] [CrossRef] [PubMed]
- FDA. PCV. Available online: https://www.fda.gov/vaccines-blood-biologics/pneumococcal-13-valent-conjugate-vaccine-diphtheria-crm197-protein (accessed on 29 March 2024).
- CDC. Pneumococcal Vaccines. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/about-vaccine.html#:~:text=Pneumococcal%20Conjugate%20Vaccines&text=PCV15%20(Vaxneuvance%C2%AE)%20is%20a,diphtheria%20toxin%20known%20as%20CRM197 (accessed on 29 March 2024).
- CDC. Recommendations Pneumococcal Vaccine. Available online: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/recommendations.html (accessed on 29 March 2024).
- Jackson, L.A.; Neuzil, K.M.; Nahm, M.H.; Whitney, C.G.; Yu, O.; Nelson, J.C.; Starkovich, P.T.; Dunstan, M.; Carste, B.; Shay, D.K.; et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007, 25, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Clutterbuck, E.; Yu, L.-M.; Bowman, J.; Bateman, E.A.; Diggle, L.; Angus, B.; Peto, T.E.; Beverley, P.C.; Mant, D.; et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin. Infect Dis. 2011, 52, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.N.; Gurtman, A.; Frenck, R.W.; Strout, C.; Jansen, K.U.; Trammel, J.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine–naïve adults 60–64 years of age. Vaccine 2014, 32, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Gurtman, A.; van Cleeff, M.; Frenck, R.W.; Treanor, J.; Jansen, K.U.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013, 31, 3594–3602. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC) Vaccine Scheduler, Pneumococcal Disease: Recommended Vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=25&SelectedCountryIdByDisease=-1 (accessed on 29 March 2024).
- Hollingsworth, R.; Isturiz, R. Pneumococcal vaccination of older adults: Conjugate or polysaccharide? Hum. Vaccin Immunother. 2014, 10, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Vila-Corcoles, A. Is the pneumococcal polysaccharide vaccine effective in preventing pneumonia? Lancet Infect. Dis. 2008, 8, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, À.; Soldevila, N.; Toledo, D.; Torner, N.; Force, L.; Pérez, M.J.; Martín, V.; Rodríguez-Rojas, L.; Astray, J.; Egurrola, M.; et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccination in preventing community-acquired pneumonia hospitalization and severe outcomes in the elderly in Spain. PLoS ONE 2017, 12, e0171943. [Google Scholar] [CrossRef] [PubMed]
- Fedson, D.S. Preventing non bacteremic pneumococcal pneumonia in older adults: Historical background and considerations for choosing between PCV13 and PPV23. Hum. Vaccin Immunother. 2014, 10, 1322–1330. [Google Scholar] [CrossRef]
- Niederman, M.S.; Folaranmi, T.; Buchwald, U.K.; Musey, L.; Cripps, A.W.; Johnson, K.D. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: A review of available evidence. Expert Rev. Vaccines 2021, 20, 243–256. [Google Scholar] [CrossRef]
- Falkenhorst, G.; Remschmidt, C.; Harder, T.; Hummers-Pradier, E.; Wichmann, O.; Bogdan, C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169368. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.Q.; Shen, N.; Yu, P.X.; Liu, B.B.; He, B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: A systematic review and meta-analysis of randomized trials. Vaccine 2016, 34, 1496–1503. [Google Scholar] [CrossRef]
- de Roux, A.; Schmöle-Thoma, B.; Siber, G.R.; Hackell, J.G.; Kuhnke, A.; Ahlers, N.; Baker, S.A.; Razmpour, A.; Emini, E.A.; Fernsten, P.D.; et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: Conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin. Infect Dis. 2008, 46, 1015–1023. [Google Scholar] [CrossRef]
- Shelly, M.A.; Pichichero, M.E.; Treanor, J.J. Low baseline antibody level to diphtheria is associated with poor response to conjugated pneumococcal vaccine in adults. Scand. J. Infect. Dis. 2001, 33, 542–544. [Google Scholar]
- Dunne, E.M.; Cilloniz, C.; von Mollendorf, C.; Lewnard, J.; Grant, L.R.; Slack, M.P.E.; Jodar, L.; Theilacker, C.; Gessner, B.D. Pneumococcal Vaccination in Adults: What Can We Learn from Observational Studies That Evaluated PCV13 and PPV23 Effectiveness in the Same Population? Arch. Bronconeumol. 2023, 59, 157–164. [Google Scholar] [CrossRef]
- Sikjær, M.G.; Pedersen, A.A.; Wik, M.S.; Stensholt, S.S.; Hilberg, O.; Løkke, A. Vaccine effectiveness of the pneumococcal polysaccharide and conjugated vaccines in elderly and high-risk populations in preventing invasive pneumococcal disease: A systematic search and meta-analysis. Eur. Clin. Respir. J. 2023, 10, 2168354. [Google Scholar] [CrossRef] [PubMed]
- Ortqvist, A.; Henckaerts, I.; Hedlund, J.; Poolman, J. Non-response to specific serotypes likely cause for failure to 23-valent pneumococcal polysaccharide vaccine in the elderly. Vaccine 2007, 25, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Romano, M.R.; Carboni, F.; Adamo, R.; Berti, F. Strengths and weaknesses of pneumococcal conjugate vaccines. Glycoconj J. 2023, 40, 135–148. [Google Scholar] [CrossRef]
- Jokinen, J.; Snellman, M.; Palmu, A.A.; Saukkoriipi, A.; Verlant, V.; Pascal, T.; Devaster, J.-M.; Hausdorff, W.P.; Kilpi, T.M. Testing Pneumonia Vaccines in the Elderly: Determining a Case Definition for Pneumococcal Pneumonia in the Absence of a Gold Standard. Am. J. Epidemiol. 2018, 187, 1295–1302. [Google Scholar] [CrossRef]
- Berild, J.D.; Winje, B.A.; Vestrheim, D.F.; Slotved, H.-C.; Valentiner-Branth, P.; Roth, A.; Storsäter, J. A Systematic Review of Studies Published between 2016 and 2019 on the Effectiveness and Efficacy of Pneumococcal Vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens 2020, 9, 259. [Google Scholar] [CrossRef]
- Tvedskov, E.S.F.; Hovmand, N.; Benfield, T.; Tinggaard, M. Pneumococcal carriage among children in low and lower-middle-income countries: A systematic review. Int. J. Infect. Dis. 2022, 115, 1–7. [Google Scholar] [CrossRef]
- Du, Q.Q.; Shi, W.; Yu, D.; Yao, K.H. Epidemiology of non-vaccine serotypes of Streptococcus pneumoniae before and after universal administration of pneumococcal conjugate vaccines. Hum. Vaccin Immunother. 2021, 17, 5628–5637. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, M.; Zhou, R.; Ou, W.; Yao, P. How heavy is the medical expense burden among the older adults and what are the contributing factors? A literature review and problem-based analysis. Front. Public Health 2023, 11, 1165381. [Google Scholar] [CrossRef]
- Sorci, G.; Faivre, B. Age-dependent virulence of human pathogens. PLoS Pathog. 2022, 18, e1010866. [Google Scholar] [CrossRef]
- Bell, M.R.; Kutzler, M.A. An old problem with new solutions: Strategies to improve vaccine efficacy in the elderly. Adv. Drug Deliv. Rev. 2022, 183, 114175. [Google Scholar] [CrossRef]
- Del Giudice, G.; Weinberger, B.; Grubeck-Loebenstein, B. Vaccines for the elderly. Gerontology 2015, 61, 203–210. [Google Scholar] [CrossRef]
| Age (Years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 18 | 50 | 60 | 64 | 65 | 74 | 75 | ≥76 | |
| EU/EEA 1 | ||||||||
| Austria | ||||||||
| Belgium | ||||||||
| Cyprus | ||||||||
| Czechia | ||||||||
| Estonia | ||||||||
| France | ||||||||
| Germany 2 | ||||||||
| Greece 3 | ||||||||
| Italy 4 | ||||||||
| Liechtenstein | ||||||||
| Luxembourg 5 | ||||||||
| Spain 6 | ||||||||
| United States 7 | ||||||||
| Age (Years) | Recommended Vaccines * | ||||||
|---|---|---|---|---|---|---|---|
| ≥18 | ≥50 | ≥55 | ≥59 | ≥60 | ≥65 | ||
| EU/EEA | |||||||
| Austria | IIV4, aIIV4, QIV-HD | ||||||
| Belgium | IIV3, IIV4 | ||||||
| Bulgaria | IIV4 | ||||||
| Croatia | IIV4 | ||||||
| Cyprus | aIIV4 | ||||||
| Czechia | IIV4 | ||||||
| Denmark | IIV4, QIV-HD | ||||||
| Estonia | IIV4 | ||||||
| Finland | IIV4 | ||||||
| France | IIV4, QIV-HD | ||||||
| Germany | IIV4, QIV-HD | ||||||
| Greece | IIV4 | ||||||
| Hungary | IIV3 | ||||||
| Iceland | IIV4 | ||||||
| Ireland | IIV4, aIIV4 | ||||||
| Italy | IIV4, aIIV4, cIIV4, rIIV4, QIV-HD | ||||||
| Latvia | IIV4 | ||||||
| Liechtenstein | IIV4 | ||||||
| Lithuania | IIV4 | ||||||
| Luxembourg | IIV4 | ||||||
| Malta | IIV4 | ||||||
| The Netherlands | IIV4 | ||||||
| Norway | IIV4 | ||||||
| Poland | IIV4 | ||||||
| Portugal | IIV4 | ||||||
| Romania | IIV4 | ||||||
| Slovakia | IIV4 | ||||||
| Slovenia | IIV4 | ||||||
| Spain | IIV4, aIIV3, aIIV4, cIIV4, QIV-HD | ||||||
| Sweden | IIV4, QIV-HD | ||||||
| United States | HD-IIV4, RIV4, aIIV4 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastassopoulou, C.; Ferous, S.; Medić, S.; Siafakas, N.; Boufidou, F.; Gioula, G.; Tsakris, A. Vaccines for the Elderly and Vaccination Programs in Europe and the United States. Vaccines 2024, 12, 566. https://doi.org/10.3390/vaccines12060566
Anastassopoulou C, Ferous S, Medić S, Siafakas N, Boufidou F, Gioula G, Tsakris A. Vaccines for the Elderly and Vaccination Programs in Europe and the United States. Vaccines. 2024; 12(6):566. https://doi.org/10.3390/vaccines12060566
Chicago/Turabian StyleAnastassopoulou, Cleo, Stefanos Ferous, Snežana Medić, Nikolaos Siafakas, Fotini Boufidou, Georgia Gioula, and Athanasios Tsakris. 2024. "Vaccines for the Elderly and Vaccination Programs in Europe and the United States" Vaccines 12, no. 6: 566. https://doi.org/10.3390/vaccines12060566
APA StyleAnastassopoulou, C., Ferous, S., Medić, S., Siafakas, N., Boufidou, F., Gioula, G., & Tsakris, A. (2024). Vaccines for the Elderly and Vaccination Programs in Europe and the United States. Vaccines, 12(6), 566. https://doi.org/10.3390/vaccines12060566










