Fasting before Intra-Gastric Dosing with Antigen Improves Intestinal Humoral Responses in Syrian Hamsters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
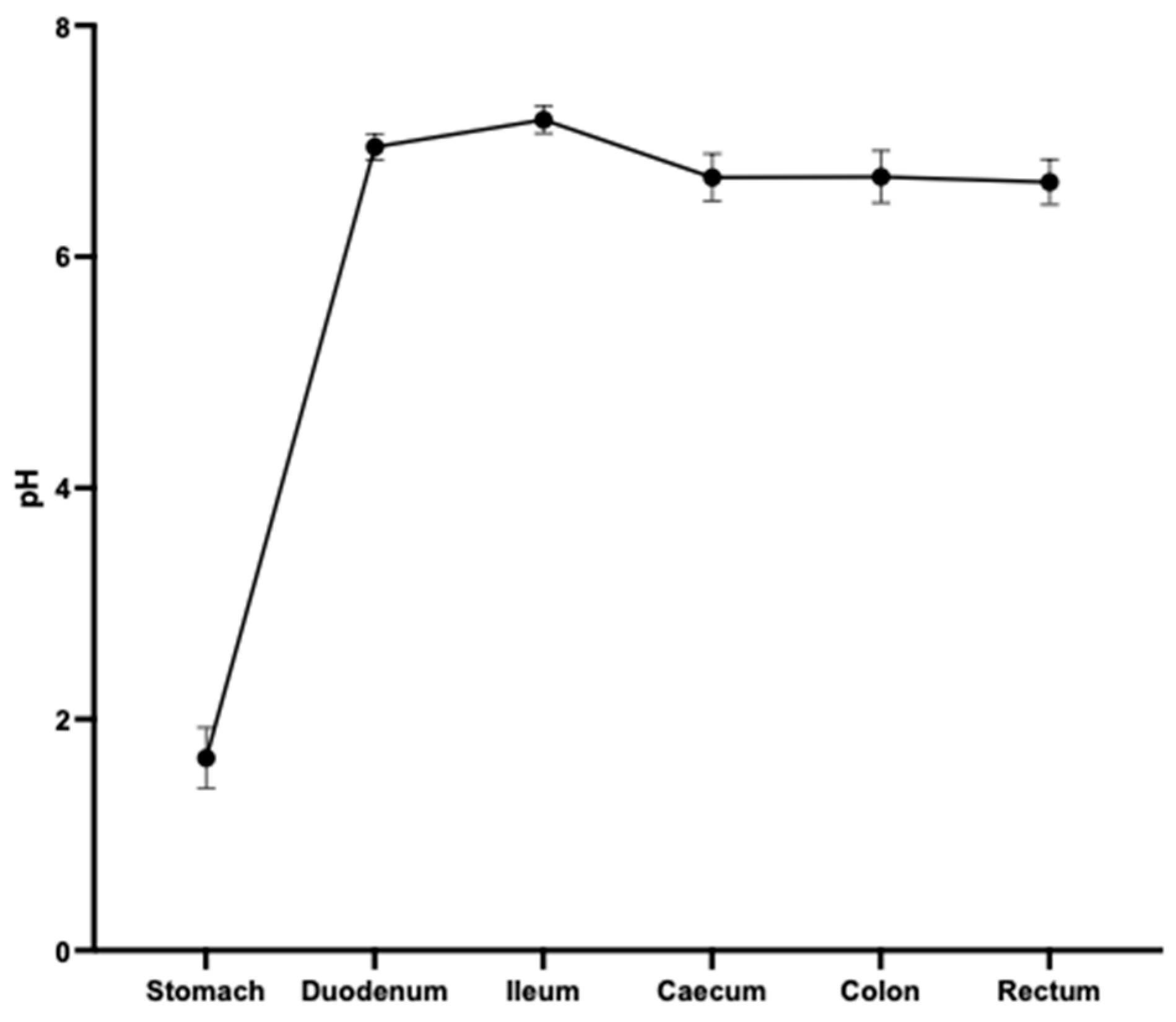
2.2. Assessment of pH of the Gastrointestinal (GI) Tract of Hamsters
2.3. Preparation of Capsules
2.4. Protein Stability Assays
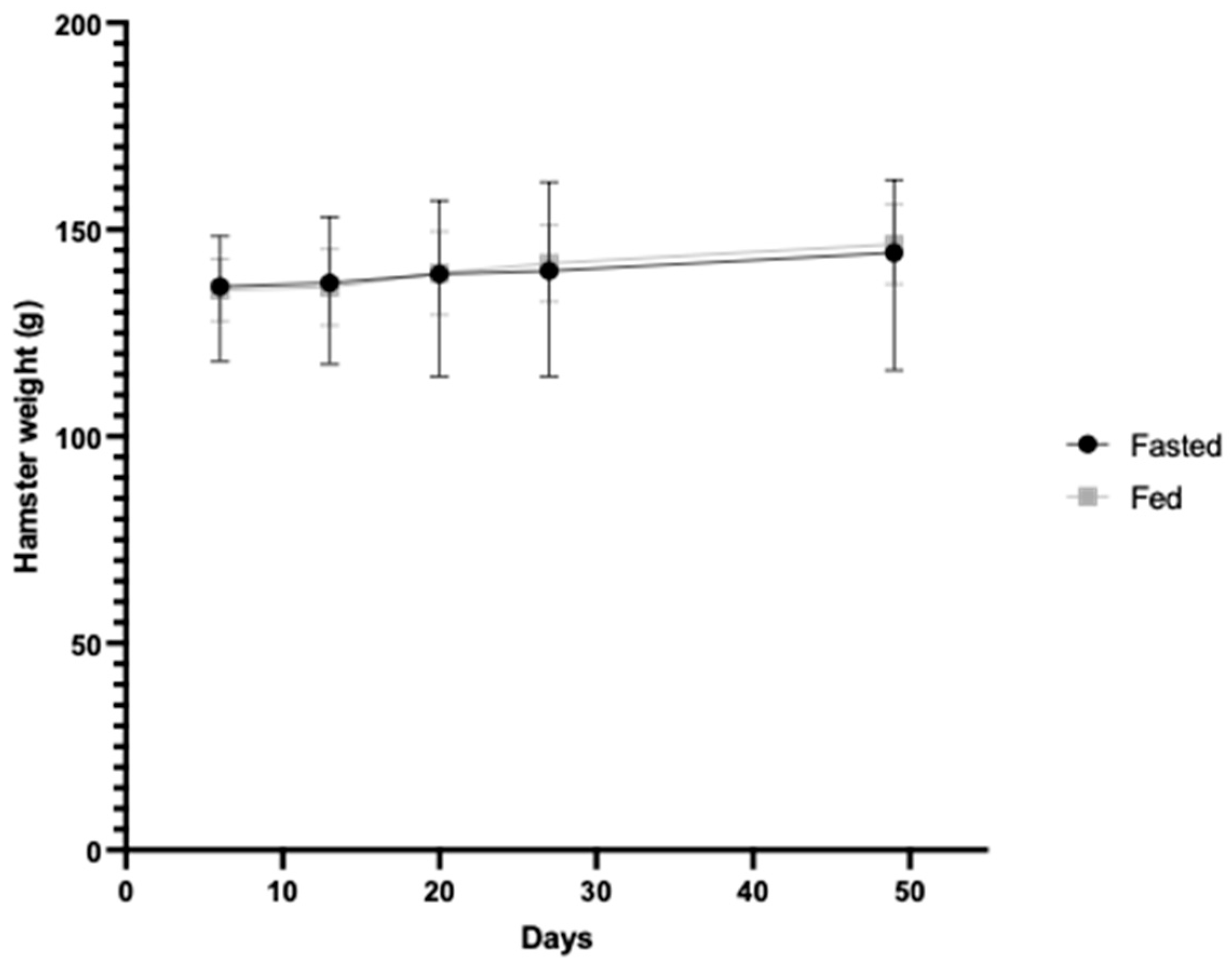
2.5. In Vivo Study
2.6. Direct ELISA for Detection of Total sIgA in Intestinal Fluid
2.7. Adherence Blocking Assay
2.8. IgG Indirect ELISA for Detection of Antigen-Specific IgG in Serum
2.9. Ethics Statement
2.10. Statistical Analysis
3. Results
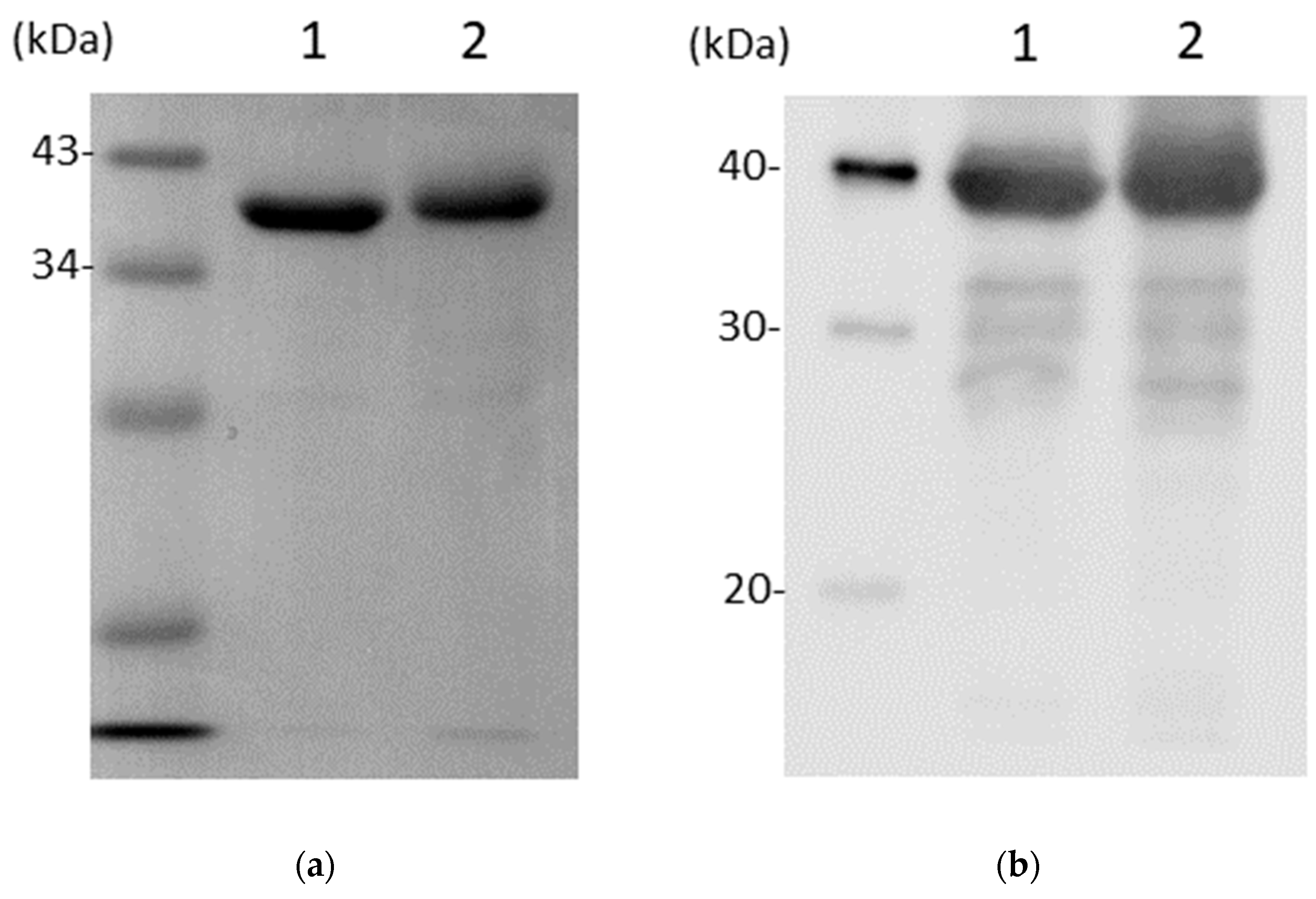
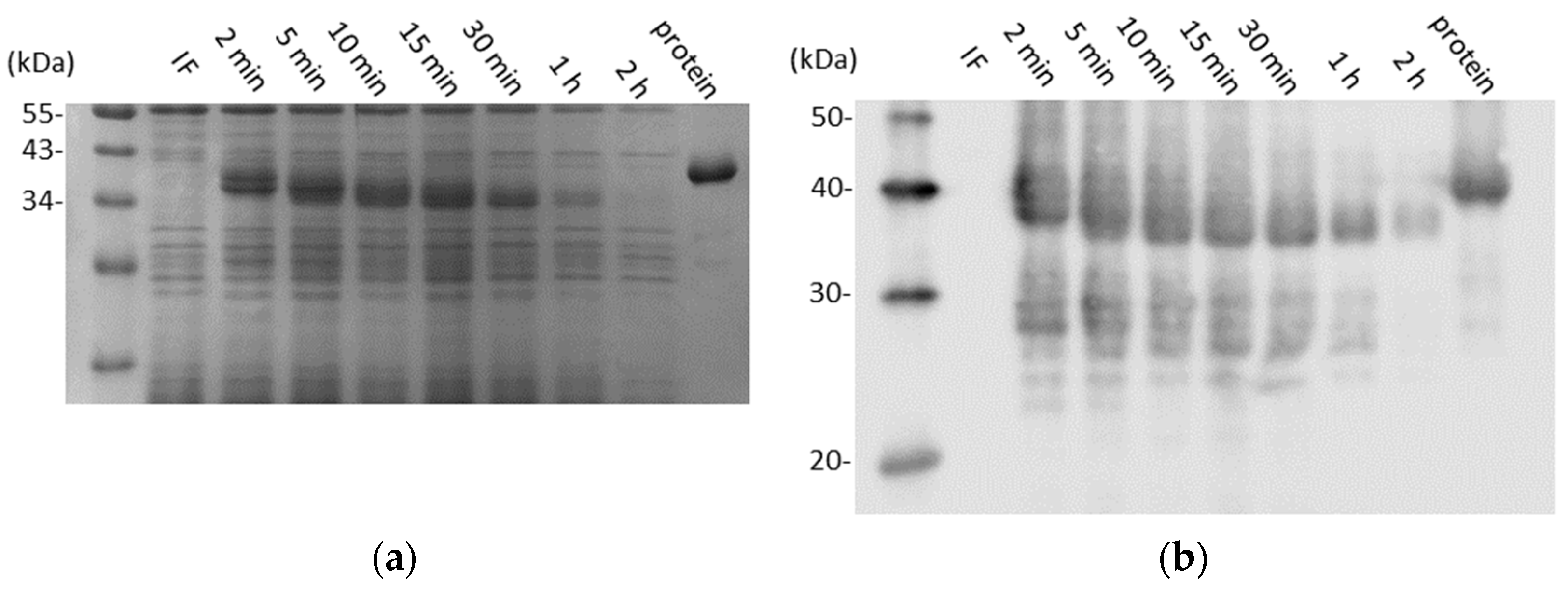
3.1. CD0873 Is Stable Following Lyophilisation and Storage and Further Incubation in Intestinal Fluid
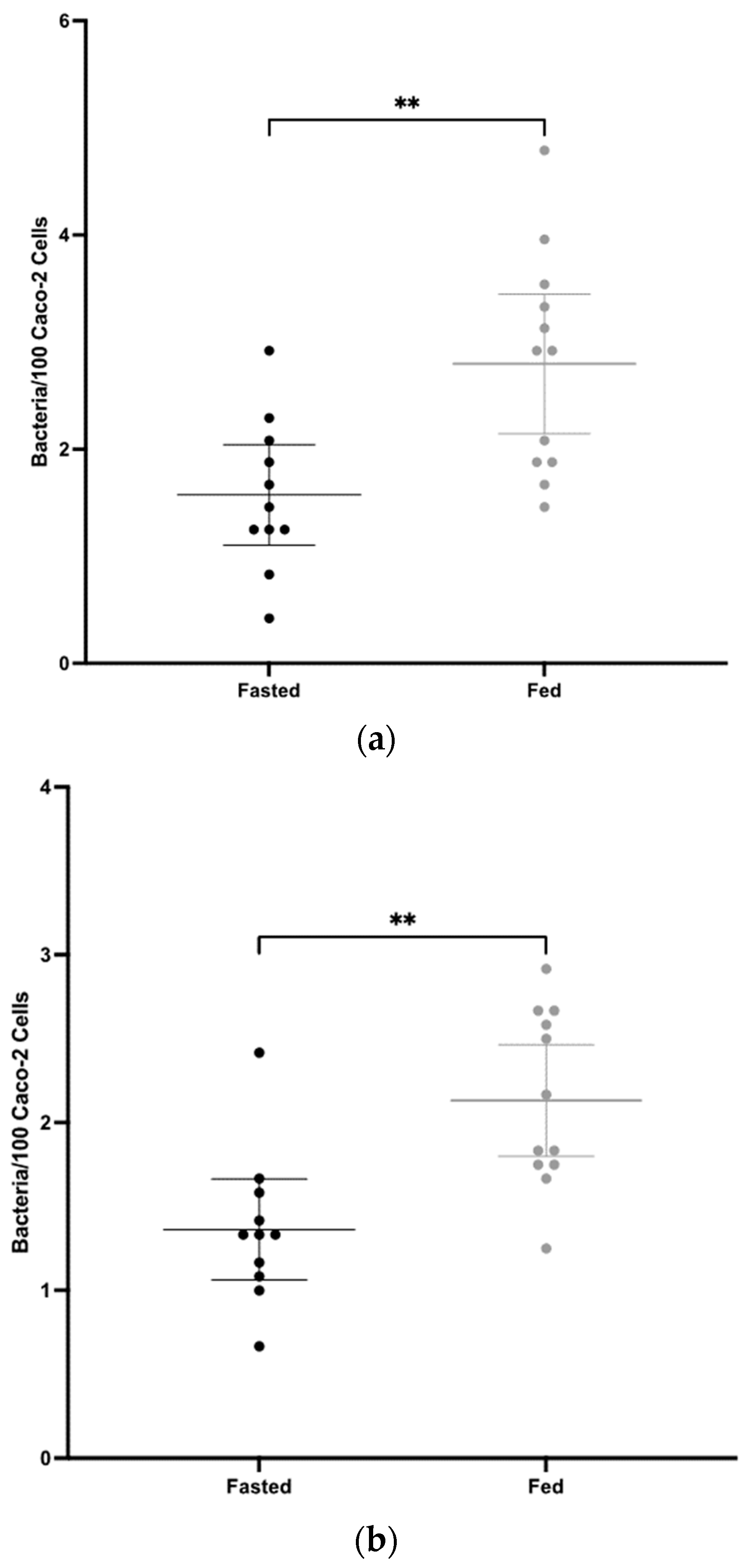
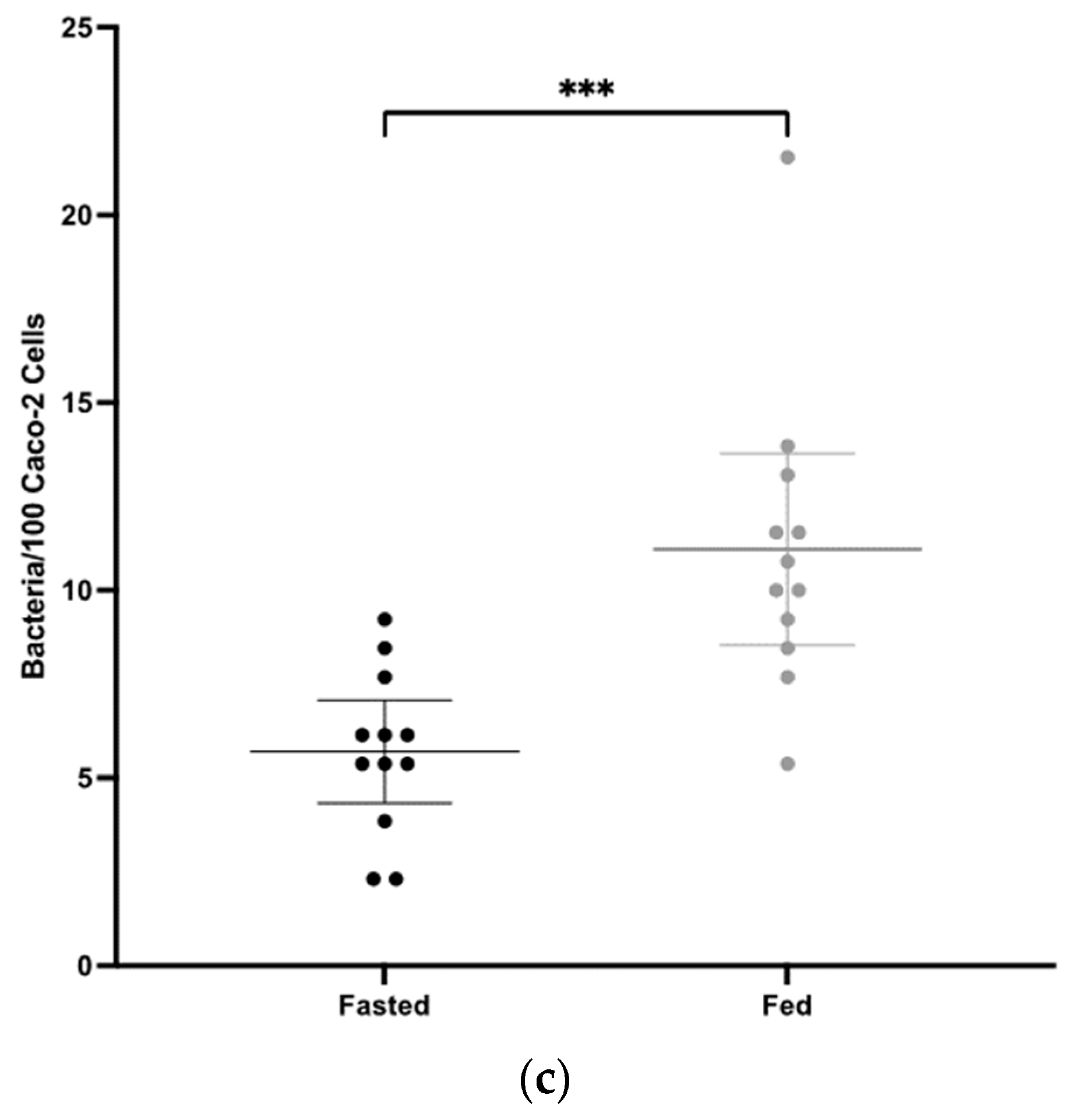
3.2. Mucosal Antibody Responses to CD0873 Given Intra-Gastrically in Capsules Are Significantly Greater in Fasted versus Fed Hamsters
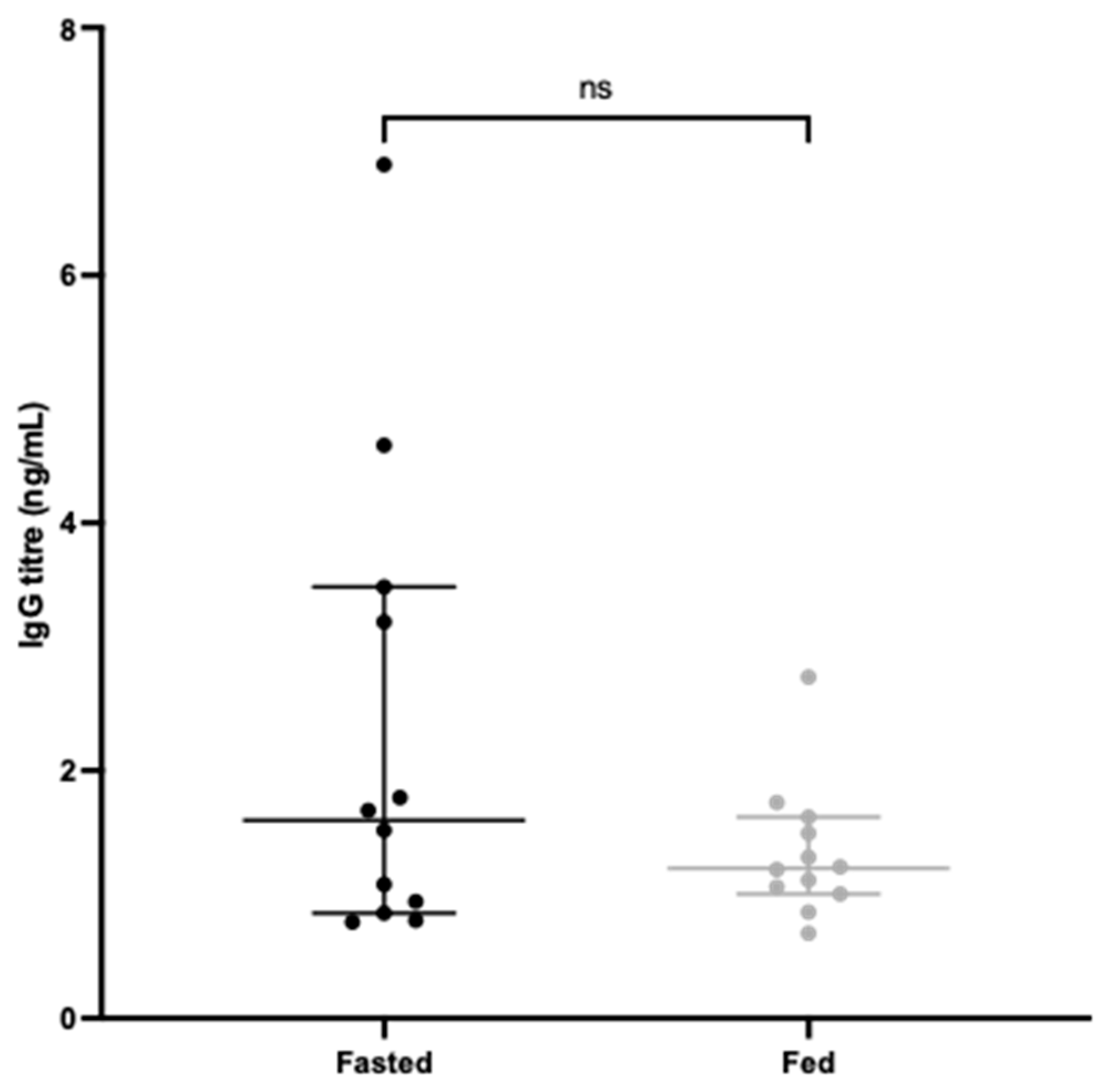
3.3. Systemic Antibody Responses to CD0873 Given Intra-Gastrically in Capsules Are Greater in Fasted versus Fed Hamsters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Hoft, D.F.; Brusic, V.; Sakala, I.G. Optimizing vaccine development. Cell Microbiol. 2011, 13, 934–942. [Google Scholar] [CrossRef]
- Kwong, K.W.-Y.; Xin, Y.; Lai, N.C.-Y.; Sung, J.C.-C.; Wu, K.-C.; Hamied, Y.K.; Sze, E.T.-P.; Lam, D.M.-K. Oral Vaccines: A Better Future of Immunization. Vaccines 2023, 11, 1232. [Google Scholar] [CrossRef]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Ogg, M.; Jonsson, C.B.; Camp, J.V.; Hooper, J.W. Ribavirin protects Syrian hamsters against lethal hantavirus pulmonary syndrome–after intranasal exposure to Andes virus. Viruses 2013, 5, 2704–2720. [Google Scholar] [CrossRef]
- Prescott, J.; DeBuysscher, B.L.; Brown, K.S.; Feldmann, H. Long-term single-dose efficacy of a vesicular stomatitis virus-based Andes virus vaccine in Syrian hamsters. Viruses 2014, 6, 516–523. [Google Scholar] [CrossRef]
- Zu Rhein, G.M. Studies of JC virus-induced nervous system tumors in the Syrian hamster: A review. Prog. Clin. Biol. Res. 1983, 105, 205–221. [Google Scholar]
- Cho, S.-A.; Park, J.-H.; Seok, S.-H.; Juhn, J.-H.; Kim, S.-J.; Ji, H.-J.; Choo, Y.-S.; Park, J.-H. Effect of granulocyte macrophage-colony stimulating factor (GM-CSF) on 5-FU-induced ulcerative mucositis in hamster buccal pouches. Exp. Toxicol. Pathol. 2006, 57, 321–328. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Yadav, P.D.; Mohandas, S.; Shete, A.M.; Nyayanit, D.A.; Gupta, N.; Patil, D.Y.; Sapkal, G.N.; Potdar, V.; Kadam, M.; Kumar, A.; et al. SARS-CoV-2 Kappa Variant Shows Pathogenicity in a Syrian Hamster Model. Vector-Borne Zoonotic Dis. 2022, 22, 289–296. [Google Scholar] [CrossRef]
- Yuan, S.; Ye, Z.-W.; Liang, R.; Tang, K.; Zhang, A.J.; Lu, G.; Ong, C.P.; Poon, V.K.M.; Chan, C.C.-S.; Mok, B.W.-Y.; et al. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 Omicron in Syrian hamsters. Science 2022, 377, 428–433. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Chau, E.C.T.; Kwong, T.C.; Pang, C.K.; Chan, L.T.; Chan, A.M.L.; Yao, X.; Tam, J.S.L.; Chan, S.W.; Leung, G.P.H.; Tai, W.C.S.; et al. A Novel Probiotic-Based Oral Vaccine against SARS-CoV-2 Omicron Variant B.1.1.529. Int. J. Mol. Sci. 2023, 24, 13931. [Google Scholar] [CrossRef]
- Bellier, B.; Saura, A.; Luján, L.A.; Molina, C.R.; Luján, H.D.; Klatzmann, D. A Thermostable Oral SARS-CoV-2 Vaccine Induces Mucosal and Protective Immunity. Front. Immunol. 2022, 13, 837443. [Google Scholar] [CrossRef]
- Jawalagatti, V.; Kirthika, P.; Hewawaduge, C.; Yang, M.-S.; Park, J.-Y.; Oh, B.; Lee, J.H. Bacteria-enabled oral delivery of a replicon-based mRNA vaccine candidate protects against ancestral and delta variant SARS-CoV-2. Mol. Ther. 2022, 30, 1926–1940. [Google Scholar] [CrossRef]
- Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Birch, S.M.; Kajon, A.E.; Werts, A.D.; Tucker, S.N. Oral Vaccination Protects Against Severe Acute Respiratory Syndrome Coronavirus 2 in a Syrian Hamster Challenge Model. J. Infect. Dis. 2022, 225, 34–41. [Google Scholar] [CrossRef]
- Lloren, K.K.S.; Jawalagatti, V.; Hewawaduge, C.; Chandran, S.; Park, J.-Y.; Lee, J.H. Salmonella-mediated oral delivery of multiple-target vaccine constructs with conserved and variable regions of SARS-CoV-2 protect against the Delta and Omicron variants in hamster. Microbes Infect. 2023, 25, 105101. [Google Scholar] [CrossRef]
- Wang, S.; Cui, H.; Zhang, C.; Li, W.; Wang, W.; He, W.; Feng, N.; Zhao, Y.; Wang, T.; Tang, X.; et al. Oral delivery of a chitosan adjuvanted COVID-19 vaccine provides long-lasting and broad-spectrum protection against SARS-CoV-2 variants of concern in golden hamsters. Antivir. Res. 2023, 220, 105765. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Onderdonk, A.B.; Cisneros, R.L.; Kasper, D.L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J. Infect. Dis. 1977, 136, 701–705. [Google Scholar] [CrossRef]
- Price, A.B.; E Larson, H.; Crow, J. Morphology of experimental antibiotic-associated enterocolitis in the hamster: A model for human pseudomembranous colitis and antibiotic-associated diarrhoea. Gut 1979, 20, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Barclay, F.E. Protection of hamsters against Clostridium difficile ileocaecitis by prior colonisation with non-pathogenic strains. J. Med. Microbiol. 1985, 19, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.M.; Spencer, J.; Candlish, D.; Irvine, J.J.; Douce, G.R. Infection of hamsters with the UK Clostridium difficile ribotype 027 outbreak strain R20291. J. Med. Microbiol. 2011, 60 Pt 8, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Razaq, N.; Sambol, S.; Nagaro, K.; Zukowski, W.; Cheknis, A.; Johnson, S.; Gerding, D.N. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J. Infect. Dis. 2007, 196, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Bruxelle, J.F.; Tsapis, N.; Hoys, S.; Collignon, A.; Janoir, C.; Fattal, E.; Péchiné, S. Protection against Clostridium difficile infection in a hamster model by oral vaccination using flagellin FliC-loaded pectin beads. Vaccine 2018, 36, 6017–6021. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Wu, C.-W.; Lien, S.-P.; Leng, C.-H.; Hsiao, K.-N.; Liu, S.-J.; Chen, H.-W.; Siu, L.-K.; Chong, P. Recombinant lipoprotein-based vaccine candidates against C. difficile infections. J. Biomed. Sci. 2015, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Aston, C.; Kelly, M.L.; Griffin, R. Towards Development of a Non-Toxigenic Clostridioides difficile Oral Spore Vaccine against Toxigenic C. difficile. Pharmaceutics 2022, 14, 1086. [Google Scholar] [CrossRef] [PubMed]
- Karyal, C.; Hughes, J.; Kelly, M.L.; Luckett, J.C.; Kaye, P.V.; Cockayne, A.; Minton, N.P.; Griffin, R. Colonisation Factor CD0873, an Attractive Oral Vaccine Candidate against Clostridioides difficile. Microorganisms 2021, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Karyal, C.; Palazi, P.; Hughes, J.; Griffiths, R.C.; Persaud, R.R.; Tighe, P.J.; Mitchell, N.J.; Griffin, R. Mimicking Native Display of CD0873 on Liposomes Augments Its Potency as an Oral Vaccine against Clostridioides difficile. Vaccines 2021, 9, 1453. [Google Scholar] [CrossRef]
- Matchett, W.E.; Anguiano-Zarate, S.; Malewana, G.B.R.; Mudrick, H.; Weldy, M.; Evert, C.; Khoruts, A.; Sadowsky, M.; Barry, M.A. A Replicating Single-Cycle Adenovirus Vaccine Effective against Clostridium difficile. Vaccines 2020, 8, 470. [Google Scholar] [CrossRef]
- Senoh, M.; Iwaki, M.; Yamamoto, A.; Kato, H.; Fukuda, T.; Shibayama, K. Development of vaccine for Clostridium difficile infection using membrane fraction of nontoxigenic Clostridium difficile. Microb. Pathog. 2018, 123, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-H.; Glenn, G.; Flyer, D.; Zhou, B.; Liu, Y.; Sullivan, E.; Wu, H.; Cummings, J.F.; Elllingsworth, L.; Smith, G. Clostridium difficile chimeric toxin receptor binding domain vaccine induced protection against different strains in active and passive challenge models. Vaccine 2017, 35, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Z.; Cai, J.; Yu, B.; Hua, Y.; Lau, C.C.; Kao, R.Y.-T.T.; Sze, K.-H.; Yuen, K.-Y.; Huang, J.-D. A DNA vaccine targeting TcdA and TcdB induces protective immunity against Clostridium difficile. BMC Infect. Dis. 2016, 16, 596. [Google Scholar] [CrossRef]
- Hensgens, M.P.M.; Goorhuis, A.; Dekkers, O.M.; Kuijper, E.J. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J. Antimicrob. Chemother. 2012, 67, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Denève, C.; Janoir, C.; Poilane, I.; Fantinato, C.; Collignon, A. New trends in Clostridium difficile virulence and pathogenesis. Int. J. Antimicrob. Agents 2009, 33 (Suppl. 1), S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Ascenzi, P.; Siarakas, S.; Petrosillo, N.; di Masi, A. Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium difficile infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Sambol, S.P.; Tang, J.K.; Merrigan, M.M.; Johnson, S.; Gerding, D.N. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J. Infect. Dis. 2001, 183, 1760–1766. [Google Scholar] [CrossRef]
- de Bruyn, G.; Gordon, D.L.; Steiner, T.; Tambyah, P.; Cosgrove, C.; Martens, M.; Bassily, E.; Chan, E.-S.; Patel, D.; Chen, J.; et al. Safety, immunogenicity, and efficacy of a Clostridioides difficile toxoid vaccine candidate: A phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect. Dis. 2021, 21, 252–262. [Google Scholar] [CrossRef]
- Pfizer.com. Phase 3 CLOVER Trial for Pfizer’s Investigational Clostridioides difficile Vaccine Indicates Strong Potential Effect in Reducing Duration and Severity of Disease Based on Secondary Endpoints. 2022. Available online: https://www.pfizer.com/news/press-release/press-release-detail/phase-3-clover-trial-pfizers-investigational-clostridioides (accessed on 18 March 2024).
- Ashford, M.; Fell, J. Targeting drugs to the colon: Delivery systems for oral administration. J. Drug Target 1994, 2, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Healey, J.N.C.; Reynolds, J.R. Evaluation of an enteric-coated delayed-release 5-aminosalicylic acid tablet in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 1987, 1, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wilding, I.R.; Hardy, J.G.; Sparrow, R.A.; Davis, S.S.; Daly, P.B.; English, J.R. In vivo evaluation of enteric-coated naproxen tablets using gamma scintigraphy. Pharm. Res. 1992, 9, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J.; Cole, E.T.; Wilding, I.R. The effect of food on the in vivo behavioiur of enteric coated starch capsules. Int. J. Pharm. 1994, 112, 207–213. [Google Scholar] [CrossRef]
- Amicizia, D.; Arata, L.; Zangrillo, F.; Panatto, D.; Gasparini, R. Overview of the impact of Typhoid and Paratyphoid fever. Utility of Ty21a vaccine (Vivotif®). J. Prev. Med. Hyg. 2017, 58, e1–e8. [Google Scholar] [PubMed]
- Murakami, T. Absorption sites of orally administered drugs in the small intestine. Expert. Opin. Drug Discov. 2017, 12, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Heading, R.C.; Nimmo, J.; Prescott, L.F.; Tothill, P. The dependence of paracetamol absorption on the rate of gastric emptying. Br. J. Pharmacol. 1973, 47, 415–421. [Google Scholar] [CrossRef]
- Saphier, S.; Rosner, A.; Brandeis, R.; Karton, Y. Gastro intestinal tracking and gastric emptying of solid dosage forms in rats using X-ray imaging. Int. J. Pharm. 2010, 388, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Simon, A.; Leuzzi, R.; Kasendra, M.; Minton, N.; Titball, R.W.; Michell, S.L. Lipoprotein CD0873 is a novel adhesin of Clostridium difficile. J. Infect. Dis. 2014, 210, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Staelens, D.; Liang, S.; Appeltans, B.; Van de Wouwer, M.; Van den Mooter, G.; Van Assche, G.; Himmelreich, U.; Velde, G.V. Visualization of delayed release of compounds from pH-sensitive capsules in vitro and in vivo in a hamster model. Contrast Media Mol. Imaging 2016, 11, 24–31. [Google Scholar] [CrossRef]
- Jacoby, H.I. Gastric Emptying. In Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hunter, E.; Fell, J.T.; Calvert, R.T.; Sharma, H. “In vivo” disintegration of hard gelatin capsules in fasting and non-fasting subjects. Int. J. Pharm. 1980, 4, 175–183. [Google Scholar] [CrossRef]
- Yamamoto, M.; Pascual, D.W.; Kiyono, H. M cell-targeted mucosal vaccine strategies. Curr. Top. Microbiol. Immunol. 2012, 354, 39–52. [Google Scholar] [PubMed]
- Dukoral Suspension and Effervescent Powder for Oral Suspension, Cholera Vaccine (Inactivated, Oral). Available online: https://www.medicines.org.uk/emc/product/5087/smpc#gref (accessed on 8 April 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wood, L.; Hughes, J.; Trussell, M.; Bishop, A.L.; Griffin, R. Fasting before Intra-Gastric Dosing with Antigen Improves Intestinal Humoral Responses in Syrian Hamsters. Vaccines 2024, 12, 572. https://doi.org/10.3390/vaccines12060572
Wood L, Hughes J, Trussell M, Bishop AL, Griffin R. Fasting before Intra-Gastric Dosing with Antigen Improves Intestinal Humoral Responses in Syrian Hamsters. Vaccines. 2024; 12(6):572. https://doi.org/10.3390/vaccines12060572
Chicago/Turabian StyleWood, Liam, Jaime Hughes, Mark Trussell, Anne L. Bishop, and Ruth Griffin. 2024. "Fasting before Intra-Gastric Dosing with Antigen Improves Intestinal Humoral Responses in Syrian Hamsters" Vaccines 12, no. 6: 572. https://doi.org/10.3390/vaccines12060572






