Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Formulation
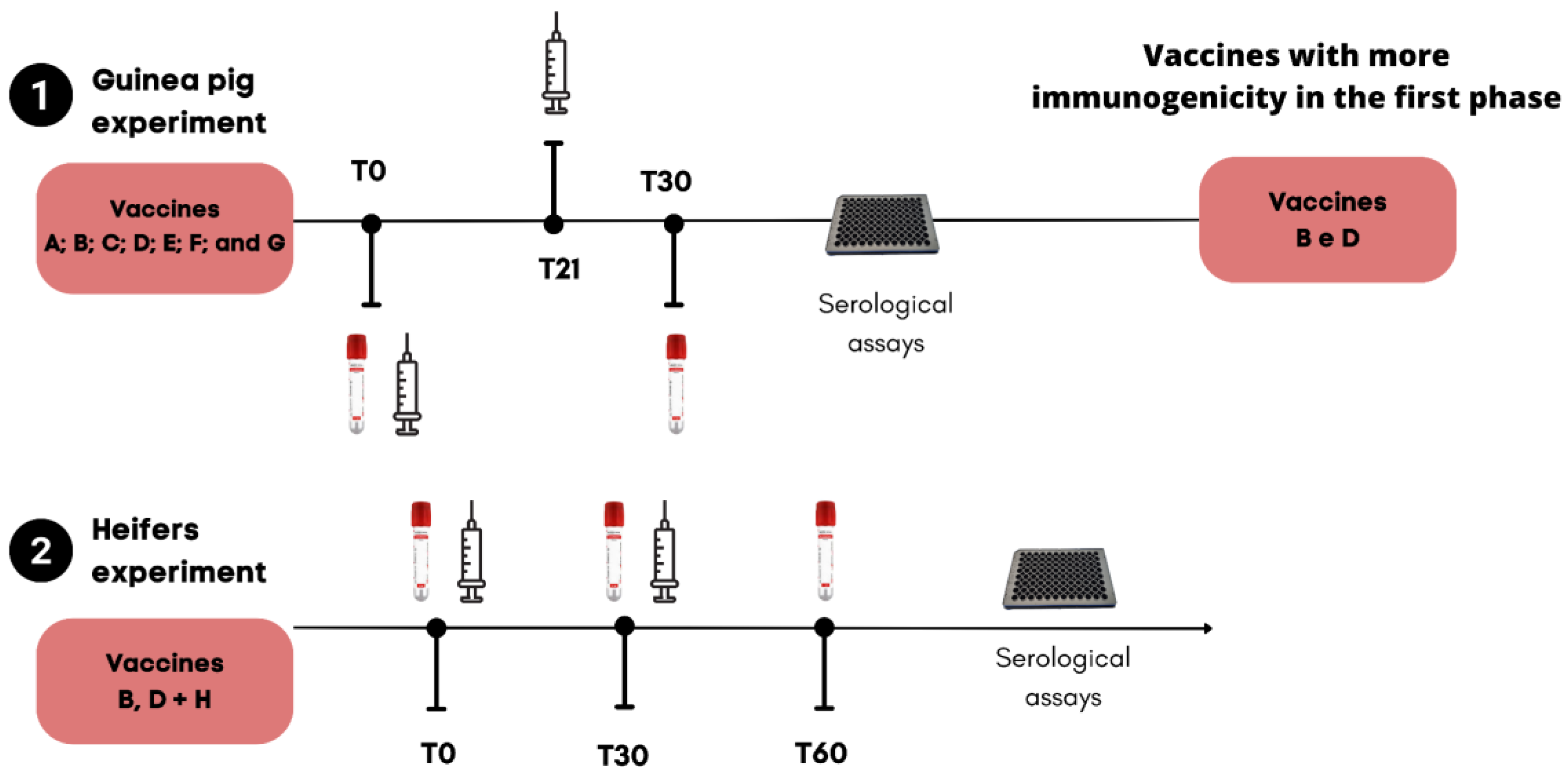
2.2. Vaccination Schemes and Sampling Times
2.2.1. Virus Neutralization
2.2.2. ELISA to Quantify Total Antibody to BoAHV-1 in Bovine
2.2.3. ELISA to Quantify Total Antibody to BoAHV-1 in Guinea Pig
2.3. Statistical Analyses
3. Results
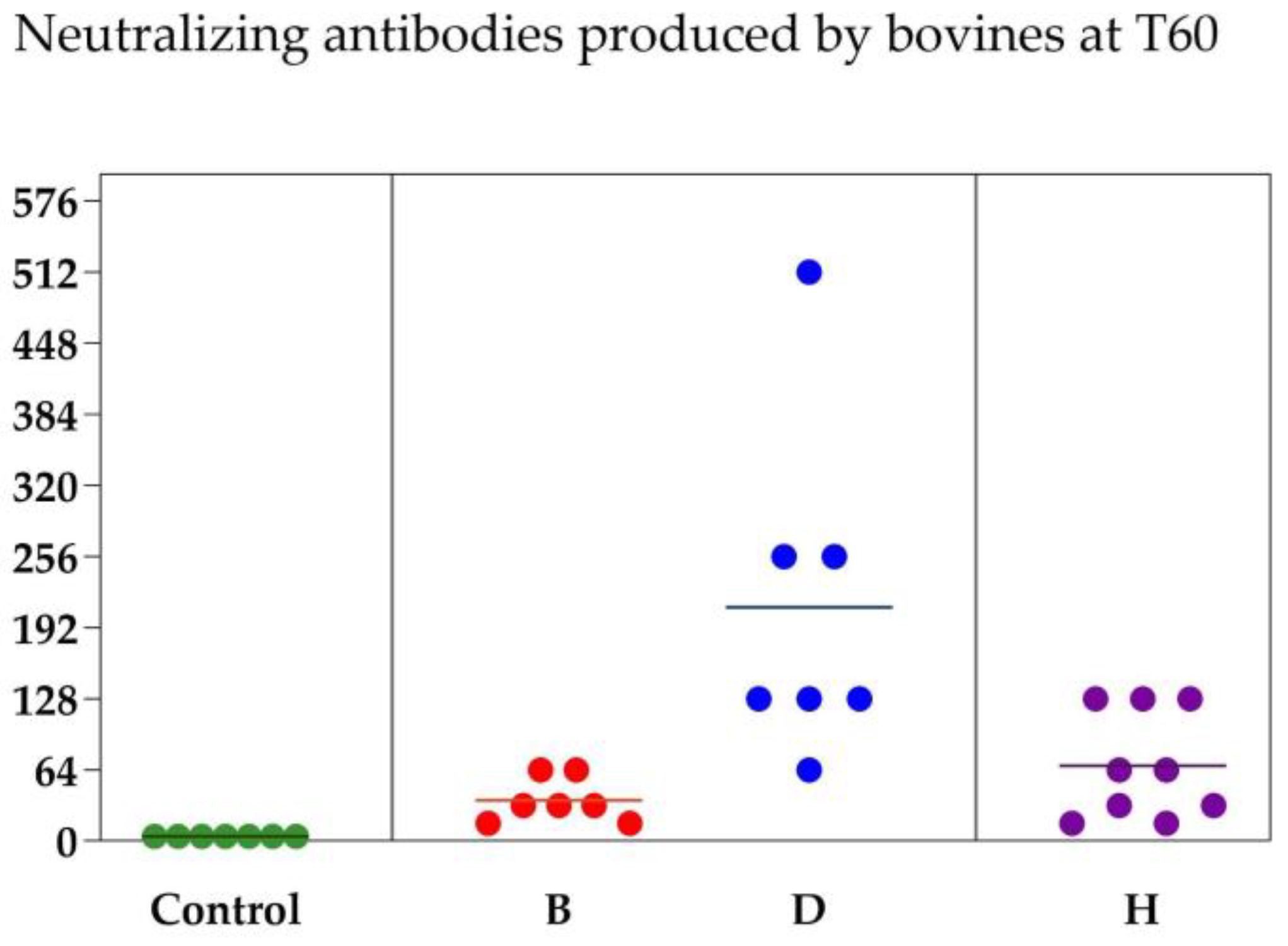
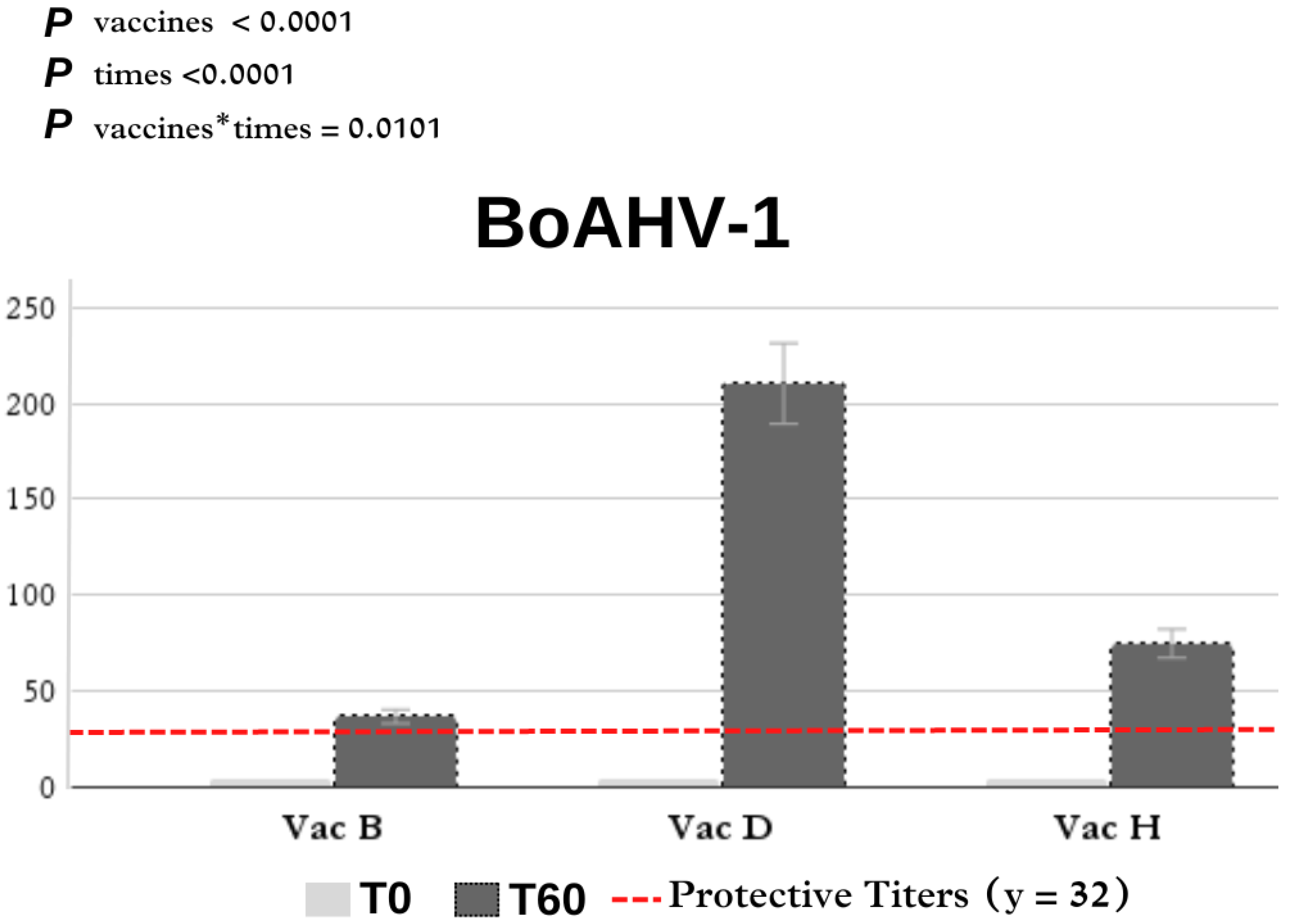
3.1. Virus Neutralization
3.2. ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vaccines | BoAHV-1 Tip | Manufacturer Recommendations |
|---|---|---|
| A | Inactivated suspension of antigens from infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD), and Leptospira pomona, L. wolfii, L. hardjo, L. icterohaemorrhagiae, L. canicola, and L. grippotyphosa bacterins. Adjuvant: Aluminum hydroxide—Contains sodium selenate 4.8 mg/mL and thimerosal. | Two doses of 5 mL, interval of 30 days, subcutaneous route |
| B | Composition per dose of 2 mL contains: Live gE-tk-double-gene deleted bovine herpes virus type 1 (BoAHV-1), strain CEDDEL: 106.3–107.3 CCID50 Abbreviations: gE-: deleted glycoprotein E; tk-: deleted thymidine kinase; CCID: cell culture infectious dose. | Administer one dose of 2 mL by intramuscular injection in the neck muscles |
| C | Inactivated suspension of vírus strains IBR 1, BVD I e II, Campylobacter fetus fetus, Campylobacter fetus venerealis, Histophilus somni (Haemophillus somnus), and Leptospira interrogans Pomona pomona. | Single dose of 5 mL, subcutaneous route |
| D | Lyophilized preparation of chemically altered thermosensitive strains of IBR and PI3 viruses and modified live BRSV plus a liquid and adjuvanted preparation of inactivated BVD virus (types 1 and 2) and inactivated cultures of the five identified Leptospira serotypes: L. canicola, L. grippotyphosa, L. hardjo, L. icterohaemorrhagiae, and L. pomona. The liquid component is used to rehydrate the lyophilized component. Viral antigens are propagated in an established cell line. The product has a unique combination of adjuvants including Amphigen®. The BVD fraction is additionally processed by an appropriate system to help ensure formulation consistency. | Two doses of 5 mL, interval of at least 21 days, subcutaneous route |
| E | Inactivated suspension of bovine herpesvirus type 1 and type 5, BVD type 1 and type 2, Leptospira interrogans sorovares pomona, wolffi, hardjo prajitno, L. icterohaemorrhagiae, L. canicola, L. copenhageni, L. bratislava, and Leptospira borgpetersenii serovar hardjo bovis, C. fetus subsp. fetus, C. fetus subsp. venerealis biotype intermedius, selenium (as selenum) sodium act) 10 mg/dose in 10% aluminum hydroxide. | Two doses of 5 mL 30 days of interval, subcutaneous or intramuscular route |
| F | Inactivated infectious bovine rhinotracheitis virus, AL strain, with a minimum titer prior to inactivation of 107,0 TCID50 (infecting dose in tissue culture). Inactivated parainuenza 3 virus, SF4 strain, with minimum titre prior to inactivation of 480 HAU (haemoagglutinating units). Inactivated bovine viral diarrhea virus, NADL strain, with a minimum titer prior to inactivation of 106,0 TCID50. Live bovine syncytial respiratory virus strain LYM P56 with a minimum titre of 104,0 TCID50. Vehicle q.s.p. | Two doses of 3 mL, 30 days of interval, subcutaneous route |
| G | Each dose of vaccine contains (before inactivation of each agent) IBR (HVB1 and HVB5), DVB virus, C. fetus subpecies fetus, C. fetus subpecies venerealis, H. somni (H. somnus), L. interrogans serovar pomona, L. interrogans serovar icterohaemorrhagiae, L. interrogans serovar caniculture, L. interrogans serovar wolffi, L. rogans serovar Hardjo, L. borgpetersenii serovar Tarassovi, L. kirschneri serovar Gryppotyphosa. With Pilatus GHA500 adjuvant (aqueous), high-protein-adsorption aluminum hydroxide, buffered physiological solution q.s.p 5 mL. | Two doses of 5 mL, 30 days of interval, subcutaneous route |
| H | Modified live samples of IBR virus (passage strain C-13), BVD virus type 1 (NADL strain), BVD virus Type 2 (strain 53637), bovine parainfluenza virus type 3 (AL-IM strain), bovine respiratory syncytial virus (BRSV/375 strain) and Mannheimia haemolytica toxoid (NL 1009 strain). | Single dose of 2 mL, subcutaneous route |
| Control Guinea pig experiment | Non-vaccinated group | Saline solution |
| Control bovine experiment | Non-vaccinated group | Saline solution |
| Titles | Vac B (n = 7) | Vac D (n = 7) | Vac H (n = 9) |
|---|---|---|---|
| 4 | 28.6% | 0% | 0% |
| 16 | 14.3% | 0% | 0% |
| 32 | 42.9% | 0% | 22.2% |
| 64 | 14.3% | 14.3% | 44.4% |
| ≥128 | 0% | 85.7% | 33.3% |
| ≥32 | 57.2% | 100% | 100% |
References
- ICTV. International Committee on Taxonomy of Viruses. Available online: https://ictv.global/taxonomy/taxondetails?taxnode_id=202201440&taxon_name=Varicellovirus%20bovinealpha1 (accessed on 6 March 2024).
- D’offay, J.M.; Eberle, R.; Fulton, R.W.; Kirkland, P.D. Complete genomic sequence and comparative analysis of four genital and respiratory isolates of bovine herpesvirus subtype 1.2b (BoHV-1.2b), including the prototype virus strain K22. Arch. Virol. 2022, 161, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Del Médico, Z.M.P.; Ladelfa, M.F.; Kotsias, F.; Muylkens, B.; Thiry, J.; Thiry, E.; Romera, S.A. Biology of bovine herpesvirus 5. Vet. J. 2010, 184, 138–145. [Google Scholar] [CrossRef]
- Campos, F.S.; Franco, A.C.; Hübner, S.O.; Oliveira, M.T.; Silva, A.D.; Esteves, P.A.; Roehe, P.M.; Rijsewijk, F.A. High prevalence of co-infections with bovine herpesvirus 1 and 5 found in cattle in southern Brazil. Vet. Microbiol. 2009, 139, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R. The ‘direct costs’ of livestock disease: The development of a system of models for the analysis of 30 endemic livestock diseases in Great Britain. J. Agric. Econ. 2003, 54, 55–71. [Google Scholar] [CrossRef]
- Statham, J.M.; Randall, L.V.; Archer, S.C. Reduction in daily milk yield associated with subclinical bovine herpesvirus 1 infection. Vet. Rec. 2015, 177, 339. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, M.; Weynants, V.; Godfroid, J.; Schynts, F.; Meyer, G.; Letesson, J.J.; Thiry, E. Effects of bovine herpesvirus type 1 infection in calves with maternal antibodies on immune response and virus latency. J. Clin. Microbiol. 2000, 38, 1885–1894. [Google Scholar] [CrossRef]
- Babiuk, L.A.; Tikoo, S.K. Immunology of bovine herpesvirus 1 infection. Vet. Microbiol. 1996, 53, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.; Ackermann, M. Pathogenesis of ruminant herpesvirus infections. Vet. Microbiol. 1996, 53, 3–15. [Google Scholar] [CrossRef]
- Chase, C.C.; Fulton, R.W.; O’Toole, D.; Gillette, B.; Daly, R.F.; Perry, G.; Clement, T. Bovine herpesvirus 1 modified live virus vaccines for cattle reproduction: Balancing protection with undesired effects. Vet. Microbiol. 2017, 206, 69–77. [Google Scholar] [CrossRef]
- De Brun, L.; Leites, M.; Furtado, A.; Campos, F.; Roehe, P.; Puentes, R. Field evaluation of commercial vaccines against infectious bovine rhinotracheitis (Ibr) virus using different immunization protocols. Vaccines 2021, 9, 408. [Google Scholar] [CrossRef]
- Kreutz, L.C. Immune response against viruses. In Veterinary Virology: General Virology and Viral Diseases, 2nd ed.; Flores, E.F., Ed.; UFSM: Santa Maria, Brazil, 2012; pp. 237–261. [Google Scholar]
- Chase, C.C. Acceptable young calf vaccination strategies—What, when, and how? Vet. Clin. Food Anim. Pract. 2022, 38, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Engels, M. Pro and contra IBR-eradication. Vet. Microbiol. 2006, 113, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Parreño, V.; López, M.V.; Rodriguez, D.; Vena, M.M.; Izuel, M.; Filippi, J.; Romera, A.; Faverin, C.; Bellinzoni, R.; Fernandez, F.; et al. Development and statistical validation of a guinea pig model for vaccine potency testing against infectious bovine rhinothracheitis (IBR) virus. Vaccine 2010, 28, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, Z.; Krejci, J.; Jinek, P.; Lany, P.; Zendulkova, D.; Cihal, P. Development of a disease control programme based on the use of an inactivated vaccine against infectious bovine rhinotracheitis. Vet. Microbiol. 1996, 53, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, D.R.; Clough, N.C. Correlation of Clostridium botulinum type C antitoxin titers in mink and guinea pigs to protection against type C intoxication in mink. Anaerobe 2008, 14, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.D.; Weiblen, R.; Flores, E.F. Imunogenicidade de vacinas comerciais inativadas contra o herpesvírus bovino tipo 1. Ciência Rural 2007, 37, 1471–1474. [Google Scholar] [CrossRef]
- Baccili, C.C.; Martin, C.C.; Silva, K.N.; Nichi, M.; Flores, E.F.; Filho, A.E.V.; Pituco, E.M.; Gomes, V. Serological response against bovine herpesvirus and bovine viral diarrhea virus induced by commercial vaccines in Holstein heifers. Pesq. Vet. Bras. 2019, 39, 870–878. [Google Scholar] [CrossRef]
- Rodning, S.P.; Marley, M.S.D.; Zhang, Y.; Eason, A.B.; Nunley, C.L.; Walz, P.H.; Riddell, K.P.; Galik, P.K.; Brodersen, B.W.; Givens, M.D. Comparison of three commercial vaccines for preventing persistent infection with bovine viral diarrhea virus. Theriogenology 2010, 73, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.D.; Buterbaugh, R.E.; Herbert, J.M.; Hass, J.M.; Frank, N.E.; Luempert, L.G., III; Chase, C.C. Efficacy of bovine herpesvirus-1 inactivated vaccine against abortion and stillbirth in pregnant heifers. J. Am. Vet. Med. Assoc. 2007, 231, 1386–1389. [Google Scholar] [CrossRef]
- Walz, P.H.; Givens, M.D.; Rodning, S.P.; Riddell, K.P.; Brodersen, B.W.; Scruggs, D.; Short, T.; Grotelueschen, D. Evaluation of reproductive protection against bovine viral diarrhea virus and bovine herpesvirus-1 afforded by annual revaccination with modified-live viral or combination modified-live/killed viral vaccines after primary vaccination with modified-live viral vaccine. Vaccine 2017, 35, 1046–1054. [Google Scholar] [CrossRef]
- Petrini, S.; Iscaro, C.; Righi, C. Antibody responses to bovine alphaherpesvirus 1 (BoHV-1) in passively immunized calves. Viruses 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.A.; Geary, T.W.; Walker, J.A.; Rich, J.J.J.; Northrop, E.J.; Perkins, S.D.; Mogck, C.L.; Van Emon, M.L.; Zezeski, A.L.; Daly, R.F. Influence of vaccination with a combined chemically altered/inactivated BHV-1/BVD vaccine or a modified-live BHV-1/BVD vaccine on reproductive performance in beef cows and heifers. Bov. Pract. 2018, 52, 53–58. [Google Scholar] [CrossRef]

| Vaccine Formulations | Guinea Pig Experiment | Bovine Experiment |
|---|---|---|
| Effect of vaccines formulation | <0.0001 | <0.0001 |
| Effect of time | 0.0017 | <0.0001 |
| Interaction vaccine × time | <0.0001 | <0.0001 |
| Vaccine A | 4.00 ± 0.00 | Reproved |
| Vaccine B | 20.00 ± 6.66 | 20.00 ± 4.51 |
| Vaccine C | 5.42 ± 0.90 | Reproved |
| Vaccine D | 28.66 ± 8.10 | 84.76 ± 27.53 |
| Vaccine E | 4.00 ± 0.00 | Reproved |
| Vaccine F | 6.33 ± 1.34 | Reproved |
| Vaccine G | 4.00 ± 0.00 | Reproved |
| Vaccine H | No-tested | 48.74 ± 9.27 |
| Non-vaccinated Guinea pig and bovine model | 4.00 ± 0.00 | |
| Vaccine | Guinea Pig—BoAHV-1 (Cooper) | |
| T0 | T30 | |
| Non-vaccinated | 4.00 ± 0.00 A | 4.00 ± 0.00 B |
| A | 4.00 ± 0.00 A | 4.00 ± 0.00 B |
| B | 4.00 ± 0.00 Ab | 36.0 ± 9.63 Aba |
| C | 4.00 ± 0.00 Ab | 6.85 ± 1.68 Ca |
| D | 4.00 ± 0.00 Ab | 53.33 ± 6.74 Aa |
| E | 4.00 ± 0.00 A | 4.00 ± 0.00 B |
| F | 4.00 ± 0.00 Ab | 8.66 ± 2.40 BCa |
| G | 4.00 ± 0.00 A | 4.00 ± 0.00 B |
| Vaccine | Bovine—BoAHV-1 (Cooper) | |
| T0 | T60 | |
| Non-vaccinated | 4.00 ± 0.00 A | 4.00 ± 0.00 B |
| B | 4.00 ± 0.00 Ac | 36.57 ± 7.58 Ba |
| D | 4.00 ± 0.00 Ac | 210.28 ± 57.09 Aa |
| H | 4.00 ± 0.00 Ac | 74.66 ± 14.11 Ba |
| Vaccine Formulations | Guinea Pig Experiment | Bovine Experiment |
|---|---|---|
| Effect of vaccine formulation | 0.0005 | 0.0005 |
| Effect of time | 0.0162 | <0.0001 |
| Interaction of vaccine × time | 0.0050 | <0.0001 |
| Vaccine A | 7.71 ± 7.71 | Reproved |
| Vaccine B | 533.33 ± 282.21 | 426.66 ± 164.86 |
| Vaccine C | 320.00 ± 96.48 | Reproved |
| Vaccine D | 693.33 ± 329.16 | 780.95 ± 229.19 |
| Vaccine E | 40.00 ± 40.00 | Reproved |
| Vaccine F | 70.00 ± 53.51 | Reproved |
| Vaccine G | 11.42 ± 5.01 | Reproved |
| Vaccine H | No-tested | 657.77 ± 165.01 |
| Non-vaccinated Guinea pig and bovine model | 0.00 ± 0.00 | |
| Vaccines | Guinea Pig—BoAHV-1 | |
| T0 | T30 | |
| Non-vaccinated | 0.00 ± 0.00 A | 0.00 ± 0.00 B |
| A | 0.00 ± 0.00 A | 0.00 ± 0.00 B |
| B | 0.00 ± 0.00 Ab | 1066.67 ± 486.47 Aba |
| C | 0.00 ± 0.00 Ab | 140.00 ± 103.15 Ca |
| D | 0.00 ± 0.00 Ab | 1386.67 ± 533.33 C |
| E | 0.00 ± 0.00 A | 26.66 ± 26.66 B |
| F | 0.00 ± 0.00 Ab | 80.00 ± 80.00 BC |
| G | 0.00 ± 0.00 A | 0.00 ± 0.00 B |
| Vaccines | Bovine—BoAHV-1 | |
| T0 | T60 | |
| Non-vaccinated | 0.00 ± 0.00 A | 0.00 ± 0.00 C |
| B | 5.71 ± 5.71 A | 1188.57 ± 354.10 Ca |
| D | 5.71 ± 5.71 A | 2011.43 ± 354.10 Ca |
| H | 0.00 ± 0.00 Ac | 1440.00 ± 357.77 Ca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, L.; Franklin, Y.V.; da Silva, G.F.R.; Santos, J.F.; Parreño, V.G.; Wigdorovitz, A.; Gomes, V. Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations. Vaccines 2024, 12, 615. https://doi.org/10.3390/vaccines12060615
Camargo L, Franklin YV, da Silva GFR, Santos JF, Parreño VG, Wigdorovitz A, Gomes V. Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations. Vaccines. 2024; 12(6):615. https://doi.org/10.3390/vaccines12060615
Chicago/Turabian StyleCamargo, Luana, Yasmin Vieira Franklin, Gustavo Feliciano Resende da Silva, Janaína Ferreira Santos, Viviana Gladys Parreño, Andrés Wigdorovitz, and Viviani Gomes. 2024. "Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations" Vaccines 12, no. 6: 615. https://doi.org/10.3390/vaccines12060615
APA StyleCamargo, L., Franklin, Y. V., da Silva, G. F. R., Santos, J. F., Parreño, V. G., Wigdorovitz, A., & Gomes, V. (2024). Serological Responses of Guinea Pigs and Heifers to Eight Different BoAHV-1 Vaccine Formulations. Vaccines, 12(6), 615. https://doi.org/10.3390/vaccines12060615







