COVID-19 Vaccine Uptake and Effectiveness by Time since Vaccination in the Western Cape Province, South Africa: An Observational Cohort Study during 2020–2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Study Period, and Data Sources
2.2. Definition of Outcomes
2.3. Definition of Vaccine States
2.4. Statistical Methods
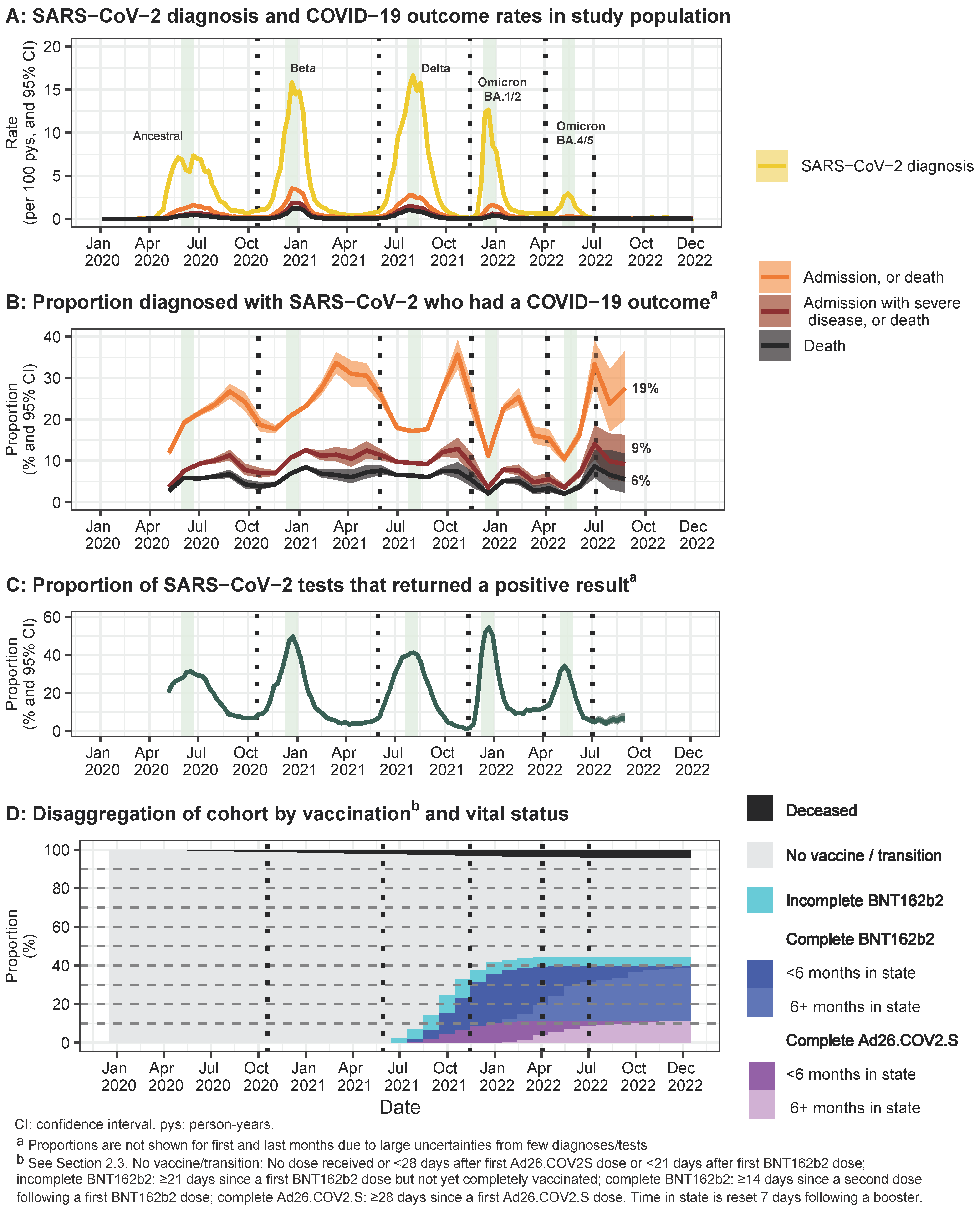
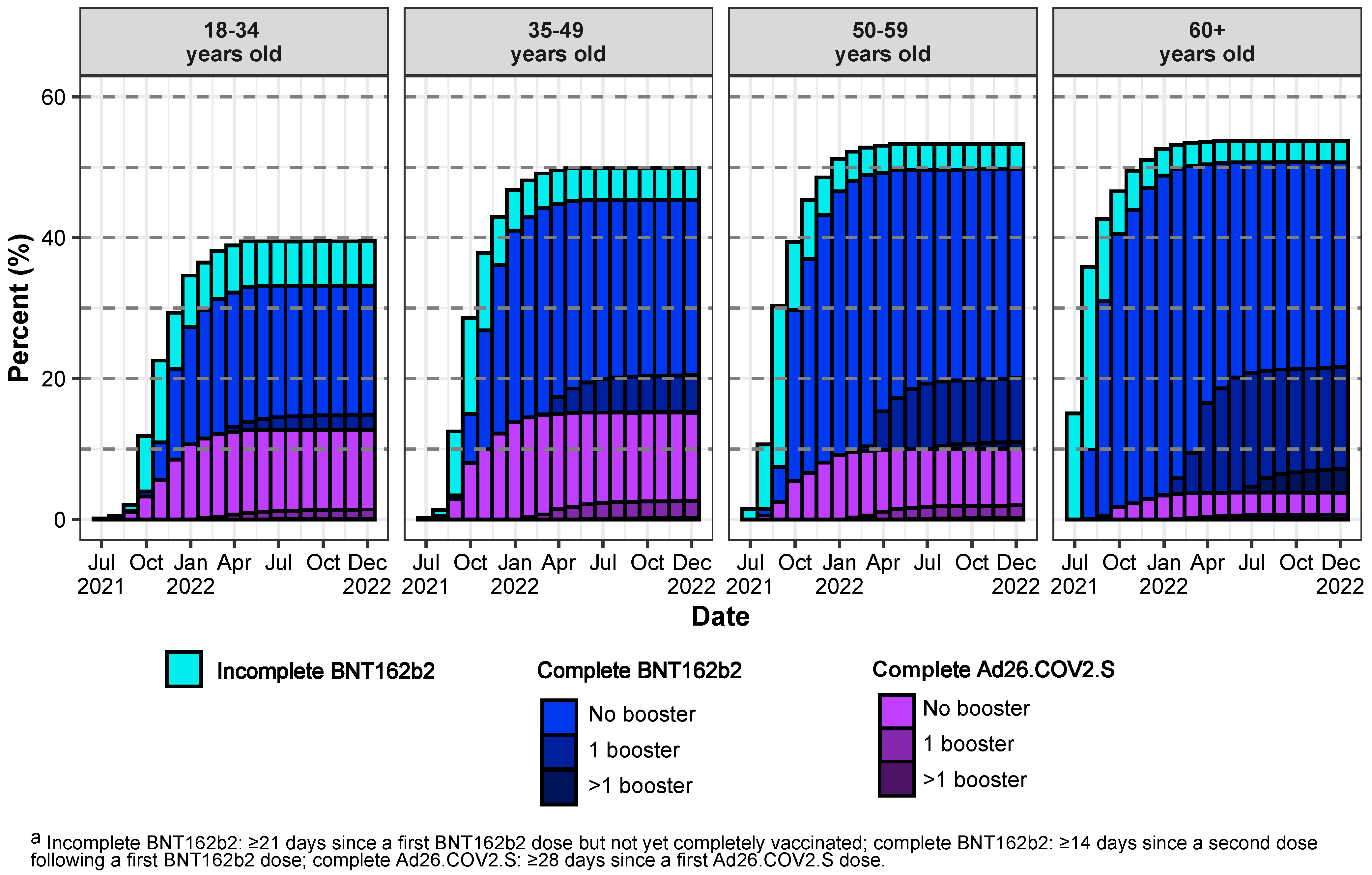
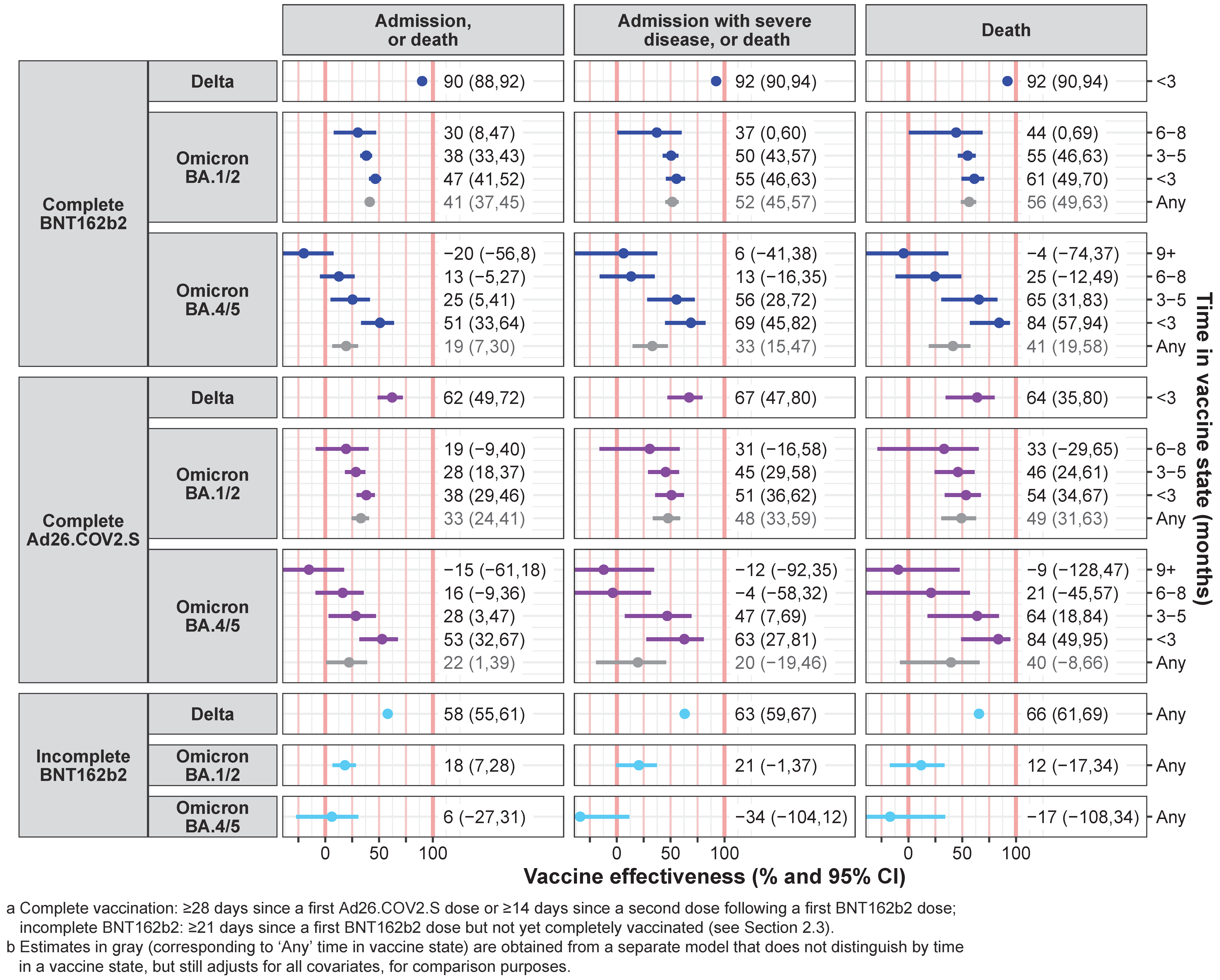
3. Results
3.1. Description of Cohort Characteristics, Vaccination, and COVID-19 Outcomes
3.2. Vaccine Effectiveness Estimates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, J. COVID-19: WHO declares end of global health emergency. BMJ 2023, 381, 1041. [Google Scholar] [CrossRef] [PubMed]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; d’Andrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of waning of SARS-CoV-2 vaccine-induced immunity: A systematic review and meta-analysis. JAMA Netw. Open 2023, 6, e2310650. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after COVID-19 vaccination and previous infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Abu-Raddad, L.J. Waning effectiveness of COVID-19 vaccines. Lancet 2022, 399, 771–773. [Google Scholar] [CrossRef]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population immunity and COVID-19 severity with Omicron variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Mutombo, P.N.; Fallah, M.P.; Munodawafa, D.; Kabel, A.; Houeto, D.; Goronga, T.; Mweemba, O.; Balance, G.; Onya, H.; Kamba, R.S.; et al. COVID-19 vaccine hesitancy in Africa: A call to action. Lancet Glob. Health 2022, 10, e320–e321. [Google Scholar] [CrossRef]
- Brazier, E.; Ajeh, R.; Maruri, F.; Musick, B.; Freeman, A.; Wester, C.W.; Lee, M.; Shamu, T.; Ramírez, B.C.; D’Almeida, M.; et al. Service delivery challenges in HIV care during the first year of the COVID-19 pandemic: Results from a site assessment survey across the global IeDEA consortium. J. Int. AIDS Soc. 2022, 25, e26036. [Google Scholar] [CrossRef]
- Harris, T.G.; Jaszi, E.; Lamb, M.R.; Laudari, C.A.; Furtado, M.L.M.; Nijirazana, B.; Aimé, N.; Ekali, G.L.; Lifanda, L.E.; Brou, H.; et al. Effects of the Coronavirus Disease 2019 Pandemic on Human Immunodeficiency Virus Services: Findings from 11 Sub-Saharan African Countries. Clin. Infect. Dis. 2022, 75, e1046–e1053. [Google Scholar] [CrossRef] [PubMed]
- Wagner, Z.; Mukasa, B.; Nakakande, J.; Stecher, C.; Saya, U.; Linnemayr, S. Impact of the COVID-19 pandemic on use of HIV care, Antiretroviral therapy adherence, and viral suppression: An observational cohort study from Uganda. JAIDS 2021, 88, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Dear, N.; Duff, E.; Esber, A.; Parikh, A.; Iroezindu, M.; Bahemana, E.; Kibuuka, H.; Owuoth, J.; Maswai, J.; Crowell, T.A.; et al. Transient reductions in Human Immunodeficiency Virus (HIV) clinic attendance and food security during the Coronavirus Disease 2019 (COVID-19) pandemic for people living with HIV in 4 African countries. Clin. Infect. Dis. 2021, 73, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Hussey, H.; Vreede, H.; Davies, M.A.; Heekes, A.; Kalk, E.; Hardie, D.; Van Zyl, G.; Naidoo, M.; Morden, E.; Bam, J.L.; et al. Epidemiology and outcomes of SARS-CoV-2 infection associated with anti-nucleocapsid seropositivity in Cape Town, South Africa. medRxiv 2022, 2022.12.01.22282927. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.; Cable, R.; Coleman, C.; Glatt, T.N.; Grebe, E.; Mhlanga, L.; Nyano, C.; Pieterson, N.; Swanevelder, R.; Swarts, A.; et al. Estimates of prevalence of anti-SARS-CoV-2 antibodies among blood donors in South Africa in March 2022. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Collie, S.; Nayager, J.; Bamford, L.; Bekker, L.G.; Zylstra, M.; Gray, G. Effectiveness and durability of the BNT162b2 vaccine against Omicron sublineages in South Africa. N. Engl. J. Med. 2022, 387, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Boulle, A.; Heekes, A.; Tiffin, N.; Smith, M.; Mutemaringa, T.; Zinyakatira, N.; Phelanyane, F.; Pienaar, C.; Buddiga, K.; Coetzee, E.; et al. Data Centre Profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int. J. Popul. Data Sci. 2019, 4, 1143. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, N.; Gray, A.; Mkhabela, B.; Myende, N.; Day, C. Health and related indicators 2022. S. Afr. Health Rev. 2023, 25. [Google Scholar] [CrossRef]
- South African Government Electronic Vaccination Data System (EVDS). Self Registration Portal. Available online: https://www.gov.za/covid-19/vaccine/evds (accessed on 10 May 2024).
- National Institute for Communicable Diseases. COVID-19 Surveillance Reports. Available online: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports (accessed on 13 October 2023).
- Jassat, W.; Cohen, C.; Tempia, S.; Masha, M.; Goldstein, S.; Kufa, T.; Murangandi, P.; Savulescu, D.; Walaza, S.; Bam, J.-L.; et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: A cohort study. Lancet HIV 2021, 8, e554–e567. [Google Scholar] [CrossRef]
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2020, 73, e2005–e2015. [Google Scholar] [CrossRef]
- Western Cape Government. Western Cape Coronavirus (COVID-19) Partners—Western Cape Government: Health (WCGH) Circulars. Available online: https://www.westerncape.gov.za/general-publication/western-cape-coronavirus-covid-19-partners-western-cape-government-health-wcgh-circulars (accessed on 10 May 2024).
- Carazo, S.; Skowronski, D.M.; Brisson, M.; Barkati, S.; Sauvageau, C.; Brousseau, N.; Gilca, R.; Fafard, J.; Talbot, D.; Ouakki, M.; et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: A test-negative case-control study. Lancet Infect. Dis. 2023, 23, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, M.; Berec, L.; Přibylová, L.; Májek, O.; Pavlík, T.; Jarkovský, J.; Weiner, J.; Barusová, T.; Trnka, J. Protection by vaccines and previous infection against the Omicron variant of Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2022, 226, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; de Araujo Oliveira, V.; Paixão, E.S.; Florentino, P.T.; Penna, G.O.; Pearce, N.; Werneck, G.L.; Barreto, M.L.; Boaventura, V.S.; Barral-Netto, M. Vaccination plus previous infection: Protection during the Omicron wave in Brazil. Lancet Infect. Dis. 2022, 22, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Kahn, F. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants—Surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill. 2022, 27, 2200322. [Google Scholar] [CrossRef] [PubMed]
- Plumb, I.D.; Feldstein, L.R.; Barkley, E.; Posner, A.B.; Bregman, H.S.; Hagen, M.B.; Gerhart, J.L. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. MMWR Morb. Mortal. Wkly Rep. 2022, 71, 549–555. [Google Scholar] [CrossRef]
- Nichol, K.L. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine 2009, 27, 6305–6311. [Google Scholar] [CrossRef]
- Paleker, M.; Davies, M.A.; Raubenheimer, P.; Naude, J.; Boulle, A.; Hussey, H. Change in profile of COVID-19 deaths in Western Cape Province, South Africa, during the fourth wave. S. Afr. Med. J. 2022, 112, 185–186. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Changes to the Way We Report on COVID-19 Deaths. Available online: https://ukhsa.blog.gov.uk/2023/01/27/changes-to-the-way-we-report-on-covid-19-deaths/ (accessed on 8 January 2024).
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Kitano, T.; Thompson, D.A.; Engineer, L.; Dudley, M.Z.; Salmon, D.A. Risk and benefit of mRNA COVID-19 Vaccines for the Omicron variant by age, sex, and presence of comorbidity: A quality-adjusted life years analysis. Am. J. Epidemiol. 2023, 192, 1137–1147. [Google Scholar] [CrossRef]
- Schoub, B.D.; Ngcobo, N.J.; Madhi, S. The National Advisory Group on Immunization (NAGI) of the Republic of South Africa. Vaccine 2010, 28 (Suppl. S1), A31–A34. [Google Scholar] [CrossRef] [PubMed]
- Western Cape Department of Health and Wellness. Circular 39/2024 COVID-19 Vaccine Update. Available online: https://www.westerncape.gov.za/assets/departments/health/COVID-19/wcghw_circular_h39_of_2024_-_covid-19_vaccine_update.pdf (accessed on 10 May 2024).
- World Health Organization. WHO Roadmap on Uses of COVID-19 Vaccines in the Context of Omicron and High Population Immunity. Available online: https://iris.who.int/bitstream/handle/10665/373987/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2023.2-eng.pdf?sequence=1 (accessed on 10 May 2024).
| Overall N = 2,429,927 | Complete Vaccination by the End of 2022 a | ||
|---|---|---|---|
| Characteristics and Conditions (2020–2022) | No N = 1,455,385 | Yes N = 974,542 | |
| Age category at the start of 2020 (years) | |||
| 18–34 | 41.6 (1,009,672) | 46.6 (677,813) | 34.1 (331,859) |
| 35–49 | 29.3 (711,077) | 27.2 (395,497) | 32.4 (315,580) |
| 50–59 | 14.1 (342,308) | 12.4 (179,961) | 16.7 (162,347) |
| 60+ | 15.1 (366,870) | 13.9 (202,114) | 16.9 (164,756) |
| Sex | |||
| Female | 59.7 (1,450,660) | 57.8 (841,578) | 62.5 (609,082) |
| Male | 40.3 (979,267) | 42.2 (613,807) | 37.5 (365,460) |
| Hypertension | |||
| Absent at the end of 2022 | 76.5 (1,858,079) | 79.8 (1,161,884) | 71.4 (696,195) |
| Onset during 2020–2022 | 3.4 (83,174) | 2.8 (40,473) | 4.4 (42,701) |
| Present before 2020 | 20.1 (488,674) | 17.4 (253,028) | 24.2 (235,646) |
| Diabetes | |||
| Absent at the end of 2022 | 89.5 (2,174,691) | 90.8 (1,321,701) | 87.5 (852,990) |
| Onset during 2020–2022 | 1.9 (46,111) | 1.6 (22,634) | 2.4 (23,477) |
| Present before 2020 | 8.6 (209,125) | 7.6 (111,050) | 10.1 (98,075) |
| Chronic kidney disease | |||
| Absent at the end of 2022 | 95.7 (2,324,768) | 95.9 (1,396,419) | 95.3 (928,349) |
| Onset during 2020–2022 | 0.7 (16,445) | 0.5 (7707) | 0.9 (8738) |
| Present before 2020 | 3.7 (88,714) | 3.5 (51,259) | 3.8 (37,455) |
| Chronic respiratory disease | |||
| Absent at the end of 2022 | 92.2 (2,241,094) | 92.5 (1,346,451) | 91.8 (894,643) |
| Onset during 2020–2022 | 1.5 (36,451) | 1.4 (20,454) | 1.6 (15,997) |
| Present before 2020 | 6.3 (152,382) | 6.1 (88,480) | 6.6 (63,902) |
| HIV | |||
| Absent at the end of 2022 | 85.4 (2,075,796) | 85.3 (1,241,925) | 85.6 (833,871) |
| Onset during 2020–2022 | 1.4 (32,986) | 1.4 (21,048) | 1.2 (11,938) |
| Present before 2020 | 13.2 (321,145) | 13.2 (192,412) | 13.2 (128,733) |
| Tuberculosis (at any time in past) b | |||
| Absent at the end of 2022 | 90.2 (2,190,920) | 89.2 (1,298,552) | 91.6 (892,368) |
| (First) onset during 2020–2022 | 1.7 (42,068) | 2.0 (28,783) | 1.4 (13,285) |
| Present before 2020 | 8.1 (196,939) | 8.8 (128,050) | 7.1 (68,889) |
| Tuberculosis (ongoing episode) | |||
| Absent throughout 2020–2022 | 96.8 (2,351,220) | 96.2 (1,400,628) | 97.5 (950,592) |
| Experienced an episode during 2020–2022 | 3.2 (78,707) | 3.8 (54,757) | 2.5 (23,950) |
| Complete Vaccination by the End of 2022 a | |||
|---|---|---|---|
| State or Events Experienced as at the End of 2022 | Overall N = 2,429,927 | No N = 1,455,385 | Yes N = 974,542 |
| COVID-19 vaccination status a and boosters | |||
| None | 55.0 (1,336,826) | 91.9 (1,336,826) | 0.0 (0) |
| Incomplete BNT162b2 | 4.9 (118,559) | 8.1 (118,559) | 0.0 (0) |
| Complete Ad26.COV2.S (no booster) | 9.7 (236,725) | 0.0 (0) | 24.3 (236,725) |
| Complete Ad26.COV2.S (and booster) | 1.7 (41,196) | 0.0 (0) | 4.2 (41,196) |
| Complete BNT162b2 (no booster) | 22.6 (548,914) | 0.0 (0) | 56.3 (548,914) |
| Complete BNT162b2 (and booster) | 6.1 (147,707) | 0.0 (0) | 15.2 (147,707) |
| SARS-CoV-2 diagnosis | |||
| Not experienced | 92.1 (2,237,396) | 93.3 (1,357,422) | 90.3 (879,974) |
| Experienced | 7.9 (192,531) | 6.7 (97,963) | 9.7 (94,568) |
| Number of negative SARS-CoV-2 tests | |||
| 0 | 81.8 (1,987,267) | 84.3 (1,227,129) | 78.0 (760,138) |
| 1 | 13.1 (317,758) | 11.4 (165,723) | 15.6 (152,035) |
| 2+ | 5.1 (124,902) | 4.3 (62,533) | 6.4 (62,369) |
| COVID-19-related hospitalization, or death | |||
| Not experienced | 98.4 (2,391,586) | 98.2 (1,429,692) | 98.7 (961,894) |
| Experienced | 1.6 (38,341) | 1.8 (25,693) | 1.3 (12,648) |
| COVID-19-related hospitalization with severe disease, or death | |||
| Not experienced | 99.3 (2,412,279) | 99.0 (1,441,138) | 99.7 (971,141) |
| Experienced | 0.7 (17,648) | 1.0 (14,247) | 0.3 (3401) |
| COVID-19-related death and vital status b | |||
| Alive | 95.4 (2,318,859) | 93.3 (1,357,339) | 98.7 (961,520) |
| Died: not COVID-19-related | 4.1 (99,525) | 6.0 (87,001) | 1.3 (12,524) |
| Died: COVID-19-related | 0.5 (11,543) | 0.8 (11,045) | 0.1 (498) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassanjee, R.; Davies, M.-A.; Heekes, A.; Mahomed, H.; Hawkridge, A.J.; Morden, E.; Jacobs, T.; Cohen, C.; Moultrie, H.; Lessells, R.J.; et al. COVID-19 Vaccine Uptake and Effectiveness by Time since Vaccination in the Western Cape Province, South Africa: An Observational Cohort Study during 2020–2022. Vaccines 2024, 12, 628. https://doi.org/10.3390/vaccines12060628
Kassanjee R, Davies M-A, Heekes A, Mahomed H, Hawkridge AJ, Morden E, Jacobs T, Cohen C, Moultrie H, Lessells RJ, et al. COVID-19 Vaccine Uptake and Effectiveness by Time since Vaccination in the Western Cape Province, South Africa: An Observational Cohort Study during 2020–2022. Vaccines. 2024; 12(6):628. https://doi.org/10.3390/vaccines12060628
Chicago/Turabian StyleKassanjee, Reshma, Mary-Ann Davies, Alexa Heekes, Hassan Mahomed, Anthony J. Hawkridge, Erna Morden, Theuns Jacobs, Cheryl Cohen, Harry Moultrie, Richard J. Lessells, and et al. 2024. "COVID-19 Vaccine Uptake and Effectiveness by Time since Vaccination in the Western Cape Province, South Africa: An Observational Cohort Study during 2020–2022" Vaccines 12, no. 6: 628. https://doi.org/10.3390/vaccines12060628
APA StyleKassanjee, R., Davies, M.-A., Heekes, A., Mahomed, H., Hawkridge, A. J., Morden, E., Jacobs, T., Cohen, C., Moultrie, H., Lessells, R. J., Van Der Walt, N., Arendse, J. O., Wolter, N., Walaza, S., Jassat, W., von Gottberg, A., Hannan, P. L., Feikin, D. R., Cloete, K., & Boulle, A. (2024). COVID-19 Vaccine Uptake and Effectiveness by Time since Vaccination in the Western Cape Province, South Africa: An Observational Cohort Study during 2020–2022. Vaccines, 12(6), 628. https://doi.org/10.3390/vaccines12060628






