Heterogenous Induction of Blocking Antibodies against Ragweed Allergen Molecules by Allergen Extract-Based Immunotherapy Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression and Purification of the Recombinant Allergens and Extract Preparation
2.2. Sera from Allergic Patients
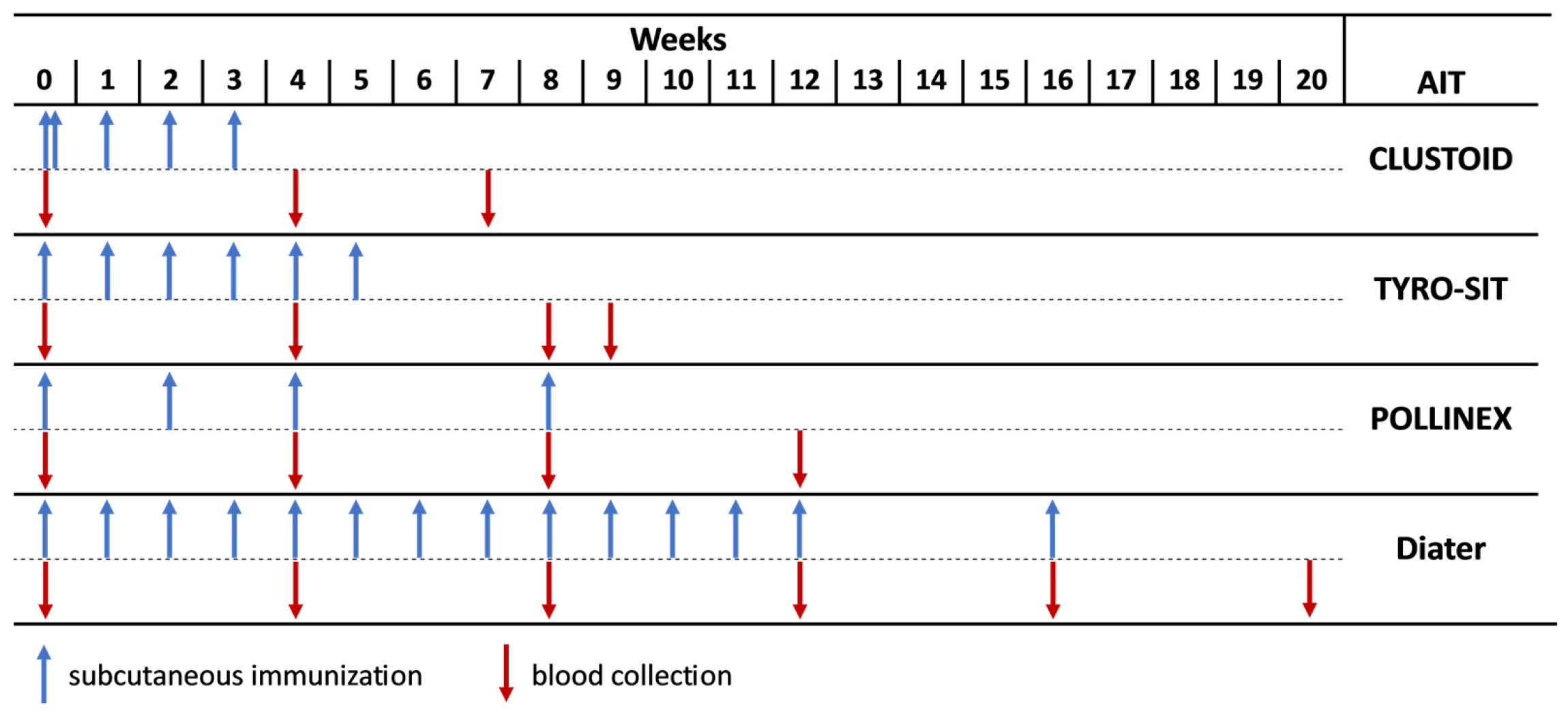
2.3. Subcutaneous Allergen-Specific Immunotherapy Vaccines for the Treatment of Ragweed Pollen Allergy
2.4. Immunization of Rabbits with AIT Vaccines and Purified Recombinant Ragweed Pollen Allergens
2.5. Measurement of Ragweed Pollen Allergen-Specific Antibodies by ELISA
2.6. Inhibition of Human IgE Binding to Recombinant Ragweed Pollen Allergens by AIT Vaccine-Induced IgG Antibodies in ELISA Competition Assays
2.7. Inhibition of Allergen-Induced Basophil Activation by AIT-Specific IgG Antibodies in Rat Basophil Leukaemia (RBL) Assays
3. Results
3.1. Only Rabbits Immunized with CLUSTOID and Diater Had Relevant Anti-Ragweed Pollen Extract-Specific IgG Responses Four Weeks after the Last Injection
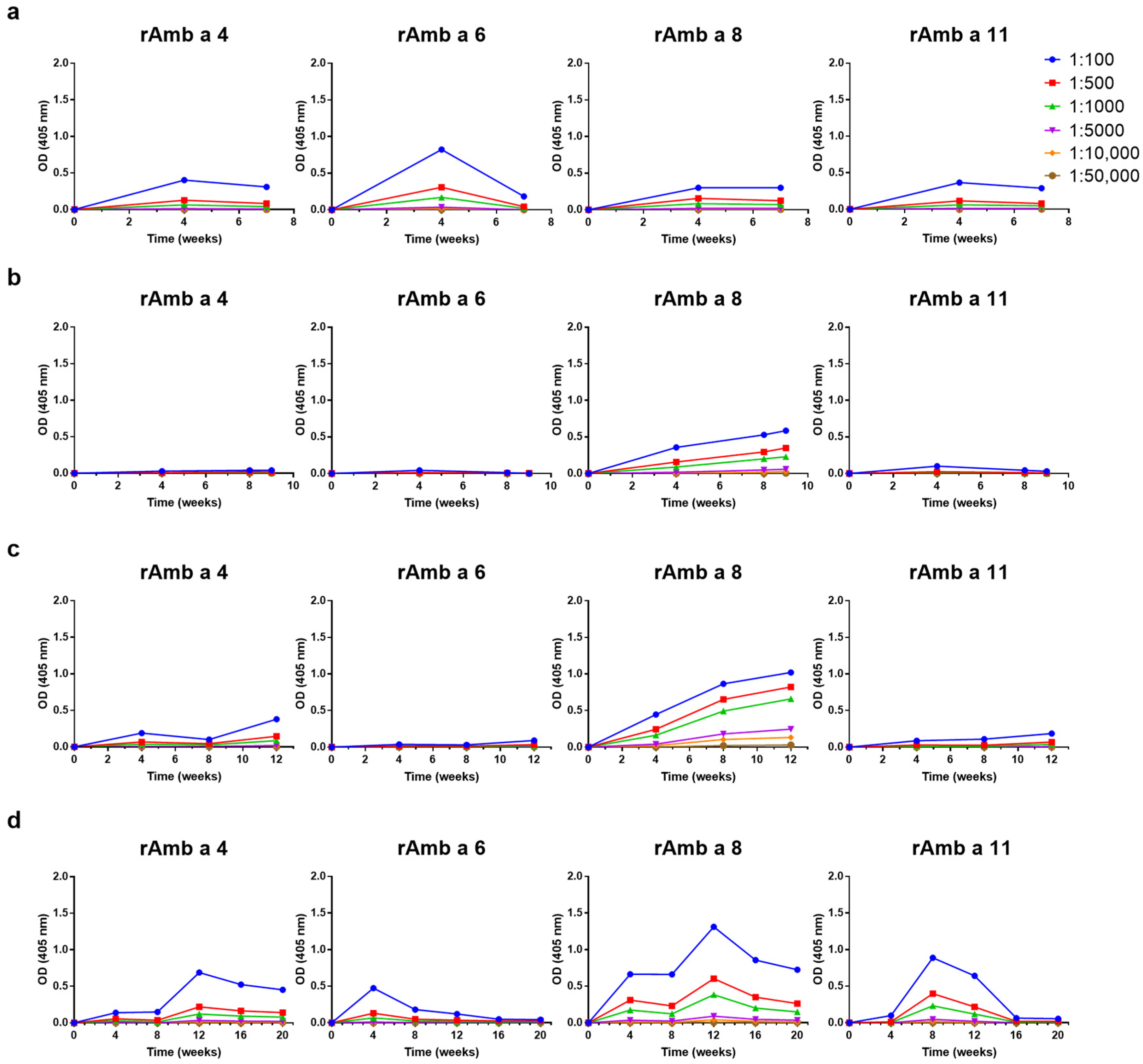
3.2. Levels and Kinetics of IgG against rAmb a 1.01, nAmb a 1.01 and rAmb a 1.03 in AIT Vaccine-Immunized Rabbits
3.3. Levels and Kinetics of Allergen-Specific IgG Responses Induced by the Four AIT Vaccines to Ragweed Pollen Allergen Molecules Vary
3.4. Highly Variable Inhibition of Patient’s IgE Binding to Ragweed Pollen Extract and Ragweed Pollen Allergens by AIT Vaccine-Induced Antibodies
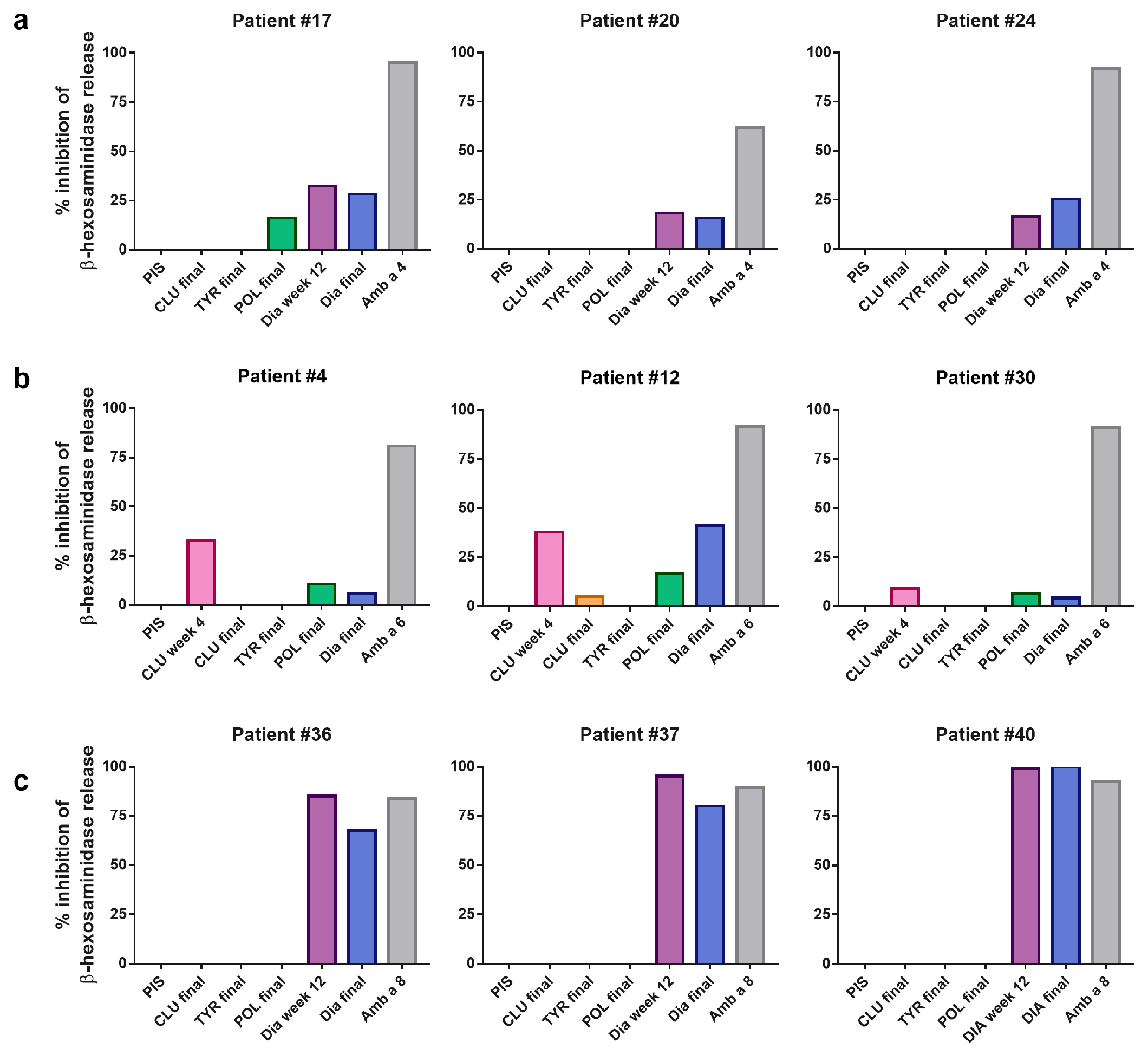
3.5. AIT Vaccine-Induced Antibodies Block Allergen-Induced Basophil Degranulation to a Varying Degree
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiss, L.; Béres, I. Anthropogenic Factors behind the Recent Population Expansion of Common Ragweed (Ambrosia artemisiifolia L.) in Eastern Europe: Is There a Correlation with Political Transitions? J. Biogeogr. 2006, 33, 2156–2157. [Google Scholar] [CrossRef]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common Ragweed: A Threat to Environmental Health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Oswalt, M.L.; Marshall, G.D. Ragweed as an Example of Worldwide Allergen Expansion. Allergy Asthma Clin. Immunol. 2008, 4, 130–135. [Google Scholar] [CrossRef] [PubMed]
- WHO/IUIS Allergen Nomenclature Sub-Committee: Allergen Nomenclature. IUIS Database. Available online: www.allergen.org (accessed on 3 June 2024).
- Floch, B.-L.; Groeme, R.; Chabre, H.; Baron-Bodo, V.; Nony, E.; Mascarell, L.; Moingeon, P. New Insights into Ragweed Pollen Allergens. Curr. Allergy Asthma Rep. 2015, 15, 1–7. [Google Scholar]
- Wopfner, N.; Gadermaier, G.; Egger, M.; Asero, R.; Ebner, C.; Jahn-Schmid, B.; Ferreira, F. The Spectrum of Allergens in Ragweed and Mugwort Pollen. Int. Arch. Allergy Immunol. 2005, 138, 337–346. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Norman, P.S.; Connell, J.T. Isolation and Characterization of Allergens from Ragweed Pollen. II. Biochemistry 1964, 3, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Rafnar, T.; Griffith, I.J.; Kuo, M.C.; Bond, J.F.; Rogers, B.L.; Klapper, D.G. Cloning of Amb a I (Antigen E), the Major Allergen Family of Short Ragweed Pollen. J. Biol. Chem. 1991, 266, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Twaroch, T.E.; Huber, S.; Reithofer, M.; Steiner, M.; Aglas, L.; Hauser, M.; Aloisi, I.; Asam, C.; Hofer, H. Amb a 1 Isoforms: Unequal Siblings with Distinct Immunological Features. Allergy 2017, 72, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Bouley, J.; Groeme, R.; Mignon, M.L.; Jain, K.; Chabre, H.; Floch, V.B.-L.; Couret, M.-N.; Bussières, L.; Lautrette, A.; Naveau, M.; et al. Identification of the Cysteine Protease Amb a 11 as a Novel Major Allergen from Short Ragweed. J. Allergy Clin. Immunol. 2015, 136, 1055–1064. [Google Scholar] [CrossRef]
- Groeme, R.; Airouche, S.; Kopečný, D.; Jaekel, J.; Savko, M.; Berjont, N.; Bussieres, L.; Le Mignon, M.; Jagic, F.; Zieglmayer, P. Structural and Functional Characterization of the Major Allergen Amb a 11 from Short Ragweed Pollen. J. Biol. Chem. 2016, 291, 13076–13087. [Google Scholar] [CrossRef]
- Buzan, M.; Zbîrcea, L.; Gattinger, P.; Babaev, E.; Stolz, F.; Valenta, R.; Păunescu, V.; Panaitescu, C.; Chen, K. Complex IgE Sensitization Patterns in Ragweed Allergic Patients: Implications for Diagnosis and Specific Immunotherapy. Clin. Transl. Allergy 2022, 12, e12179. [Google Scholar] [CrossRef] [PubMed]
- Léonard, R.; Wopfner, N.; Pabst, M.; Stadlmann, J.; Petersen, B.O.; Duus, J.Ø.; Himly, M.; Radauer, C.; Gadermaier, G.; Razzazi-Fazeli, E. A New Allergen from Ragweed (Ambrosia artemisiifolia) with Homology to Art v 1 from Mugwort. J. Biol. Chem. 2010, 285, 27192–27200. [Google Scholar] [CrossRef]
- Pablos, I.; Eichhorn, S.; Machado, Y.; Briza, P.; Neunkirchner, A.; Jahn-Schmid, B.; Wildner, S.; Soh, W.T.; Ebner, C.; Park, J. Distinct Epitope Structures of Defensin-like Proteins Linked to Proline-rich Regions Give Rise to Differences in Their Allergenic Activity. Allergy 2017, 73, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Gadermaier, G.; Wopfner, N.; Wallner, M.; Egger, M.; Didierlaurent, A.; Regl, G.; Aberger, F.; Lang, R.; Ferreira, F.; Hawranek, T. Array-based Profiling of Ragweed and Mugwort Pollen Allergens. Allergy 2008, 63, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Offermann, L.R.; Schlachter, C.R.; Perdue, M.L.; Majorek, K.A.; He, J.Z.; Booth, W.T.; Garrett, J.; Kowal, K.; Chruszcz, M. Structural, Functional, and Immunological Characterization of Profilin Panallergens Amb a 8, Art v 4, and Bet v 2. J. Biol. Chem. 2016, 291, 15447–15459. [Google Scholar] [CrossRef]
- Egger, M.; Mutschlechner, S.; Wopfner, N.; Gadermaier, G.; Briza, P.; Ferreira, F. Pollen-food Syndromes Associated with Weed Pollinosis: An Update from the Molecular Point of View. Allergy 2006, 61, 461–476. [Google Scholar] [CrossRef]
- Cudowska, B.; Kapingidza, A.B.; Pawłowicz, M.; Pampuch, A.; Hyduke, N.; Pote, S.; Schlachter, C.R.; Lebensztejn, D.M.; Chruszcz, M.; Kowal, K. Production and Use of Recombinant Profilins Amb a 8, Art v 4, Bet v 2, and Phl p 12 for Allergenic Sensitization Studies. Molecules 2020, 25, 369. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Roebber, M.; Goodfriend, L. Studies on the Immunological Relationship of Ragweed Pollen Antigens E and Ra. 3. J. Allergy Clin. Immunol. 1973, 51, 285–295. [Google Scholar] [CrossRef]
- Marsh, D.G.; Bias, W.B.; Santilli, J., Jr.; Schacter, B.; Goodfriend, L. Ragweed Allergen Ra5: A New Tool in Understanding the Genetics and Immunochemistry of Immune Response in Man. Immunochemistry 1975, 12, 539–543. [Google Scholar] [CrossRef]
- Wopfner, N.; Gruber, P.; Wallner, M.; Briza, P.; Ebner, C.; Mari, A.; Richter, K.; Vogel, L.; Ferreira, F. Molecular and Immunological Characterization of Novel Weed Pollen Pan-allergens. Allergy 2008, 63, 872–881. [Google Scholar] [CrossRef]
- Bordas-Le Floch, V.; Le Mignon, M.; Bouley, J.; Groeme, R.; Jain, K.; Baron-Bodo, V.; Nony, E.; Mascarell, L.; Moingeon, P. Identification of Novel Short Ragweed Pollen Allergens Using Combined Transcriptomic and Immunoproteomic Approaches. PLoS ONE 2015, 10, e0136258. [Google Scholar] [CrossRef] [PubMed]
- Dahl, Å.; Strandhede, S.-O.; Wihl, J.-Å. Ragweed–an Allergy Risk in Sweden? Aerobiologia 1999, 15, 293–297. [Google Scholar] [CrossRef]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; Van Cauwenberge, P. Allergenic Pollen and Pollen Allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Ihler, F.; Canis, M. Ragweed-Induced Allergic Rhinoconjunctivitis: Current and Emerging Treatment Options. J. Asthma Allergy 2015, 8, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Van Weel, C. Allergic Rhinitis and Its Impact on Asthma (ARIA) 2008. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.; Niggemann, B.; Dreborg, S.; Ferdousi, H.A.; Halken, S.; Høst, A.; Koivikko, A.; Norberg, L.A.; Valovirta, E.; Wahn, U.; et al. Specific Immunotherapy Has Long-Term Preventive Effect of Seasonal and Perennial Asthma: 10-Year Follow-up on the PAT Study. Allergy 2007, 62, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Casale, T.B.; Stokes, J.R. Immunotherapy: What Lies Beyond. J. Allergy Clin. Immunol. 2014, 133, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.R.; Shamji, M.H. Allergen Immunotherapy: Past, Present and Future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of Allergen-Specific Immunotherapy and Immune Tolerance to Allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating Cat Allergy with Monoclonal IgG Antibodies That Bind Allergen and Prevent IgE Engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef]
- Gevaert, P.; De Craemer, J.; De Ruyck, N.; Rottey, S.; de Hoon, J.; Hellings, P.W.; Volckaert, B.; Lesneuck, K.; Orengo, J.M.; Atanasio, A. Novel Antibody Cocktail Targeting Bet v 1 Rapidly and Sustainably Treats Birch Allergy Symptoms in a Phase 1 Study. J. Allergy Clin. Immunol. 2022, 149, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Cappella, A.; Durham, S.R. Allergen Immunotherapy for Allergic Respiratory Diseases. Hum. Vaccin. Immunother. 2012, 8, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Komlósi, Z.I.; Kovács, N.; Sokolowska, M.; van de Veen, W.; Akdis, M.; Akdis, C.A. Mechanisms of Subcutaneous and Sublingual Aeroallergen Immunotherapy: What Is New? Immunol. Allergy Clin. 2020, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, G.; Bagnasco, D.; Canonica, G.W. 30 Years of Sublingual Immunotherapy. Allergy 2020, 75, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Jarolim, E.; Bachmann, M.F.; Bonini, S.; Jacobsen, L.; Jutel, M.; Klimek, L.; Mahler, V.; Mösges, R.; Moingeon, P.; O’ Hehir, R.E. State-of-the-art in Marketed Adjuvants and Formulations in Allergen Immunotherapy: A Position Paper of the European Academy of Allergy and Clinical Immunology (EAACI). Allergy 2020, 75, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, Presence, and Future of Allergen Immunotherapy Vaccines. Allergy Eur. J. Allergy Clin. Immunol. 2020, 76, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.E. The Use of Allergoids and Adjuvants in Allergen Immunotherapy. Arch. Asthma Allergy Immunol. 2017, 1, 40–60. [Google Scholar] [CrossRef]
- Becker, S.; Zieglmayer, P.; Canto, G.; Fassio, F.; Yong, P.; Acikel, C.; Raskopf, E.; Steveling-Klein, E.H.; Allekotte, S.; Mösges, R. A Meta-analysis on Allergen-specific Immunotherapy Using MCT®(MicroCrystalline Tyrosine)-adsorbed Allergoids in Pollen Allergic Patients Suffering from Allergic Rhinoconjunctivitis. Clin. Transl. Allergy 2021, 11, e12037. [Google Scholar] [CrossRef]
- Baldrick, P.; Richardson, D.; Woroniecki, S.R.; Lees, B. Pollinex® Quattro Ragweed: Safety Evaluation of a New Allergy Vaccine Adjuvanted with Monophosphoryl Lipid A (MPL®) for the Treatment of Ragweed Pollen Allergy. J. Appl. Toxicol. An. Int. J. 2007, 27, 399–409. [Google Scholar] [CrossRef]
- Patel, P.; Holdich, T.; von Weikersthal-Drachenberg, K.J.F.; Huber, B. Efficacy of a Short Course of Specific Immunotherapy in Patients with Allergic Rhinoconjunctivitis to Ragweed Pollen. J. Allergy Clin. Immunol. 2014, 133, 121–129. [Google Scholar] [CrossRef]
- Codina, R.; Lockey, R.F. Pollen Used to Produce Allergen Extracts. Ann. Allergy Asthma Immunol. 2017, 118, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Vieths, S.; Kaul, S. Standardization and Regulation of Allergen Products in the European Union. Curr. Allergy Asthma Rep. 2016, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.G.; Lichtenstein, L.M.; Campbell, D.H. Studies Onallergoids’ Prepared from Naturally Occurring Allergens: I. Assay of Allergenicity and Antigenicity of Formalinized Rye Group I Component. Immunology 1970, 18, 705. [Google Scholar] [PubMed]
- Lund, L.; Henmar, H.; Würtzen, P.A.; Lund, G.; Hjortskov, N.; Larsen, J.N. Comparison of Allergenicity and Immunogenicity of an Intact Allergen Vaccine and Commercially Available Allergoid Products for Birch Pollen Immunotherapy. Clin. Exp. Allergy 2007, 37, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Zhernov, Y.; Elisyutina, O.; Campana, R.; Focke-Tejkl, M.; Curin, M.; Namazova-Baranova, L.; Wang, J.Y.; et al. Allergen Extracts for In Vivo Diagnosis and Treatment of Allergy: Is There a Future? J. Allergy Clin. Immunol. Pract. 2018, 6, 1845–1855.e2. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Niespodziana, K.; Linhart, B.; Neubauer, A.; Huber, H.; Henning, R.; Valenta, R.; Focke-Tejkl, M. Comparison of the Immunogenicity of BM32, a Recombinant Hypoallergenic B Cell Epitope–Based Grass Pollen Allergy Vaccine with Allergen Extract–Based Vaccines. J. Allergy Clin. Immunol. 2017, 140, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Khaitov, M.; Shilovskiy, I.; Valenta, R.; Weber, M.; Korneev, A.; Tulaeva, I.; Gattinger, P.; van Hage, M.; Hofer, G.; Konradsen, J.R. Recombinant PreS-fusion Protein Vaccine for Birch Pollen and Apple Allergy. Allergy 2024, 79, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yu, L.; Teng, F.; Wang, N.; Zhou, Y.; Zhang, C.; Yang, L. Expression of Recombinant Allergen, Der f 1, Der f 2 and Der f 4 Using Baculovirus-Insect Cell Systems. Arch. Med. Sci. 2018, 14, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Zabel, M.; Weber, M.; Kratzer, B.; Köhler, C.; Jahn-Schmid, B.; Gadermaier, G.; Gattinger, P.; Bidovec-Stojkovič, U.; Korošec, P.; Smole, U. Art v 1 IgE Epitopes of Patients and Humanized Mice Are Conformational. J. Allergy Clin. Immunol. 2022, 150, 920–930. [Google Scholar] [CrossRef]
- Lichtenstein, L.M.; Norman, P.S.; Winkenwerder, W.L.; Osler, A.G. In Vitro Studies of Human Ragweed Allergy: Changes in Cellular and Humoral Activity Associated with Specific Desensitization. J. Clin. Investig. 1966, 45, 1126–1136. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, A.; Berings, M.; Rohrbach, A.; Huang, H.-J.; Curin, M.; Gevaert, P.; Matricardi, P.M.; Valenta, R.; Vrtala, S. Molecular Profiling of Allergen-Specific Antibody Responses May Enhance Success of Specific Immunotherapy. J. Allergy Clin. Immunol. 2020, 146, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.F.; Citterio, S. The Worldwide Spread, Success, and Impact of Ragweed (Ambrosia Spp.). CRC Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Chapman, M.D.; Smith, A.M.; Vailes, L.D.; Arruda, L.K.; Dhanaraj, V.; Pomés, A. Recombinant Allergens for Diagnosis and Therapy of Allergic Disease. J. Allergy Clin. Immunol. 2000, 106, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Landivar, M.E.; Ruiz-García, M.; Andregnette-Rosigno, M.V.; Mahillo, I. How Molecular Diagnosis Can Change Allergen-specific Immunotherapy Prescription in a Complex Pollen Area. Allergy 2012, 67, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Hankin, C.S.; Cox, L.; Bronstone, A.; Wang, Z. Allergy Immunotherapy: Reduced Health Care Costs in Adults and Children with Allergic Rhinitis. J. Allergy Clin. Immunol. 2013, 131, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Dramburg, S.; Hilger, C.; Santos, A.F.; de Las Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-W.; Zieglmayer, P.; Zieglmayer, R.; Lemell, P.; Horak, F.; Bunu, C.P.; Valenta, R.; Vrtala, S. Selection of House Dust Mite–Allergic Patients by Molecular Diagnosis May Enhance Success of Specific Immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- James, L.K.; Shamji, M.H.; Walker, S.M.; Wilson, D.R.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Kimber, I.; Till, S.J.; Durham, S.R. Long-Term Tolerance after Allergen Immunotherapy Is Accompanied by Selective Persistence of Blocking Antibodies. J. Allergy Clin. Immunol. 2011, 127, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rosewich, M.; Schulze, J.; Eickmeier, O.; Telles, T.; Rose, M.A.; Schubert, R.; Zielen, S. Tolerance Induction after Specific Immunotherapy with Pollen Allergoids Adjuvanted by Monophosphoryl Lipid A in Children. Clin. Exp. Immunol. 2010, 160, 403–410. [Google Scholar] [CrossRef]
- Smoldovskaya, O.; Feyzkhanova, G.; Voloshin, S.; Arefieva, A.; Chubarova, A.; Pavlushkina, L.; Filatova, T.; Antonova, E.; Timofeeva, E.; Butvilovskaya, V. Allergen-Specific IgE and IgG4 Patterns among Patients with Different Allergic Diseases. World Allergy Organ. J. 2018, 11, 35. [Google Scholar] [CrossRef]
- Brazhnikov, G.; Smolnikov, E.; Litovkina, A.; Jiang, T.; Shatilov, A.; Tulaeva, I.; Tulaev, M.; Karaulov, A.; Poroshina, A.; Zhernov, Y. Natural Human Bet v 1-specific IgG Antibodies Recognize Non-conformational Epitopes Whereas IgE Reacts with Conformational Epitopes. Allergy 2023, 78, 3136–3153. [Google Scholar] [CrossRef] [PubMed]
- Zieglmayer, P.; Focke-Tejkl, M.; Schmutz, R.; Lemell, P.; Zieglmayer, R.; Weber, M.; Kiss, R.; Blatt, K.; Valent, P.; Stolz, F. Mechanisms, Safety and Efficacy of a B Cell Epitope-Based Vaccine for Immunotherapy of Grass Pollen Allergy. EBioMedicine 2016, 11, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Neubauer, A.; Gevaert, P.; Zidarn, M.; Worm, M.; Aberer, W.; Malling, H.J.; Pfaar, O.; Klimek, L.; Pfützner, W. Safety and Efficacy of Immunotherapy with the Recombinant B-Cell Epitope–Based Grass Pollen Vaccine BM32. J. Allergy Clin. Immunol. 2018, 142, 497–509. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbîrcea, L.-E.; Buzan, M.-R.; Grijincu, M.; Cotarcă, M.-D.; Tamaș, T.-P.; Haidar, L.; Tănasie, G.; Huțu, I.; Babaev, E.; Stolz, F.; et al. Heterogenous Induction of Blocking Antibodies against Ragweed Allergen Molecules by Allergen Extract-Based Immunotherapy Vaccines. Vaccines 2024, 12, 635. https://doi.org/10.3390/vaccines12060635
Zbîrcea L-E, Buzan M-R, Grijincu M, Cotarcă M-D, Tamaș T-P, Haidar L, Tănasie G, Huțu I, Babaev E, Stolz F, et al. Heterogenous Induction of Blocking Antibodies against Ragweed Allergen Molecules by Allergen Extract-Based Immunotherapy Vaccines. Vaccines. 2024; 12(6):635. https://doi.org/10.3390/vaccines12060635
Chicago/Turabian StyleZbîrcea, Lauriana-Eunice, Maria-Roxana Buzan, Manuela Grijincu, Monica-Daniela Cotarcă, Tudor-Paul Tamaș, Laura Haidar, Gabriela Tănasie, Ioan Huțu, Elijahu Babaev, Frank Stolz, and et al. 2024. "Heterogenous Induction of Blocking Antibodies against Ragweed Allergen Molecules by Allergen Extract-Based Immunotherapy Vaccines" Vaccines 12, no. 6: 635. https://doi.org/10.3390/vaccines12060635






