Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Concept
2.2. Research Strategy
2.3. Selection Criteria
- (1)
- Immunization with nirsevimab of children aged less than 2 years;
- (2)
- Nirsevimab mAb administration (any strategy and settings);
- (3)
- Comparison of nirsevimab efficacy with placebo in either a randomized controlled trial (RCT) or real-world settings;
- (4)
- Reporting on the occurrence of LRTIs in individuals treated with nirsevimab and placebo with subsequent hospitalization.
- (1)
- Immunization of children aged 2 years or more;
- (2)
- Secondary studies (i.e., systematic reviews and meta-analyses, letters, editorial comment, case reports);
- (3)
- Studies on animals (including non-human primates) or preclinical testing;
- (4)
- Outcomes other than clinical efficacy;
- (5)
- The full text was not available either through online repositories or through inter-library loan or its main text was written in a language different from English, Italian, German, French, Spanish, or Portuguese;
- (6)
- A lack of details about the geographical setting and corresponding timeframe;
- (7)
- Reporting on the occurrence of influenza-like illnesses and/or respiratory syndromes other than LRTIs;
- (8)
- Reporting on the occurrence of LRTIs that did not include the number of cases eventually admitted to the hospital settings because of respiratory syndrome;
- (9)
- Methods other than Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) or non-RT-qPCR Nucleic Acid Amplification Tests (NAATs) were applied for the laboratory diagnosis of RSV infection.
2.4. Selection Criteria
2.5. Data Extraction
2.6. Quality Assessment (Risk of Bias)
2.7. Data Analysis
2.7.1. Immunization Efficacy
2.7.2. Meta-Analysis
2.7.3. Sensitivity Analysis
2.7.4. Analysis of Publication Bias
2.7.5. Software
3. Results
3.1. Characteristics of Retrieved Studies
3.2. Risk of Bias Assessment
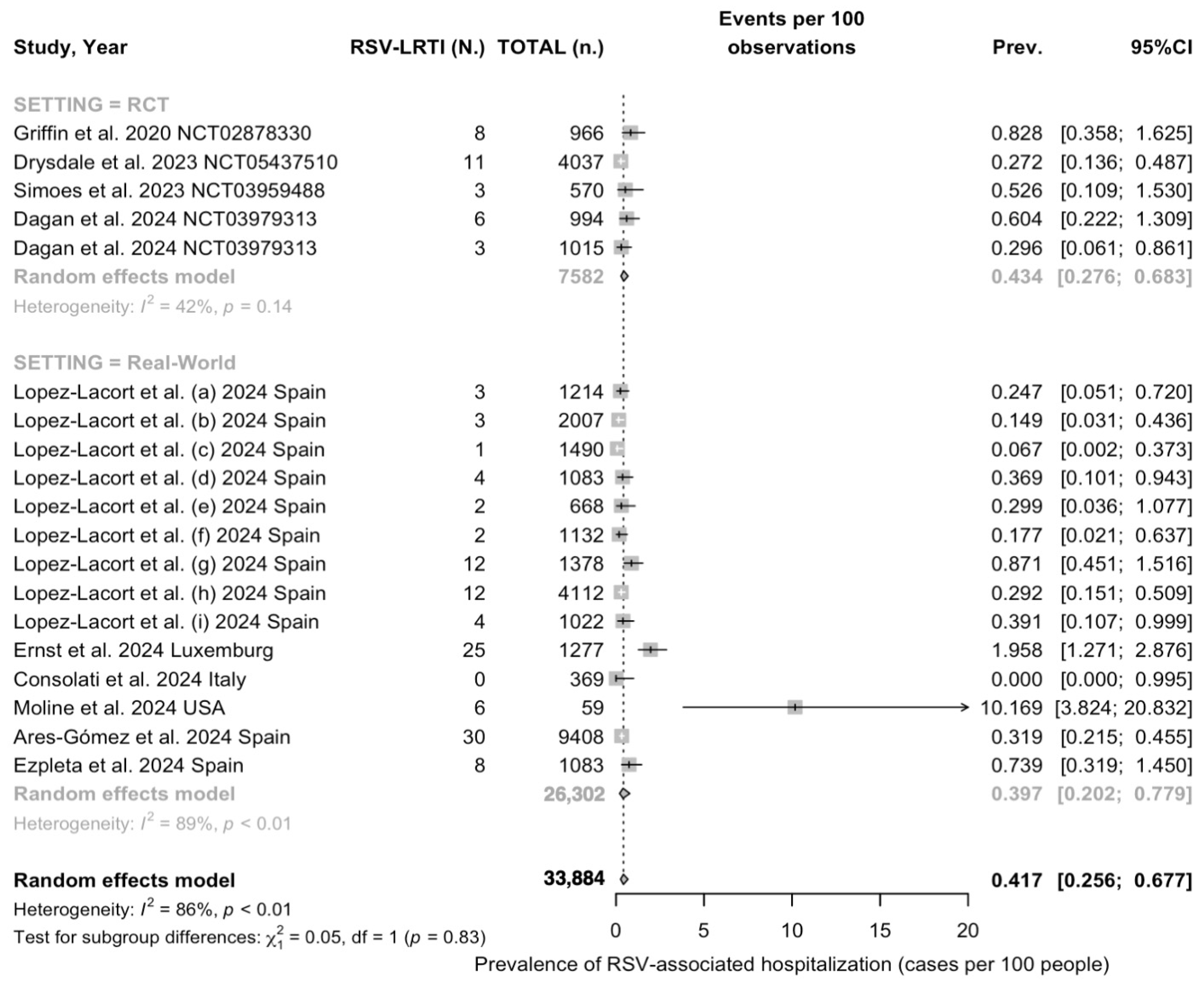
3.3. Quantative Analysis
3.3.1. Removal of Studies
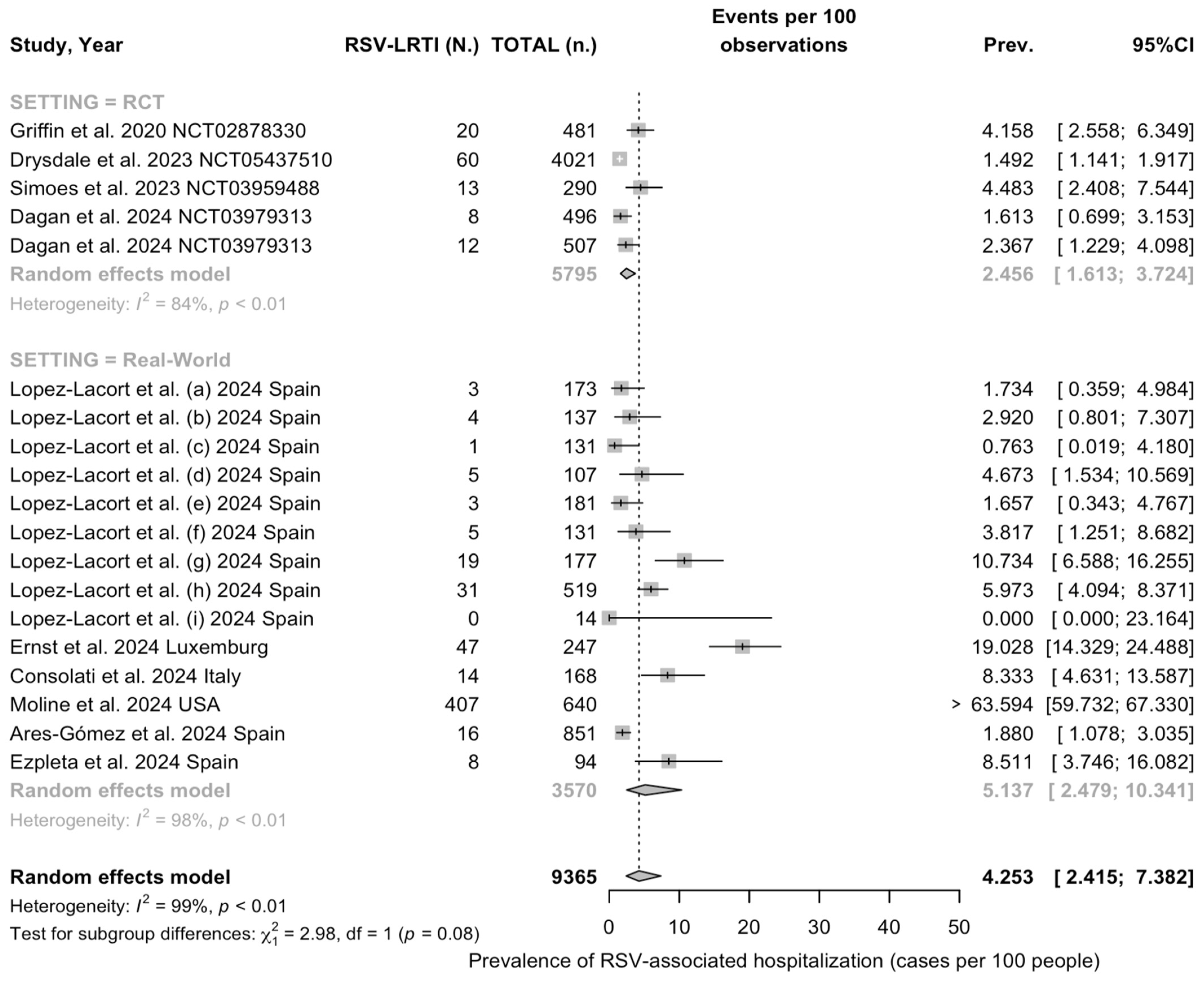
3.3.2. Descriptive Analysis
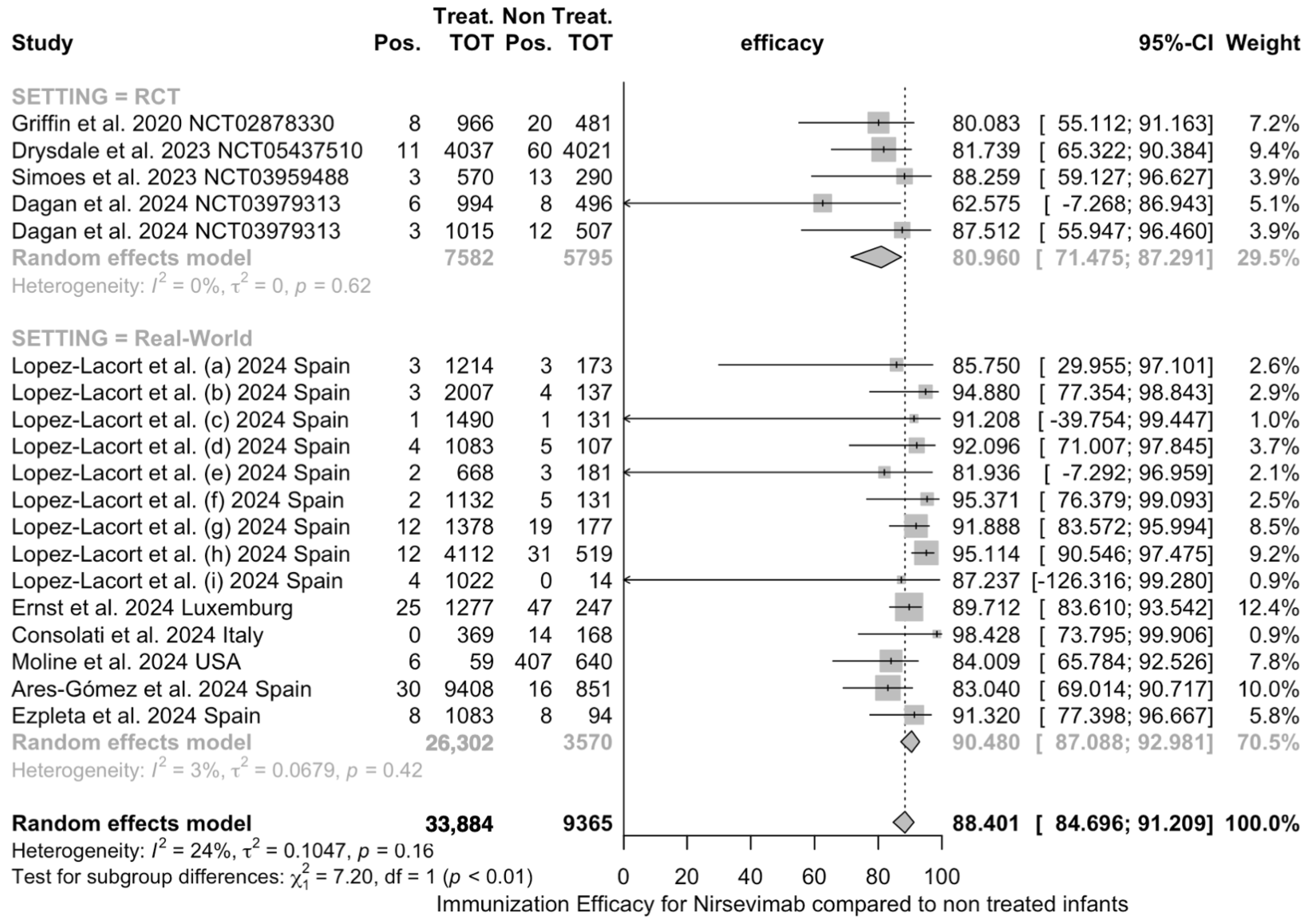
3.3.3. Meta Analysis
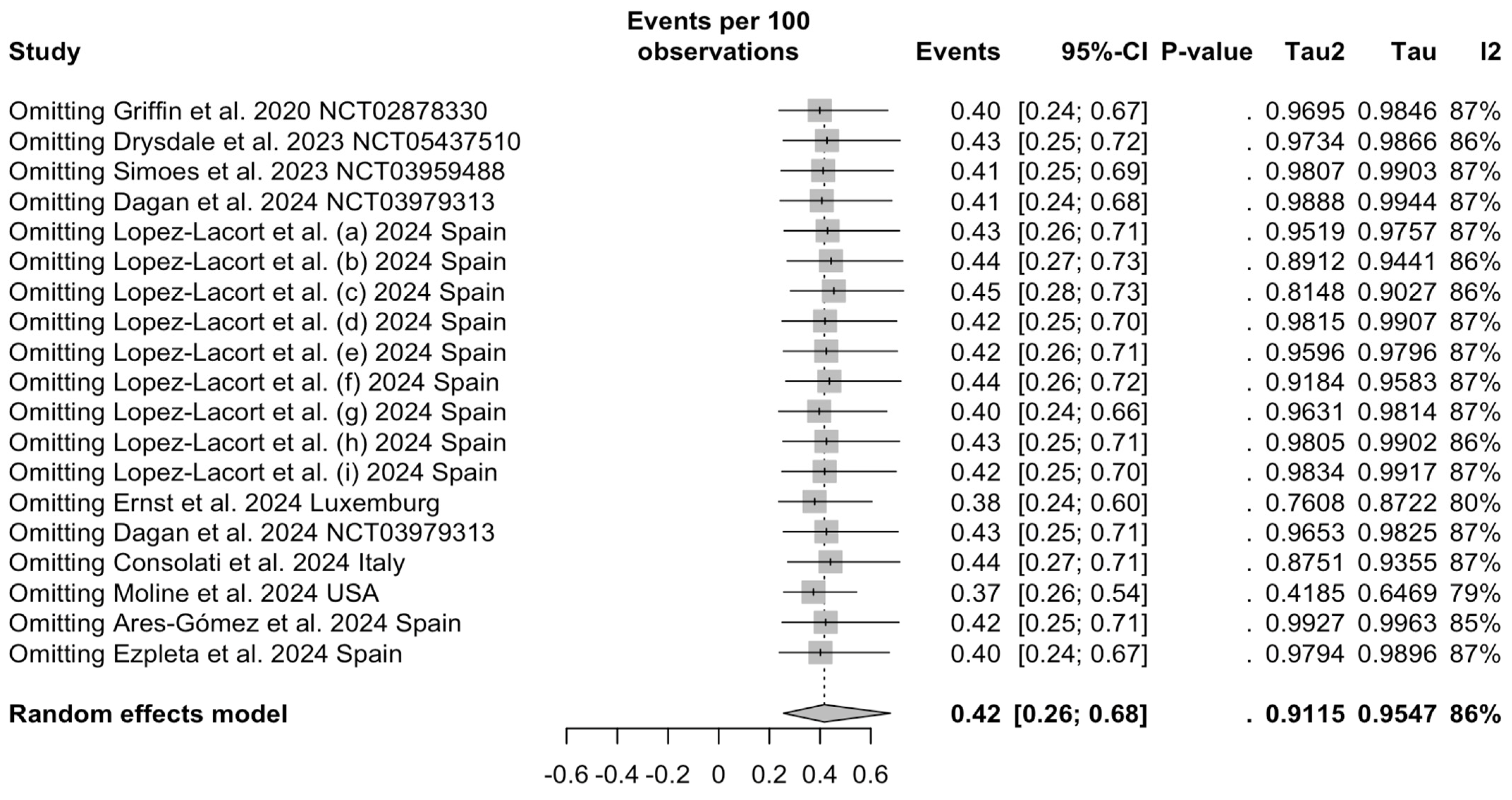
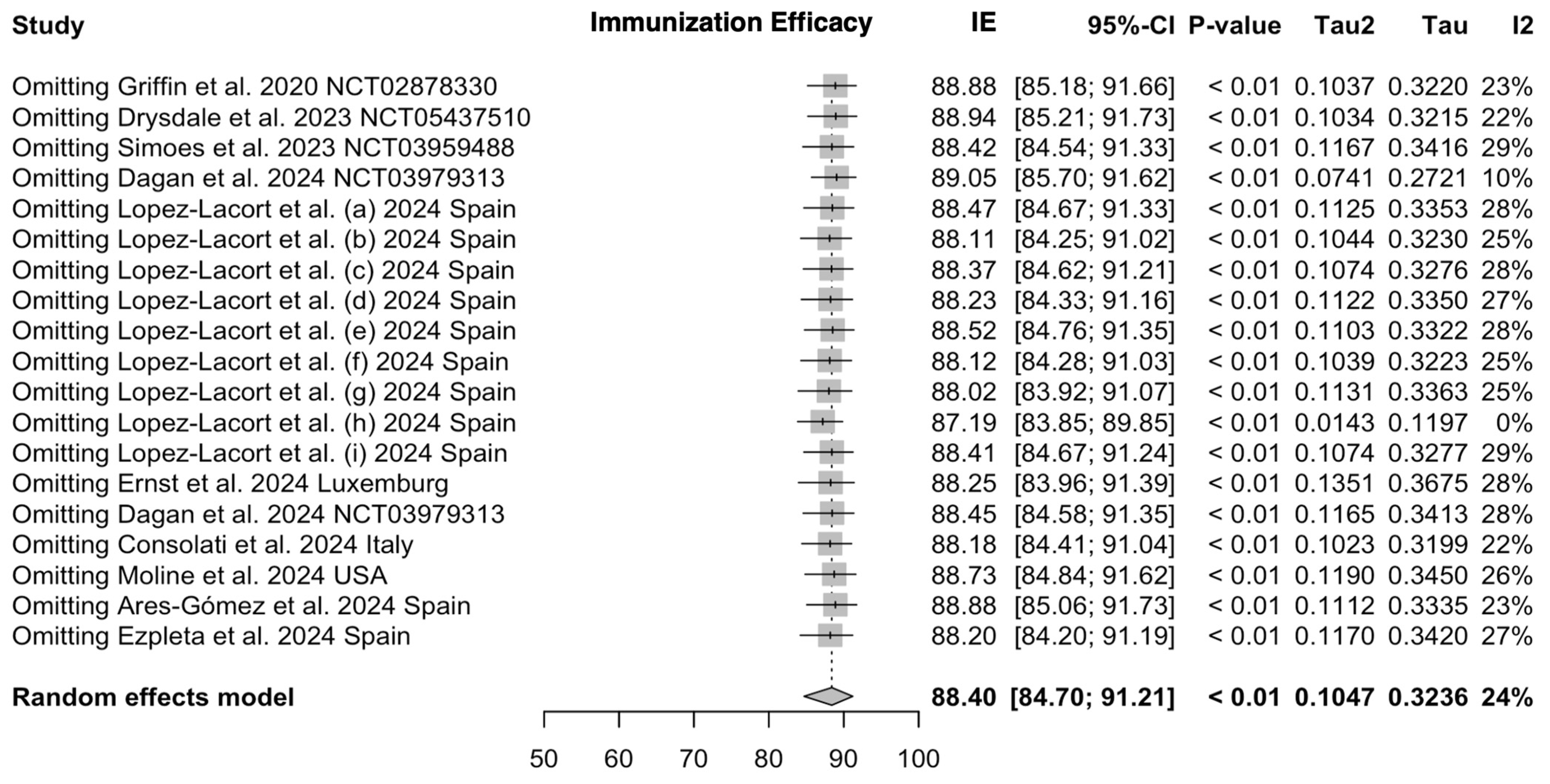
3.4. Sensitivity Analysis
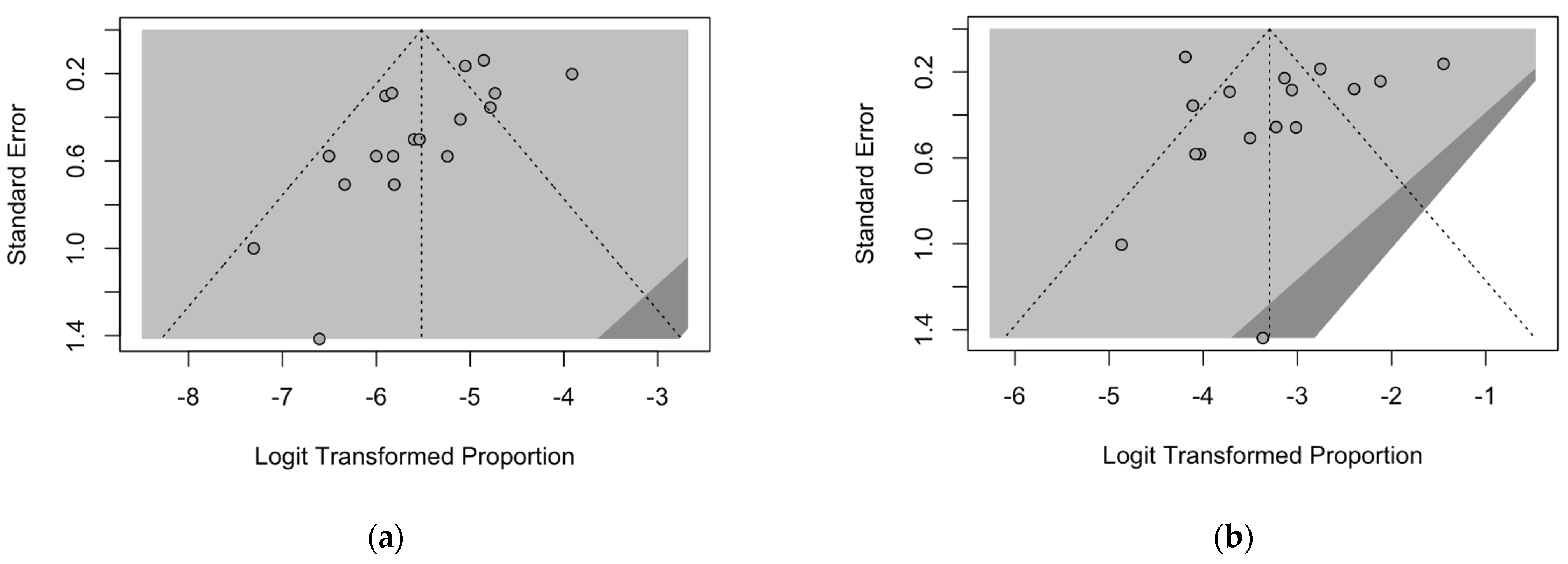
3.5. Publication Bias
4. Discussion
4.1. Summary of Main Findings
4.2. Generalizability and Implications for Daily Practice
4.3. Limitations and Implications for Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Keywords | Entries (N.) |
|---|---|---|
| PubMed | (“Respiratory Syncytial Virus, Human”[Mesh] OR bronchiolitis OR “LRTD” OR “LRTI” OR “low* respiratory tract infection*”) AND (“nirsevimab” OR MED18897 OR MED-18897) | 60 |
| Scopus | (“Respiratory Syncytial Virus, Human” OR bronchiolitis OR “LRTD” OR “LRTI” OR “low* respiratory tract infection*”) AND (“nirsevimab” OR med18897 OR med-18897) | 145 |
| EMBASE | (‘nirsevimab’/exp OR nirsevimab OR med18897 OR med-18897) AND (‘human respiratory syncytial virus’ OR bronchiolitis OR RSV OR LRTI OR LRTD OR ‘lower respiratory tract infection’ | 196 |
| Study | Year | Nirsevimab Group | Control Group | ||||
|---|---|---|---|---|---|---|---|
| Total (N.) | Treated with Palivizumab (n.N./, %) | Maternal Vaccination (n.N./, %) | Total (N.) | Treated with Palivizumab (n.N./, %) | Maternal Vaccination (n.N./, %) | ||
| Griffin et al. [78] | 2020 | 966 | 0 | 0 | 481 | 0 | 0 |
| Hammitt et al. [80] | 2022 | 994 | 0 | 0 | 496 | 0 | 0 |
| Drysdale et al. [81] | 2023 | 4037 | 0 | 0 | 4021 | 0 | 0 |
| Simoes et al. [79] | 2023 | 994 | 0 | 0 | 496 | 0 | 0 |
| 570 | 0 | 0 | 290 | 290 (100%) | 0 | ||
| López-Lacort et al. [84] | 2024 | 14,106 | N.A. | N.A. | 1570 | N.A. | N.A. |
| Ernst et al. [83] | 2024 | 1277 | N.A. | N.A. | 247 | N.A. | N.A. |
| NIRSEGAL Study [86] | 2024 | 6723 | 0 | N.A. | 555 | 0 | N.A. |
| 5824 | 0 | N.A. | 1530 | 0 | N.A. | ||
| Dagan et al. [82] | 2024 | 994 | 0 | 0 | 496 | 0 | 0 |
| 1015 | 0 | 0 | 507 | 0 | 0 | ||
| 1944 | 0 | 0 | 967 | 0 | 0 | ||
| Consolati et al. [88] | 2024 | 369 | 0 | 0 | 168 | 0 | 0 |
| Moline et al. [61] | 2024 | 59 | 0 | 0 | 640 | 0 | 0 |
| Paireau et al. [87] | 2024 | 58 | 0 | 0 | 230 | 0 | 0 |
| Ares-Gómez et al. [59] | 2024 | 9408 | 0 | N.A. | 851 | 0 | N.A. |
| Ezpeleta et al. [85] | 2024 | 1083 | N.A. | N.A. | 94 | N.A. | N.A. |
| Study | D1 | D2 | D3 | D4 | D5 | D6 |
|---|---|---|---|---|---|---|
| Griffin et al. 2020 [78] | ☺☺ | ☺ | ☺☺ | ☺ | ☺ | ☺☺ |
| Hammitt et al. 2022 [80] | ☺☺ | ☹ | ☺☺ | ☺ | ☺ | ☺ |
| Drysdale et al. 2023 [81] | ☺☺ | ☺☺ | ☺ | ☺ | ☺☺ | ☺☺ |
| Simoes et al. 2023 [79] | ☺☺ | ☺ | ☺☺ | ☺ | ☺ | ☺☺ |
| López-Lacort et al. 2024 [84] | ☺☺ | ☺☺ | ☺ | ☺☺ | ☺☺ | ☺☺ |
| Ernst et al. 2024 [83] | ☺☺ | ☺☺ | ☺ | ☺☺ | ☺☺ | ☺☺ |
| NIRSEGAL Study Group 2024 [86] | ☹ | ☺ | ☺☺ | ☺ | ☺☺ | ☺ |
| Dagan et al. 2024 [82] | ☺☺ | ☺ | ☺☺ | ☺ | ☺ | ☺☺ |
| Consolati et al. 2024 [88] | ☺☺ | ☺☺ | ☺ | ☺ | ☺☺ | ☺☺ |
| Moline et al. 2024 [61] | ☺ | ☺☺ | ☺ | ☺ | ☺ | ☺ |
| Paireau et al. 2024 [87] | ☹☹ | ☺☺ | ☺ | ☹ | ☺ | ☺ |
| Ares-Gómez et al. 2024 [59] | ☺☺ | ☺☺ | ☺ | ☺☺ | ☺☺ | ☺☺ |
| Ezpeleta et al. 2024 [85] | ☺☺ | ☺☺ | ☺ | ☺☺ | ☺☺ | ☺☺ |
| Study | Hospitalizations | % Reduction | |||
|---|---|---|---|---|---|
| 2023–2024 | 2022–2024 | ||||
| Total (N.) | Nirsevimab (n./N., %) | Conventional (n./N., %) | Total (N.) | ||
| Consolati et al. [88] | 14 | 0, - | 14, 100% | 61 | 77.05% |
| Ernst et al. [83] | 272 | 25, 9.19% | 247, 90.81% | 389 | 30.08% |
| NIRSEGAL Study (1) [86] | 242 | 671 (2) | 63.93% | ||
| Study | Controls | Cases | Effectiveness | ||
|---|---|---|---|---|---|
| Not Treated | Treated | Not Treated | Treated | (95% CI) | |
| Paireau et al. [87] | 29 | 21 | 201 | 37 | 74.6% (50.7; 86.9) |
| Moline et al. [61] | 401 | 53 | 239 | 6 | 81.0% (55.1; 92.0) |

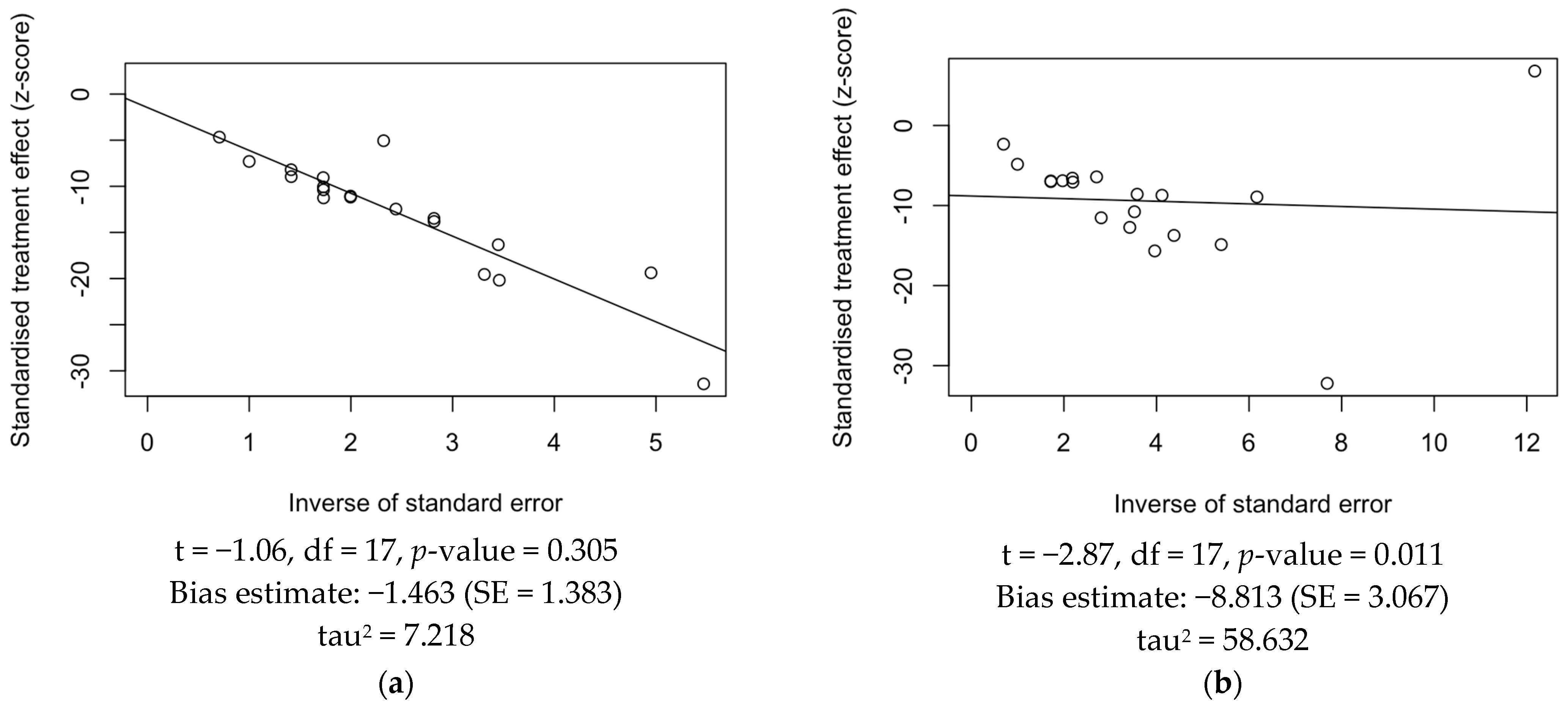
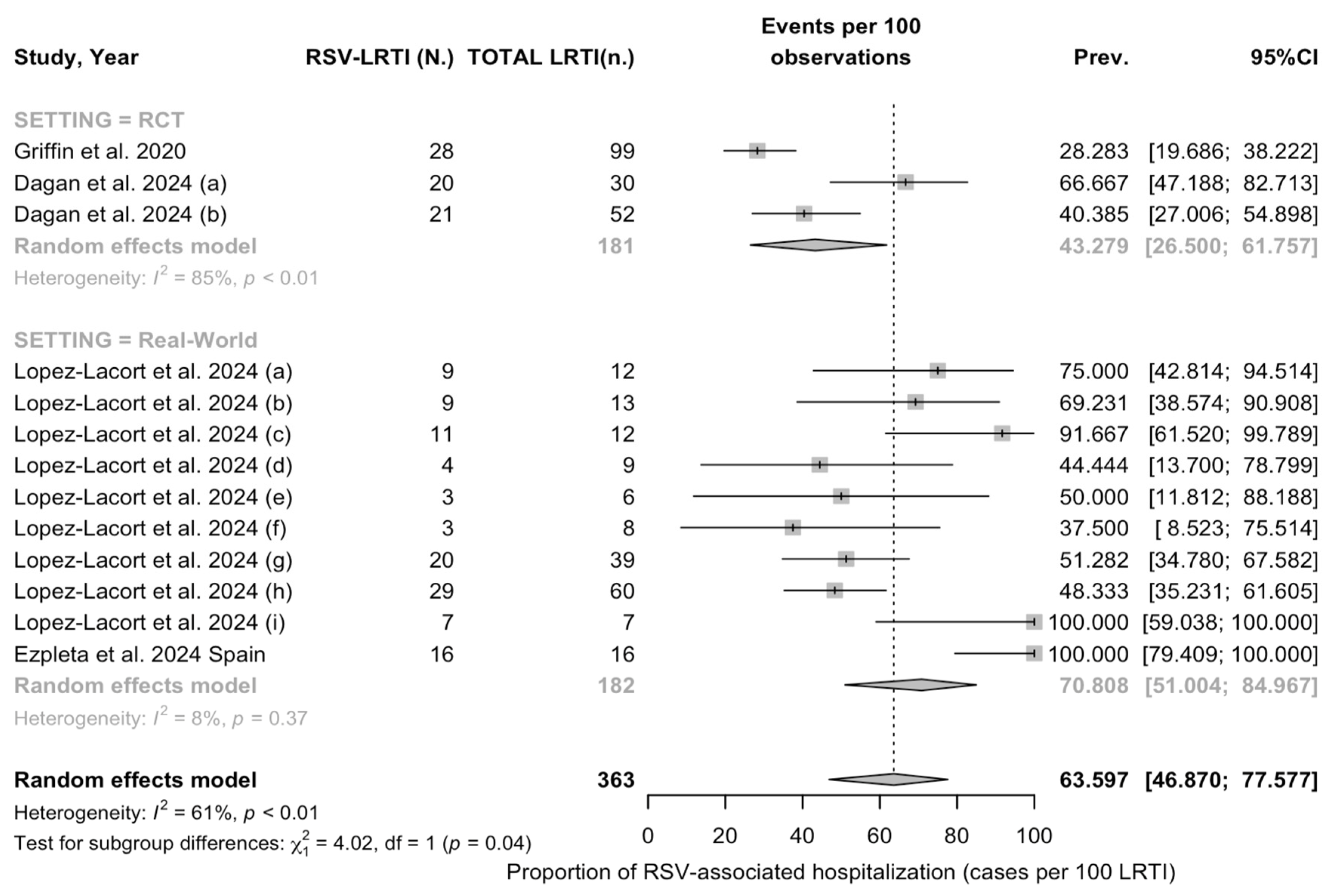
References
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, L.; Xie, Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front. Cell. Infect. Microbiol. 2022, 12, 858629. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L.; et al. ICTV Virus Taxonomy Profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, P.; Chin, A.A.; Tan, S.; Jeon, A.H.; Ackerley, C.A.; Siu, K.K.; Lee, J.E.; Hegele, R.G. Identification of Rsv Fusion Protein Interaction Domains on the Virus Receptor, Nucleolin. Viruses 2021, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Battles, M.B.; McLellan, J.S. Respiratory Syncytial Virus Entry and How to Block It. Nat. Rev. Microbiol. 2019, 17, 233–245. [Google Scholar] [CrossRef] [PubMed]
- San-Juan-Vergara, H.; Peeples, M.E. Importance of Virus Characteristics in Respiratory Syncytial Virus-Induced Disease. Immunol. Allergy Clin. N. Am. 2019, 39, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Sullender, W.M. Respiratory Syncytial Virus Genetic and Antigenic Diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The Journey to a Respiratory Syncytial Virus Vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, G.; Perez, M.K. Respiratory Syncytial Virus Infection and Bronchiolitis Practice Gaps. Pediatr. Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel Antigens for RSV Vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Dondi, A.; Scarpini, S.; Rocca, A.; Vandini, S.; Poletti, G.; Lanari, M. Current State and Challenges in Developing Respiratory Syncytial Virus Vaccines. Vaccines 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S. Vaccine Development for Respiratory Syncytial Virus. Curr. Opin. Virol. 2017, 23, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tan, J. Clonal Wars: Monoclonal Antibodies Against Infectious Pathogens. DNA Cell Biol. 2022, 41, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Baral, R.; Higgins, D.; Khan, S.; Kochar, S.; Li, Y.; Ortiz, J.R.; Cherian, T.; Feikin, D.; Jit, M.; et al. Value Profile for Respiratory Syncytial Virus Vaccines and Monoclonal Antibodies. Vaccine 2023, 41, S7–S40. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccines Immunother. 2022, 18, 2079322. [Google Scholar] [CrossRef]
- Del Riccio, M.; Spreeuwenberg, P.; Osei-Yeboah, R.; Johannesen, C.K.; Fernandez, L.V.; Teirlinck, A.C.; Wang, X.; Heikkinen, T.; Bangert, M.; Caini, S.; et al. Burden of Respiratory Syncytial Virus in the European Union: Estimation of RSV-Associated Hospitalizations in Children under 5 Years. J. Infect. Dis. 2023, 228, 1528–1538. [Google Scholar] [CrossRef]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus–Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef]
- Li, Y.; Reeves, R.M.; Wang, X.; Bassat, Q.; Brooks, W.A.; Cohen, C.; Moore, D.P.; Nunes, M.; Rath, B.; Campbell, H.; et al. Global Patterns in Monthly Activity of Influenza Virus, Respiratory Syncytial Virus, Parainfluenza Virus, and Metapneumovirus: A Systematic Analysis. Lancet Glob. Health 2019, 7, e1031–e1045. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Shi, T.; Bont, L.J.; Chu, H.Y.; Zar, H.J.; Wahi-Singh, B.; Ma, Y.; Cong, B.; Sharland, E.; et al. Global Disease Burden of and Risk Factors for Acute Lower Respiratory Infections Caused by Respiratory Syncytial Virus in Preterm Infants and Young Children in 2019: A Systematic Review and Meta-Analysis of Aggregated and Individual Participant Data. Lancet 2024, 403, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Palmieri, S.; Marchesi, F. Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children 2023, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.; Xu, Q.; Chen, Z.; Jiang, W.; Wang, T.; Zhou, Y.; Yu, H.; Yan, Y.; Pan, T. Respiratory Syncytial Virus Infection in Children and Its Correlation with Climatic and Environmental Factors. J. Int. Med. Res. 2021, 49, 3000605211044593. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Raybould, J.E.; Sastry, S.; de la Cruz, O. Respiratory Viruses in Transplant Recipients: More than Just a Cold. Clinical Syndromes and Infection Prevention Principles. Int. J. Infect. Dis. 2017, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Ciarlitto, C.; Guolo, S.; Brusco, C.; Cerone, G.; Antilici, L.; Schettini, L.; Piscitelli, A.L.; Chiara Vittucci, A.; Cutrera, R.; et al. Respiratory Syncytial Virus Bronchiolitis in Infancy: The Acute Hospitalization Cost. Front. Pediatr. 2021, 8, 594898. [Google Scholar] [CrossRef] [PubMed]
- Rha, B.; Curns, A.T.; Lively, J.Y.; Campbell, A.P.; Englund, J.A.; Boom, J.A.; Azimi, P.H.; Weinberg, G.A.; Staat, M.A.; Selvarangan, R.; et al. Respiratory Syncytial Virus—Associated Hospitalizations among Young Children: 2015–2016. Pediatrics 2020, 146, e20193611. [Google Scholar] [CrossRef] [PubMed]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Leader, S.; Kohlhase, K.; Pearlman, M.H.; Williams, J.V.; Engle, W.A. Recent Trends in Severe Respiratory Syncytial Virus (RSV) among US Infants, 1997 to 2000. J. Pediatr. 2003, 143, S127–S132. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yan, R.; Wu, X.; Zhang, X.; Chen, C.; Jiang, D.; Yang, M.; Cao, K.; Chen, M.; You, Y.; et al. Global Burden and Trends of Respiratory Syncytial Virus Infection across Different Age Groups from 1990 to 2019: A Systematic Analysis of the Global Burden of Disease 2019 Study. Int. J. Infect. Dis. 2023, 135, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Parisi, S.; Corrado, S.; Marchesi, F.; Bottazzoli, M.; Gori, D. Respiratory Syncytial Virus Infections in Recipients of Bone Marrow Transplants: A Systematic Review and Meta-Analysis. Infect. Dis. Rep. 2024, 16, 317–355. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A Phase 1, Randomized, Placebo-Controlled Study to Evaluate the Safety and Immunogenicity of an MRNA-Based RSV Prefusion F Protein Vaccine in Healthy Younger and Older Adults. Hum. Vaccines Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Regassa, B.T.; Gebrewold, L.A.; Mekuria, W.T.; Kassa, N.A. Molecular Epidemiology of Respiratory Syncytial Virus in Children with Acute Respiratory Illnesses in Africa: A Systematic Review and Meta-Analysis. J. Glob. Health 2023, 13, 04001. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Y.; Chmaisse, A.; Boutros, C.; Chamseddine, S.; Fayad, D.; Zaraket, H.; Dbaibo, G. The Burden of Respiratory Syncytial Virus (RSV) Infection in the Middle East and North Africa (MENA) Region across Age Groups: A Systematic Review. Vaccine 2021, 39, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F. Respiratory Syncytial Virus, Influenza and SARS-CoV-2 in Homeless People from Urban Shelters: A Systematic Review and Meta-Analysis (2023). Epidemiologia 2024, 5, 41–79. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, Q.; Zhou, Y.; Ran, Y.; Liu, Z.; Hou, W.; Pei, S.; Lai, S. Spatiotemporal Variations of “Triple-Demic” Outbreaks of Respiratory Infections in the United States in the Post-COVID-19 Era. BMC Public Health 2023, 23, 2452. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.A.; Jain, B.; Raifman, J. Revamping Public Health Systems: Lessons Learned from the Tripledemic. Am. J. Prev. Med. 2023, 66, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Mazela, J.; Jackowska, T.; Czech, M.; Helwich, E.; Martyn, O.; Aleksiejuk, P.; Smaga, A.; Glazewska, J.; Wysocki, J. Epidemiology of Respiratory Syncytial Virus Hospitalizations in Poland: An Analysis from 2015 to 2023 Covering the Entire Polish Population of Children Aged under Five Years. Viruses 2024, 16, 704. [Google Scholar] [CrossRef] [PubMed]
- Scarpaci, M.; Bracaloni, S.; Esposito, E.; De Angelis, L.; Baglivo, F.; Casini, B.; Panatto, D.; Ogliastro, M.; Loconsole, D.; Chironna, M.; et al. RSV Disease Burden in Primary Care in Italy: A Multi-Region Pediatric Study, Winter Season 2022–2023. Influenza Other Respir Viruses 2024, 18, e13282. [Google Scholar] [CrossRef] [PubMed]
- Kenmoe, S.; Bigna, J.J.; Well, E.A.; Simo, F.B.N.; Penlap, V.B.; Vabret, A.; Njouom, R. Prevalence of Human Respiratory Syncytial Virus Infection in People with Acute Respiratory Tract Infections in Africa: A Systematic Review and Meta-Analysis. Influenza Other Respir. Viruses 2018, 12, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 713. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatr. Rep. 2022, 14, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Cerviere, M.P.; Ranzieri, S.; Marchesi, F. Respiratory Syncytial Virus Prevention through Monoclonal Antibodies: A Cross-Sectional Study on Knowledge, Attitudes, and Practices of Italian Pediatricians. Pediatr. Rep. 2023, 15, 154–174. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Gianluca, P.; Gori, D.; Manzoni, P. Efficacy of Respiratory Syncytial Virus Vaccination to Prevent Lower Respiratory Tract Illness in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2024, 12, 500. [Google Scholar] [CrossRef]
- Frogel, M.P.; Stewart, D.L.; Hoopes, M.; Fernandes, A.W.; Mahadevia, P.J. A Systematic Review of Compliance with Palivizumab Administration for RSV Immunoprophylaxis. J. Manag. Care Pharm. 2010, 16, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Olchanski, N.; Hansen, R.N.; Pope, E.; D’Cruz, B.; Fergie, J.; Goldstein, M.; Krilov, L.R.; McLaurin, K.K.; Nabrit-Stephens, B.; Oster, G.; et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence around Value. Open Forum Infect. Dis. 2018, 5, ofy031. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Luna, M.; Manzoni, P.; Paes, B.; Baraldi, E.; Cossey, V.; Kugelman, A.; Chawla, R.; Dotta, A.; Rodríguez Fernández, R.; Resch, B.; et al. Expert Consensus on Palivizumab Use for Respiratory Syncytial Virus in Developed Countries. Paediatr. Respir. Rev. 2020, 33, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, 20184064. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Padula, W.V.; Yieh, L.; Gong, C.L. Cost-Effectiveness of Nirsevimab and Palivizumab for Respiratory Syncytial Virus Prophylaxis in Preterm Infants 29–34 6/7 Weeks’ Gestation in the United States. Pediatr. Neonatol. 2023, 65, 152–158. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics; Committee on Infectious Diseases and Bronchiolitis Guidelines Committee; Brady, M.T.; Byington, C.L.; Davies, H.D.; Edwards, K.M.; Jackson, M.A.; Maldonado, Y.A.; Murray, D.L.; Orenstein, W.A.; et al. Updated Guidance for Palivizumab Prophylaxis among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 2014, 134, e620–e638. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.H. Respiratory Syncytial Virus Infection and Palivizumab: Are Families Receiving Accurate Information? Am. J. Perinatol. 2010, 27, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Nirsevimab: First Approval. Drugs 2023, 83, 181–187. [Google Scholar] [CrossRef]
- Talha, M.; Ali, M.H. Latest FDA-Approved Drug Nirsevimab-Alip (Beyfortus): A Gamechanger for Treatment of Respiratory Syncytial Virus. J. Med. Virol. 2023, 95, e29169. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, F.; Mirás-Carballal, S.; Durán-Parrondo, C. Early Lessons from the Implementation of Universal Respiratory Syncytial Virus Prophylaxis in Infants with Long-Acting Monoclonal Antibodies, Galicia, Spain, September and October 2023. Eurosurveillance 2023, 28, 2300606. [Google Scholar] [CrossRef] [PubMed]
- Ares-Gómez, S.; Mallah, N.; Santiago-Pérez, M.-I.; Pardo-Seco, J.; Pérez-Martínez, O.; Otero-Barrós, M.-T.; Suárez-Gaiche, N.; Kramer, R.; Jin, J.; Platero-Alonso, L.; et al. Effectiveness and Impact of Universal Prophylaxis with Nirsevimab in Infants against Hospitalisation for Respiratory Syncytial Virus in Galicia, Spain: Initial Results of a Population-Based Longitudinal Study. Lancet Infect. Dis. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Francisco, L.; Cruz-Cañete, M.; Pérez, C.; Couceiro, J.A.; Otheo, E.; Launes, C.; Rodrigo, C.; Jiménez, A.B.; Llorente, M.; Montesdeoca, A.; et al. Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease in Children: Statement of the Spanish Society of Paediatric Infectious Disease (SEIP). An. Pediatr. Engl. Ed. 2023, 99, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Tannis, A.; Toepfer, A.P.; Williams, J.V.; Boom, J.A.; Englund, J.A.; Halasa, N.B.; Staat, M.A.; Weinberg, G.A.; Selvarangan, R.; et al. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Vi-rus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season-New Vaccine Surveillance Network, Oc-tober 2023–February 2024. Morb. Mortal. Wkly. Rev. 2024, 73, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Cantais, A.; Annino, N.; Thuiller, C.; Tripodi, L.; Cesana, P.; Seigle-Ferrand, E.; Zekre, F.; Pillet, S.; Pozzetto, B. First RSV Epidemic with Nirsevimab. Older Children than Previous Epidemics, Even When Hospitalized. J. Med. Virol. 2024, 96, e29483. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A Framework for Formulating Good Questions to Explore the Association of Environmental and Other Exposures with Health Outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Mintzker, Y.; Blum, D.; Adler, L. Replacing PICO in Non-Interventional Studies. BMJ Evid. Based Med. 2022, 28, 284. [Google Scholar] [CrossRef] [PubMed]
- Turalde-Mapili, M.W.R.; Mapili, J.A.L.; Turalde, C.W.R.; Pagcatipunan, M.R. The Efficacy and Safety of Nirsevimab for the Prevention of RSV Infection among Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 2023, 11, 1132740. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Von Hippel, P.T. The Heterogeneity Statistic I2 Can Be Biased in Small Meta-Analyses. BMC Med. Res. Methodol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Krumpal, I. Determinants of Social Desirability Bias in Sensitive Surveys: A Literature Review. Qual. Quant. 2013, 47, 2025–2047. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). OHAT Risk of Bias Rating Tool for Human and Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2015. [Google Scholar]
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing Risk of Bias in Human Environmental Epidemiology Studies Using Three Tools: Different Conclusions from Different Tools. Syst. Rev. 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A Re-Evaluation of Random-Effects Meta-Analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2008, 172, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.A.F.; Madhi, S.A.; Muller, W.J.; Atanasova, V.; Bosheva, M.; Cabañas, F.; Baca Cots, M.; Domachowske, J.B.; Garcia-Garcia, M.L.; Grantina, I.; et al. Efficacy of Nirsevimab against Respiratory Syncytial Virus Lower Respiratory Tract Infections in Preterm and Term Infants, and Pharmacokinetic Extrapolation to Infants with Congenital Heart Disease and Chronic Lung Disease: A Pooled Analysis of Randomised Controlled Trials. Lancet Child Adolesc. Health 2023, 7, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Hammitt, L.L.; Nuñez, B.S.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Chang, Y.; Currie, A.; et al. Infants Receiving a Single Dose of Nirsevimab to Prevent RSV Do Not Have Evidence of Enhanced Disease in Their Second RSV Season. J. Pediatr. Infect. Dis. Soc. 2024, 13, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Bejko, D.; Gaasch, L.; Hannelas, E.; Kahn, I.; Pierron, C.; Del Lero, N.; Schalbar, C.; Do Carmo, E.; Kohnen, M.; et al. Impact of Nirsevimab Prophylaxis on Paediatric Respiratory Syncytial Virus (RSV)-Related Hospitalisations during the Initial 2023/24 Season in Luxembourg. Eurosurveillance 2024, 29, 2400033. [Google Scholar] [CrossRef] [PubMed]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; Xavier López-Labrador, F.; Mengual-Chuliá, B.; Fernández-García, C.; Carballido-Fernández, M.; Pineda-Caplliure, A.; Mollar-Maseres, J.; Shalabi Benavent, M.; et al. Early Estimates of Nirsevimab Immunoprophylaxis Effectiveness against Hospital Admission for Respiratory Syncytial Virus Lower Respiratory Tract Infections in Infants, Spain, October 2023 to January 2024. Eurosurveillance 2024, 29, 2400046. [Google Scholar] [CrossRef] [PubMed]
- Ezpeleta, G.; Navascués, A.; Viguria, N.; Herranz-Aguirre, M.; Juan Belloc, S.E.; Gimeno Ballester, J.; Muruzábal, J.C.; García-Cenoz, M.; Trobajo-Sanmartín, C.; Echeverria, A.; et al. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines 2024, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Dirección Xeral de Saúde Pública. Follow-Up Report on Immunization with Nirsevimab in Galicia-Data up to Week 13, 2024 (31-03-2024); Dirección Xeral de Saúde Pública: Santiago de Compostela, Spain, 2024. [Google Scholar]
- Paireau, J.; Durand, C.; Raimbault, S.; Cazaubon, J.; Mortamet, G.; Viriot, D.; Milesi, C.; Daudens-Vaysse, E.; Ploin, D.; Tessier, S.; et al. Nirsevimab Effectiveness against Cases of Respiratory Syncytial Virus Bronchiolitis Hospitalised in Pediatric Intensive Care Units in France September 2023–January 2024. HAL Open Sci. (Prepr.) 2024, pasteur04501286. Available online: https://hal.science/pasteur-04501286 (accessed on 30 April 2024).
- Consolati, A.; Farinelli, M.; Serravalle, P.; Rollandin, C.; Apprato, L.; Esposito, S.; Bongiorno, S. Safety and Efficacy of Nirsevimab in a Universal Prevention Program of Respiratory Syncytial Virus Bronchiolitis in Newborns and Infants in the First Year of Life in the Valle d’Aosta Region, Italy, in the 2023–2024 Epidemic Season. Vaccines 2024, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Tunis, S.R.; Stryer, D.B.; Clancy, C.M. Practical Clinical Trials Increasing the Value of Clinical Research for Decision Making in Clinical and Health Policy. JAMA 2003, 290, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, I.; Li, A.; Bjornson, C.L.; Lanctot, K.L.; Paes, B.A. Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada. Am. J. Perinatol. 2021, 39, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Voirin, N.; Virlogeux, V.; Demont, C.; Kieffer, A. Potential Impact of Nirsevimab on RSV Transmission and Medically Attended Lower Respiratory Tract Illness Caused by RSV: A Disease Transmission Model. Infect. Dis. Ther. 2021, 11, 277–292. [Google Scholar] [CrossRef]
- Busack, B.; Shorr, A.F. Going Viral—RSV as the Neglected Adult Respiratory Virus. Pathogens 2022, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Comini, S.; Bianco, G.; Cavallo, R.; Costa, C. From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults. Viruses 2024, 16, 531. [Google Scholar] [CrossRef] [PubMed]
- Lee Mortensen, G.; Harrod-Lui, K. Parental Knowledge about Respiratory Syncytial Virus (RSV) and Attitudes to Infant Immunization with Monoclonal Antibodies. Expert. Rev. Vaccines 2022, 21, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, S.; Kawada, J.; Yamashita, D.; Yasufuku, C.; Akano, T.; Kato, M.; Suzuki, K.; Tano, C.; Matsumoto, K.; Mizutani, S.; et al. Impact of the Coronavirus Disease 2019 Pandemic on the Clinical Features of Pediatric Respiratory Syncytial Virus Infection in Japan. Open Forum Infect. Dis. 2022, 9, ofac562. [Google Scholar] [CrossRef] [PubMed]
- Stamm, P.; Sagoschen, I.; Weise, K.; Plachter, B.; Münzel, T.; Gori, T.; Vosseler, M. Influenza and RSV Incidence during COVID-19 Pandemic—An Observational Study from in-Hospital Point-of-Care Testing. Med. Microbiol. Immunol. 2021, 210, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, A.; Donà, D.; Barbieri, E.; Giaquinto, C.; Bont, L.J.; Baraldi, E. COVID-19 Lesson for Respiratory Syncytial Virus (RSV): Hygiene Works. Children 2021, 8, 1144. [Google Scholar] [CrossRef]
- Castagno, E.; Raffaldi, I.; Del Monte, F.; Garazzino, S.; Bondone, C. New Epidemiological Trends of Respiratory Syncytial Virus Bronchiolitis during COVID-19 Pandemic. World J. Pediatr. 2022, 19, 502–504. [Google Scholar] [CrossRef]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during Covid-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef] [PubMed]
- Loconsole, D.; Centrone, F.; Rizzo, C.; Caselli, D.; Orlandi, A.; Cardinale, F.; Serio, C.; Giordano, P.; Lassandro, G.; Milella, L.; et al. Out-of-Season Epidemic of Respiratory Syncytial Virus during the COVID-19 Pandemic: The High Burden of Child Hospitalization in an Academic Hospital in Southern Italy in 2021. Children 2022, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Billard, M.; van de Ven, P.M.; Baraldi, B.; Kragten-Tabatabaie, L.; Bont, L.J.; Wildenbeest, J.G. International Changes in Respiratory Syncytial Virus (RSV) Epidemiology during the COVID-19 Pandemic: Association with School Closures. Influenza Other Respir. Viruses 2022, 16, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Keipp Talbot, H.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Jones, J.M.; Roper, L.E.; Prill, M.M.; Ortega-Sanchez, I.R.; Moulia, D.L.; Wallace, M.; Godfrey, M.; Broder, K.R.; Tepper, N.K.; et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine during Pregnancy for the Prevention of Respiratory Syncytial Virus-Associated Lower Respiratory Tract. Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1115–1122. [Google Scholar] [PubMed]
- Vidal Valero, M. “A Good Day”: FDA Approves World’s First RSV Vaccine. Nature 2023, 617, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Am. J. Transplant. 2023, 23, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, J.E.; Borghans, J.A.M.; Bont, L.J.; Drylewicz, J. Disagreement FDA and EMA on RSV Maternal Vaccination: Possible Consequence for Global Mortality. Pediatr. Infect. Dis. J. 2024, 43, E1–E2. [Google Scholar] [CrossRef] [PubMed]
- Phijffer, E.W.; de Bruin, O.; Ahmadizar, F.; Bont, L.J.; Van der Maas, N.A.; Sturkenboom, M.C.; Wildenbeest, J.G.; Bloemenkamp, K.W. Respiratory Syncytial Virus Vaccination during Pregnancy for Improving Infant Outcomes. Cochrane Database Syst. Rev. 2024, 2024, CD015134. [Google Scholar] [CrossRef] [PubMed]
- Dieussaert, I.; Hyung Kim, J.; Luik, S.; Seidl, C.; Pu, W.; Stegmann, J.-U.; Swamy, G.K.; Webster, P.; Dormitzer, P.R. RSV Prefusion F Protein–Based Maternal Vaccine—Preterm Birth and Other Outcomes. N. Engl. J. Med. 2024, 390, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.; Madhi, S.A.; Simões, E.A.F.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.A.; Dudley, M.Z.; Glanz, J.M.; Omer, S.B. Vaccine Hesitancy: Causes, Consequences, and a Call to Action. Am. J. Prev. Med. 2015, 49, S391–S398. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Jarrett, C.; Eckersberger, E.; Smith, D.M.D.; Paterson, P. Understanding Vaccine Hesitancy around Vaccines and Vaccination from a Global Perspective: A Systematic Review of Published Literature, 2007–2012. Vaccine 2014, 32, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Asplin, P.; Keeling, M.J.; Mancy, R.; Hill, E.M. Epidemiological and Health Economic Implications of Symptom Propagation in Respiratory Pathogens: A Mathematical Modelling Investigation. PLoS Comput. Biol. 2024, 20, e1012096. [Google Scholar] [CrossRef] [PubMed]
- Wimalasena, N.N.; Chang-Richards, A.; Wang, K.I.K.; Dirks, K.N. Housing Risk Factors Associated with Respiratory Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2815. [Google Scholar] [CrossRef]
- Ares-Gómez, S.; Mallah, N.; Pardo-Seco, J.; Malvar-Pintos, A.; Pérez-Martínez, O.; Otero-Barrós, M.; Súarez-Gaiche, N.; Santiago-Pérez, M.; González-Pérez, J.; López-Pérez, L.; et al. Short- and Mid-term Morbidity and Primary-care Burden Due to Infant Respiratory Syncytial Virus Infection: A Spanish 6-year Population-based Longitudinal Study. Pediatr. Allergy Immunol. 2024, 35, e14131. [Google Scholar] [CrossRef]
- Ma, J.; Chen, L.; Tang, S.F.; Shi, Y. Efficacy and Safety of Respiratory Syncytial Virus Vaccination during Pregnancy to Prevent Lower Respiratory Tract Illness in Newborns and Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 2023, 11, 1260740. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.; Vadivelan, A.; Kovvuri, M.; Garg, N.; Thangavelu, M.; Wang, Y.; Raj, S. Association between Multiple Respiratory Viral Infections and Pediatric Intensive Care Unit Admission among Infants with Bronchiolitis. Arch. De Pediatr. 2020, 27, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Hung, E.; Tang, J.; Chow, C.M.; Leung, T.F.; Cheung, K.L.; Ng, P.C. Premorbid Factors and Outcome Associated with Respiratory Virus Infections in a Pediatric Intensive Care Unit. Pediatr. Pulmonol. 2008, 43, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 Public Health Measures and Respiratory Syncytial Virus. Lancet Child Adolesc. Health 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Di Mattia, G.; Nenna, R.; Mancino, E.; Rizzo, V.; Pierangeli, A.; Villani, A.; Midulla, F. During the COVID-19 Pandemic Where Has Respiratory Syncytial Virus Gone? Pediatr. Pulmonol. 2021, 56, 3106–3109. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.J.; Madhi, S.A.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Dagan, R.; Hammitt, L.L.; Llapur, C.J.; Novoa, J.M.; Saez Llorens, X.; et al. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N. Engl. J. Med. 2023, 388, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Ahani, B.; Tuffy, K.M.; Aksyuk, A.A.; Wilkins, D.; Abram, M.E.; Dagan, R.; Domachowske, J.B.; Guest, J.D.; Ji, H.; Kushnir, A.; et al. Molecular and Phenotypic Characteristics of RSV Infections in Infants during Two Nirsevimab Randomized Clinical Trials. Nat. Commun. 2023, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Bianchi, F.P.; Frisicale, E.M.; Guicciardi, S.; Fiacchini, D.; Tafuri, S.; et al. RSV Infection in Refugees and Asylum Seekers: A Systematic Review and Meta-Analysis. Epidemiologia 2024, 5, 221–249. [Google Scholar] [CrossRef]
- Wishaupt, J.O.; van der Ploeg, T.; Smeets, L.C.; de Groot, R.; Versteegh, F.G.A.; Hartwig, N.G. Pitfalls in Interpretation of CT-Values of RT-PCR in Children with Acute Respiratory Tract Infections. J. Clin. Virol. 2017, 90, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-Analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Demont, C.; Petrica, N.; Bardoulat, I.; Duret, S.; Watier, L.; Chosidow, A.; Lorrot, M.; Kieffer, A.; Lemaitre, M. Economic and Disease Burden of RSV-Associated Hospitalizations in Young Children in France, from 2010 through 2018. BMC Infect. Dis. 2021, 21, 730. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, A.; DeVincenzo, J.P.; Ambrose, C.S.; Krilov, L.R. RSV and Non-RSV Illness Hospitalization in RSV Immunoprophylaxis Recipients: A Systematic Literature Review. J. Clin. Virol. 2020, 129, 104339. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Duclos, A.; Lina, B.; Casalegno, J.S. Cost and Burden of RSV Related Hospitalisation from 2012 to 2017 in the First Year of Life in Lyon, France. Vaccine 2018, 36, 6591–6593. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, D.; Langedijk, A.C.; Lebbink, R.J.; Morehouse, C.; Abram, M.E.; Ahani, B.; Aksyuk, A.A.; Baraldi, E.; Brady, T.; Chen, A.T.; et al. Nirsevimab Binding-Site Conservation in Respiratory Syncytial Virus Fusion Glycoprotein Worldwide between 1956 and 2021: An Analysis of Observational Study Sequencing Data. Lancet Infect. Dis. 2023, 23, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Schaerlaekens, S.; Jacobs, L.; Stobbelaar, K.; Cos, P.; Delputte, P. All Eyes on the Prefusion-Stabilized F Construct, but Are We Missing the Potential of Alternative Targets for Respiratory Syncytial Virus Vaccine Design? Vaccines 2024, 12, 97. [Google Scholar] [CrossRef] [PubMed]
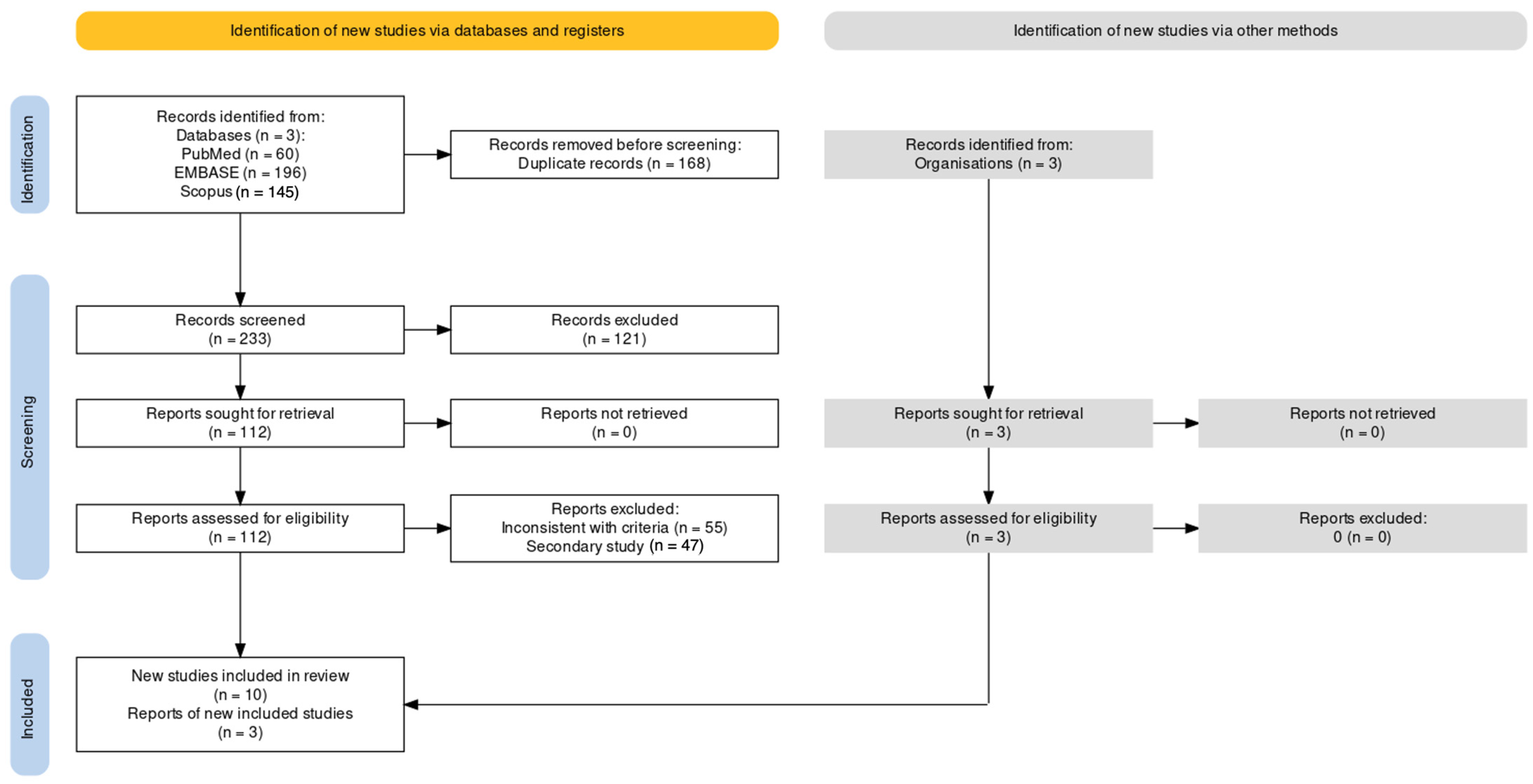
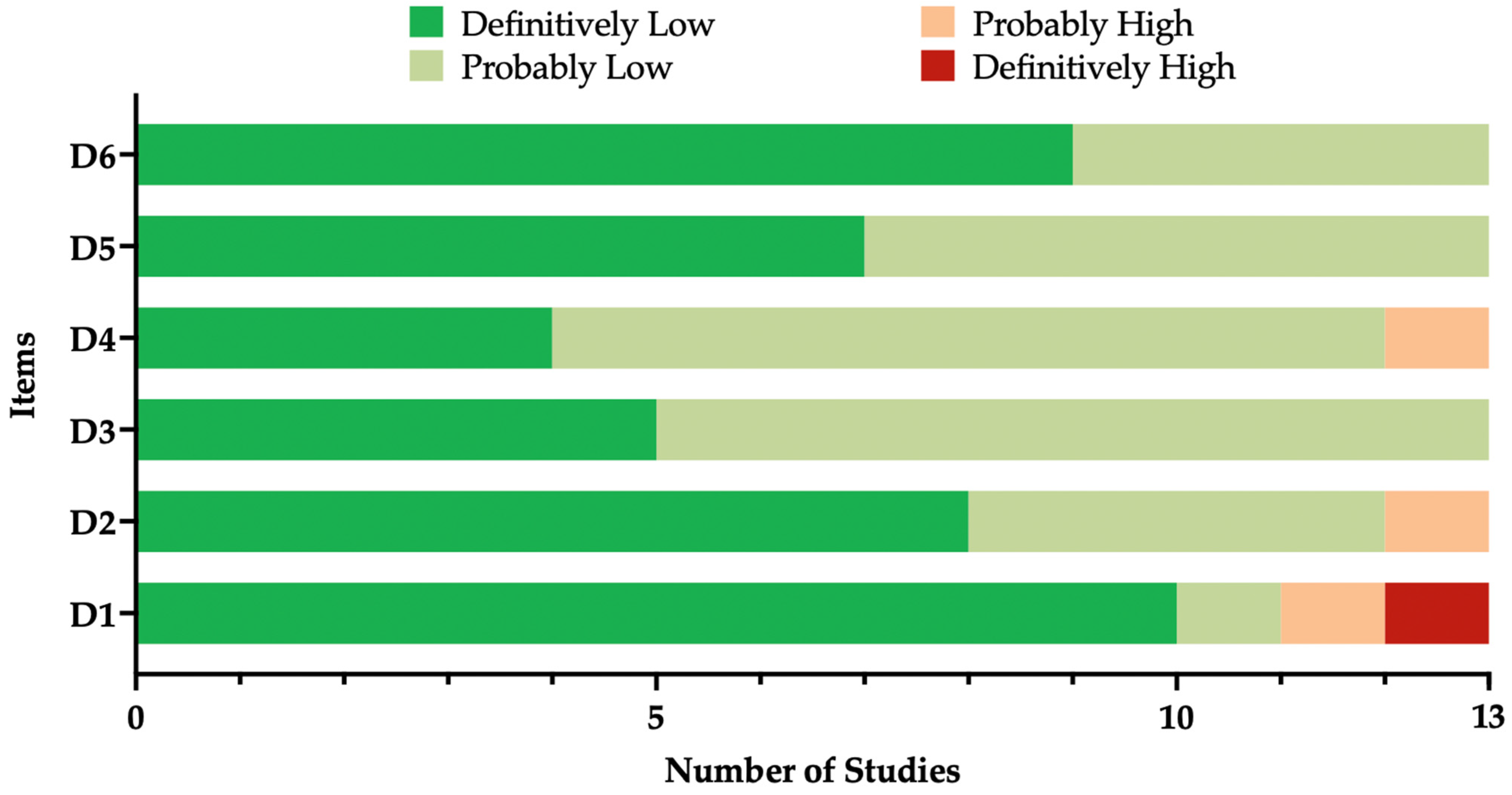



| Item | Definition |
|---|---|
| Population of Interest | Children who had received at least one dose of nirsevimab for the prevention of respiratory syncytial virus infection |
| Exposure | exposed to RSV infection during the subsequent RSV season |
| Control/Comparator | Children who had not received nirsevimab or palivizumab for the prevention of respiratory syncytial virus infection (placebo) |
| Outcome | Occurrence of lower respiratory tract infection |
| Study | Year | Settings | Timeframe | Design | Obs. Time (Days) | Total Sample (N.) | Nirsevimab | Controls | IE (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n./N., %) | RSV-Associated LRTI Cases with Hospital Admission (n./n.) | Total (n./N., %) | RSV-Associated LRTI Cases with Hospital Admission (n./n.) | ||||||||
| Griffin et al. [78] | 2020 | RCT (NCT02878330) | 3 November 2016 to 1 December 2017 | MC 23 countries | 393 | 1453 | 966 (66.48%) | 8 (0.83%) | 481 (33.52%) | 20 (4.16%) | 80.1% (55.1 to 91.2) |
| Hammitt et al. [80] | 2022 | RCT * (NCT03979313) | 23 July 2019 to 15 March 2020 | MC 20 countries | 236 | 1490 | 994 (66.71%) | 6 (0.60%) | 496 (33.29%) | 8 (1.61%) | 62.6% (−7.3 to 86.9) |
| Drysdale et al. [81] | 2023 | RCT (NCT05437510) | 8 August 2022 to 28 February 2023 | MC France, Germany, UK | 204 | 8058 | 4037 (50.10%) | 11 (0.27%) | 4021 (49.90%) | 60 (1.49%) | 81.7% (65.3 to 90.4) |
| Simoes et al. [79] | 2023 | RCT * (NCT03979313) | 23 July 2019 to 15 March 2020 | MC 20 countries | 236 | 1490 | 994 (66.71%) | 6 (0.60%) | 496 (33.29%) | 8 (1.61%) | 62.6% (−7.3 to 86.9) |
| RCT (NCT03959488) | 2019–2021 | MC 23 countries | >150 | 860 | 570 (66.28%) | 3 (0.53%) | 290 (33.72%) | 13 (4.48%) | 88.4% (59.8 to 96.7) | ||
| López-Laco et al. [84] | 2024 | Real World | 1 October 2023 to 10 January 2024 | Spain, MC 8 centers | 91 to 101 | 15,676 | 14,106 (89.98%) | 43 (0.30%) | 1570 (10.02%) | 52 (3.31%) | 92.2% (88.6 to 94.6) |
| Ernst et al. [83] | 2024 | Real World | 1 October 2023 to 31 December 2023 | Luxemburg, SC | 91 | 1524 | 1277 (83.80%) | 25 (1.96%) | 247 (16.20%) | 47 (19.03%) | 89.7% (83.6 to 93.5) |
| NIRSEGAL Study [86] | 2024 | Real World (at birth) | 1 October 2023 to 3 March 2024 | Spain, MC (Galicia) | 154 | 7278 | 6723 (92.37%) | 52 (0.77%) | 555 (7.63%) | N.A. | - |
| Real World (catch-up) | 7354 | 5824 (79.19%) | 37 (0.64%) | 1530 (20.81%) | N.A. | - | |||||
| Dagan et al. [82] | 2024 | RCT * (NCT03979313) (original study) | 23 July 2019 to 15 March 2020 | MC 31 countries | 236 | 1490 | 994 (66.71%) | 6 (0.60%) | 496 (33.29%) | 8 (1.61%) | 62.6% (−7.3 to 86.9) |
| RCT (NCT03979313) (completed) | 23 July 2019 to 9 April 2021 | 626 | 1522 | 1015 (66.69%) | 3 (0.30%) | 507 (33.21%) | 12 (2.37%) | 87.5% (55.9 to 96.5) | |||
| RCT (NCT03979313) (second season) | 2911 | 1944 (66.78%) | 3 (0.15%) | 967 (33.22%) | 3 (0.31%) | 50.3% (−146.9 to 89.9) | |||||
| Consolati et al. [88] | 2024 | Real World | 20 December 2023 to 15 February 2024 | Italy, SC (Val d’Aosta) | 57 | 537 | 369 (68.72%) | 0, - | 168 (31.28%) | 14 (8.33%) | 98.4% (72.9 to 99.9) |
| Moline et al. [61] | 2024 | Real World (only cases with hospital admission) | 1 October 2023 to 29 April 2024 | USA, MC | 211 | 699 | 59 (8.44%) | 6 (10.17%) | 640 (91.56%) | 407 (63.59%) | 84.0% (65.8 to 92.5) |
| Paireau et al. [87] | 2024 | Real World (only cases with hospital admission) | 15 September 2023 to 31 January 2024 | France, MC | 138 | 288 | 58 (20.14%) | 37 (12.85%) | 230 (79.86%) | 201 (87.40%) | 73.0% (59.8 to 89.2) |
| Ares-Gómez et al. [59] | 2024 | Real World (NCT06180993) | 25 September 2023 to 31 March 2024 | Spain, MC (Galicia) | 154 | 10,259 | 9408 (91.70%) | 30 (0.32%) | 851 (8.30%) | 16 (1.88%) | 73.0% (69.0 to 90.7) |
| Ezpeleta et al. [85] | 2024 | Real World | 1 October 2023 to 28 January 2024 | Spain, MC (Navarre) | 119 | 1177 | 1083 (92.01%) | 8 (0.74%) | 94 (8.99%) | 8 (8.51%) | 91.3% (77.4 to 96.7) |
| Variable | % | Hospital Admissions Due to LRTIs (n./N., %) | RR | 95% CI | |
|---|---|---|---|---|---|
| Stage | N./45,238 | ||||
| First year | 43,294 | 95.70% | 819, 1.89% | REFERENCE | |
| Follow-up | 1944 | 4.30% | 6, 0.31% | 0.163 | 0.073 to 0.364 |
| Treatment | N./43,249 | ||||
| Nirsevimab | 33,884 | 78.35% | 143, 0.42% | 0.058 | 0.049 to 0.070 |
| Non-treated | 9365 | 21.65% | 676, 7.22% | REFERENCE | |
| Design of the study | N./43,249 | ||||
| RCT | 13,377 | 30.93% | 144, 1.08% | REFERENCE | |
| Real world | 29,872 | 69.07% | 675, 2.26% | 2.099 | 1.756 to 2.510 |
| Length of observation period | N./43,249 | ||||
| <150 days | 18,914 | 43.73% | 216, 1.14% | REFERENCE | |
| ≥150 days | 24,335 | 56.27% | 603, 2.48% | 2.170 | 1.860 to 2.532 |
| Status regarding COVID-19 pandemic | N./43,249 | ||||
| Before COVID-19 pandemic | 3797 | 8.78% | 58, 1.53% | REFERENCE | |
| During or after COVID-19 pandemic | 39,452 | 91.22% | 761, 1.93% | 1.263 | 0.969 to 1.646 |
| Settings | N./43,249 | ||||
| RCT | 13,377 | 30.93% | 144, 1.08% | REFERENCE | |
| Spain | 27,112 | 62.69% | 176, 0.65% | 0.603 | 0.484 to 0.751 |
| Other EU countries | 2061 | 4.77% | 86, 4.17% | 3.876 | 2.980 to 5.043 |
| USA | 699 | 1.62% | 413, 59.08% | 54.887 | 46.132 to 65.303 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Giuri, P.G.; Gori, D.; Manzoni, P. Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024). Vaccines 2024, 12, 640. https://doi.org/10.3390/vaccines12060640
Riccò M, Cascio A, Corrado S, Bottazzoli M, Marchesi F, Gili R, Giuri PG, Gori D, Manzoni P. Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024). Vaccines. 2024; 12(6):640. https://doi.org/10.3390/vaccines12060640
Chicago/Turabian StyleRiccò, Matteo, Antonio Cascio, Silvia Corrado, Marco Bottazzoli, Federico Marchesi, Renata Gili, Pasquale Gianluca Giuri, Davide Gori, and Paolo Manzoni. 2024. "Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024)" Vaccines 12, no. 6: 640. https://doi.org/10.3390/vaccines12060640








