Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Cell Lines, and Culture Media
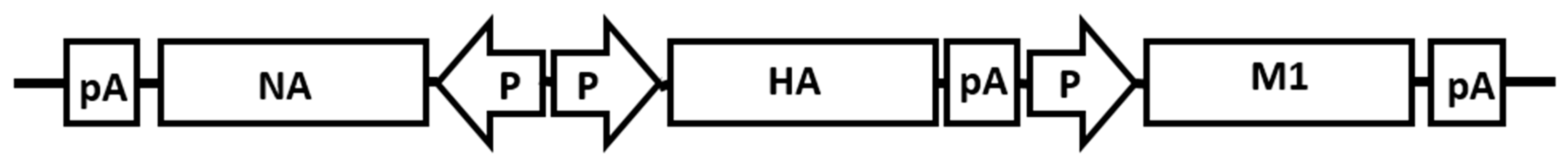
2.2. Construction of Plasmid
2.3. Production of Recombinant Baculovirus (rBV) and Virus-like Particles (VLPs)
2.4. Purification of the VLPs
2.5. Hemagglutination Assay
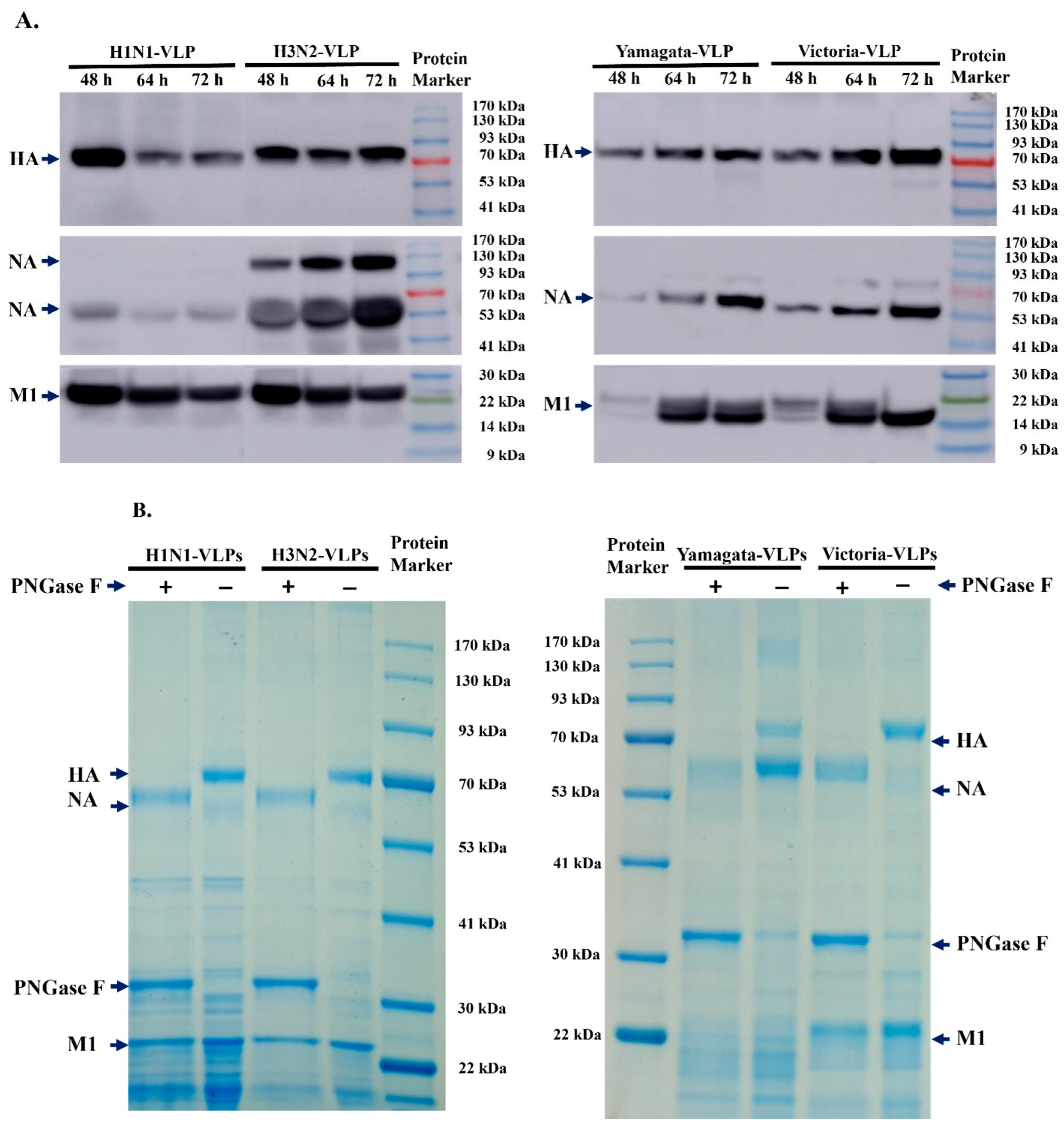
2.6. Western Blot and SDS-PAGE Analysis
2.7. Single Radial Immunodiffusion Assay (SRID)
2.8. Total Protein and HA Protein Quantification
2.9. Transmission Electron Microscopy (TEM)
2.10. Mice Immunization
2.11. Hemagglutination Inhibition (HAI) Titers
2.12. Virus Neutralization (NT) Assay
2.13. Statistical Analysis
3. Results
3.1. Expression and Characterization of Type A and Type B VLPs
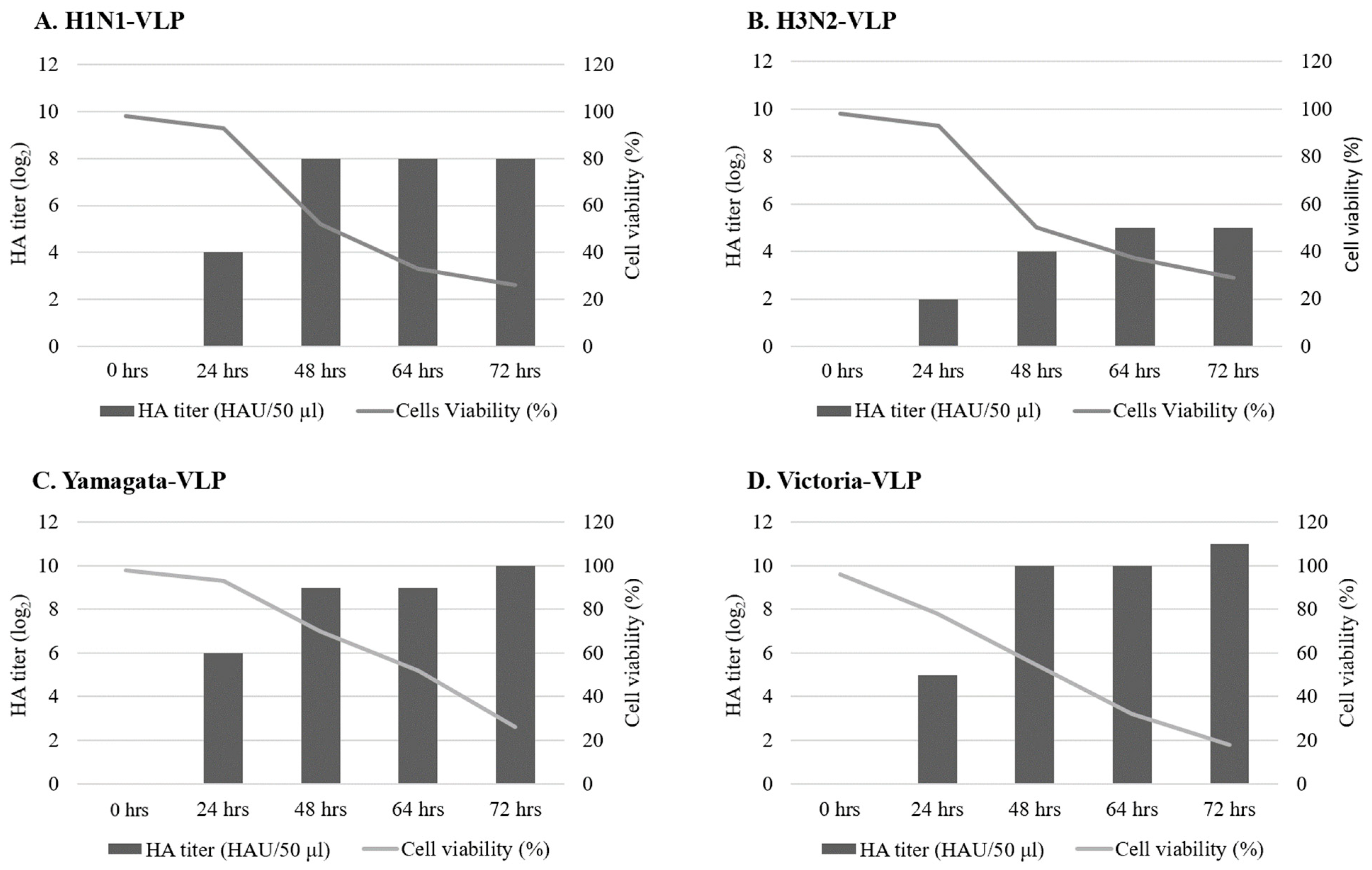
3.2. Pilot Evaluation of VLPs Stability
3.3. Immunogenicity of Quadrivalent VLPs in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Influenza (Seasonal); World Health Organization: Geneva, Switzerland, 2023.
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global Burden of Respiratory Infections Associated with Seasonal Influenza in Children under 5 Years in 2018: A Systematic Review and Modelling Study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Kaaijk, P.; Swaans, N.; Nicolaie, A.M.; Bruin, J.P.; van Boxtel, R.A.J.; de Lange, M.M.A.; Meijer, A.; Sanders, E.A.M.; van Houten, M.A.; Rots, N.Y.; et al. Contribution of Influenza Viruses, Other Respiratory Viruses and Viral Co-Infections to Influenza-Like Illness in Older Adults. Viruses 2022, 14, 797. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2021–2022 U.S. Flu Season: Preliminary in-Season Burden Estimates; Centers for Disease Control and Prevention: Antlanta, GA, USA, 2022.
- Reina, J. The Victoria and Yamagata Lineages of Influenza B Viruses, Unknown and Undervalued. Rev. Esp. Quimioter. 2022, 35, 231–235. [Google Scholar] [CrossRef]
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and Virological Characteristics of Influenza B: Results of the Global Influenza B Study. Influenza Other Respir. Viruses 2015, 9 (Suppl. S1), 3–12. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012-13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3n2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Bachmann, M.F. Virus-Like Particle Vaccinology, from Bench to Bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef]
- Sparrow, E.; Wood, J.G.; Chadwick, C.; Newall, A.T.; Torvaldsen, S.; Moen, A.; Torelli, G. Global Production Capacity of Seasonal and Pandemic Influenza Vaccines in 2019. Vaccine 2021, 39, 512–520. [Google Scholar] [CrossRef]
- Chen, J.R.; Liu, Y.M.; Tseng, Y.C.; Ma, C. Better Influenza Vaccines: An Industry Perspective. J. Biomed. Sci. 2020, 27, 33. [Google Scholar] [CrossRef]
- Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (Bevs) in Vaccine Development. Vaccines 2023, 11, 1218. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines against Emerging Infectious Diseases. Front. Microbiol. 2021, 12, 790121. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Lai, C.C.; Cheng, Y.C.; Chen, P.W.; Lin, T.H.; Tzeng, T.T.; Lu, C.C.; Lee, M.S.; Hu, A.Y. Process Development for Pandemic Influenza Vlp Vaccine Production Using a Baculovirus Expression System. J. Biol. Eng. 2019, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher. User Guide: Bac-to-Bac® Baculovirus Expression System; ThermoFisher: Waltham, MA, USA, 2018. [Google Scholar]
- World Health Organization. Who Manual on Animal Influenza Virus Diagnosis and Surveilance. 2002. Available online: https://apps.who.int/iris/bitstream/handle/10665/68026/WHO_CDS?sequence=1 (accessed on 11 June 2024).
- Wood, J.M.; Schild, G.C.; Newman, R.W.; Seagroatt, V. An Improved Single-Radial-Immunodiffusion Technique for the Assay of Influenza Haemagglutinin Antigen: Application for Potency Determinations of Inactivated Whole Virus and Subunit Vaccines. J. Biol. Stand. 1977, 5, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Routhu, N.K.; Cheedarla, N.; Gangadhara, S.; Bollimpelli, V.S.; Boddapati, A.K.; Shiferaw, A.; Rahman, S.A.; Sahoo, A.; Edara, V.V.; Lai, L.; et al. A Modified Vaccinia Ankara Vector-Based Vaccine Protects Macaques from SARS-Cov-2 Infection, Immune Pathology, and Dysfunction in the Lungs. Immunity 2021, 54, 542–556.e9. [Google Scholar] [CrossRef] [PubMed]
- Chia, M.Y.; Hu, A.Y.; Tseng, Y.F.; Weng, T.C.; Lai, C.C.; Lin, J.Y.; Chen, P.L.; Wang, Y.F.; Chao, S.R.; Chang, J.Y.; et al. Evaluation of Mdck Cell-Derived Influenza H7n9 Vaccine Candidates in Ferrets. PLoS ONE 2015, 10, e0120793. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Expert Comittee on Biological Standartisation Recommendations for the Production and Control of Influenza Vaccine (Inactivated); WHO Technical Report Series No. 927 (Annex 3); World Health Organization: Geneva, Switzerland, 2005.
- Zeng, Z.; Yau, L.F.; Lin, Z.; Xia, X.; Yang, Z.; Wang, J.R.; Song, W.; Wang, X. Characterization and Evolutionary Analysis of a Novel H3n2 Influenza a Virus Glycosylation Motif in Southern China. Front. Microbiol. 2020, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- van Baalen, C.A.; Els, C.; Sprong, L.; van Beek, R.; van der Vries, E.; Osterhaus, A.D.; Rimmelzwaan, G.F. Detection of Nonhemagglutinating Influenza a(H3) Viruses by Enzyme-Linked Immunosorbent Assay in Quantitative Influenza Virus Culture. J. Clin. Microbiol. 2014, 52, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Broszeit, F.; van Beek, R.J.; Unione, L.; Bestebroer, T.M.; Chapla, D.; Yang, J.Y.; Moremen, K.W.; Herfst, S.; Fouchier, R.A.M.; de Vries, R.P.; et al. Glycan Remodeled Erythrocytes Facilitate Antigenic Characterization of Recent a/H3n2 Influenza Viruses. Nat. Commun. 2021, 12, 5449. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Fernandes, B.; Alves, P.M.; Carrondo, M.J.T.; Roldao, A. Improving Influenza Ha-Vlps Production in Insect High Five Cells Via Adaptive Laboratory Evolution. Vaccines 2020, 8, 589. [Google Scholar] [CrossRef]
- Kong, D.; Chen, T.; Hu, X.; Lin, S.; Gao, Y.; Ju, C.; Liao, M.; Fan, H. Supplementation of H7n9 Virus-Like Particle Vaccine with Recombinant Epitope Antigen Confers Full Protection against Antigenically Divergent H7n9 Virus in Chickens. Front. Immunol. 2022, 13, 785975. [Google Scholar] [CrossRef]
- Krammer, F.; Schinko, T.; Palmberger, D.; Tauer, C.; Messner, P.; Grabherr, R. Trichoplusia Ni Cells (High Five) Are Highly Efficient for the Production of Influenza a Virus-Like Particles: A Comparison of Two Insect Cell Lines as Production Platforms for Influenza Vaccines. Mol. Biotechnol. 2010, 45, 226–234. [Google Scholar] [CrossRef]
- Matsuda, T.; Tanijima, T.; Hirose, A.; Masumi-Koizumi, K.; Katsuda, T.; Yamaji, H. Production of Influenza Virus-Like Particles Using Recombinant Insect Cells. Biochem. Eng. J. 2020, 163, 107757. [Google Scholar] [CrossRef]
- Buffin, S.; Peubez, I.; Barriere, F.; Nicolai, M.C.; Tapia, T.; Dhir, V.; Forma, E.; Seve, N.; Legastelois, I. Influenza a and B Virus-Like Particles Produced in Mammalian Cells Are Highly Immunogenic and Induce Functional Antibodies. Vaccine 2019, 37, 6857–6867. [Google Scholar] [CrossRef]
- Shin, J.I.; Park, Y.C.; Song, J.M. Influence of Temperature on the Antigenic Changes of Virus-Like Particles. Clin. Exp. Vaccine Res. 2020, 9, 126–132. [Google Scholar] [CrossRef]
- Australian Government Department of Health. National Vaccine Storage Guidelines—Strive for 5; Australian Government Department of Health: Canberra, Australia, 2019.
- Kim, S.H.; Park, Y.C.; Song, J.M. Evaluation of the Antigenic Stability of Influenza Virus Like Particles after Exposure to Acidic or Basic Ph. Clin. Exp. Vaccine Res. 2021, 10, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.; Meyers, A.E.; Williamson, A.L.; Rybicki, E.P. Stability Studies of Hiv-1 Pr55gag Virus-Like Particles Made in Insect Cells after Storage in Various Formulation Media. Virol. J. 2012, 9, 210. [Google Scholar] [CrossRef]
- Correia, R.; Meneses, L.; Richheimer, C.; Alves, P.M.; Carrondo, M.J.T.; Duarte, A.R.C.; Paiva, A.; Roldao, A. Improved Storage of Influenza Ha-Vlps Using a Trehalose-Glycerol Natural Deep Eutectic Solvent System. Vaccine 2021, 39, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Quan, F.S.; Song, J.M.; Vunnava, A.; Yoo, D.G.; Park, K.M.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Influenza Immunization with Trehalose-Stabilized Virus-Like Particle Vaccine Using Microneedles. Procedia Vaccinol. 2010, 2, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kissmann, J.; Joshi, S.B.; Haynes, J.R.; Dokken, L.; Richardson, C.; Middaugh, C.R. H1n1 Influenza Virus-Like Particles: Physical Degradation Pathways and Identification of Stabilizers. J. Pharm. Sci. 2011, 100, 634–645. [Google Scholar] [CrossRef]
- Quan, F.S.; Kim, Y.C.; Song, J.M.; Hwang, H.S.; Compans, R.W.; Prausnitz, M.R.; Kang, S.M. Long-Term Protective Immunity from an Influenza Virus-Like Particle Vaccine Administered with a Microneedle Patch. Clin. Vaccine Immunol. 2013, 20, 1433–1439. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Q.; Peng, P.; Li, R.; Li, J.; Wang, X.; Gu, M.; Hu, Z.; Hu, S.; Liu, X.; et al. Baculovirus-Derived Influenza Virus-Like Particle Confers Complete Protection against Lethal H7n9 Avian Influenza Virus Challenge in Chickens and Mice. Vet. Microbiol. 2022, 264, 109306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.V.; Massare, M.J.; Pearce, M.B.; Sun, X.; Belser, J.A.; Maines, T.R.; Creager, H.M.; Glenn, G.M.; Pushko, P.; Smith, G.E.; et al. Recombinant Virus-Like Particles Elicit Protective Immunity against Avian Influenza a(H7n9) Virus Infection in Ferrets. Vaccine 2015, 33, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Cai, M.; Hu, X.; Li, M.; Ji, Y.; Li, S.; Huang, J.; Liang, Q.; Ji, C.; Yi, H.; et al. Protection Efficacy of the H1 and H3 Bivalent Virus-Like Particle Vaccine against Swine Influenza Virus Infection. Vet. Microbiol. 2023, 280, 109719. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Hollister, J.R. Flublok, a Next Generation Influenza Vaccine Manufactured in Insect Cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Izikson, R.; Post, P.; Dunkle, L. Safety, Efficacy, and Immunogenicity of Flublok in the Prevention of Seasonal Influenza in Adults. Ther. Adv. Vaccines 2015, 3, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Treanor, J.J.; El Sahly, H.M.; Gilbert, A.; Meyer, A.L.; Patriarca, P.A.; Cox, M.M. Comparative Immunogenicity of Recombinant Influenza Hemagglutinin (Rha) and Trivalent Inactivated Vaccine (Tiv) among Persons > or =65 Years Old. Vaccine 2009, 28, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M.; Leroux-Roels, G.; Beran, J.; Devaster, J.M.; Esen, M.; Launay, O.; McElhaney, J.E.; van Essen, G.A.; Benoit, A.; Claeys, C.; et al. Immunogenicity of As03-Adjuvanted and Non-Adjuvanted Trivalent Inactivated Influenza Vaccines in Elderly Adults: A Phase 3, Randomized Trial and Post-Hoc Correlate of Protection Analysis. Hum. Vaccin. Immunother. 2016, 12, 3043–3055. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Schiff, G.M.; Hayden, F.G.; Brady, R.C.; Hay, C.M.; Meyer, A.L.; Holden-Wiltse, J.; Liang, H.; Gilbert, A.; Cox, M. Safety and Immunogenicity of a Baculovirus-Expressed Hemagglutinin Influenza Vaccine: A Randomized Controlled Trial. JAMA 2007, 297, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Heinimaki, S.; Tamminen, K.; Malm, M.; Vesikari, T.; Blazevic, V. Live Baculovirus Acts as a Strong B and T Cell Adjuvant for Monomeric and Oligomeric Protein Antigens. Virology 2017, 511, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Seguin, A.; Couillard, J.; Trepanier, S.; Landry, N. Phase Iii: Randomized Observer-Blind Trial to Evaluate Lot-to-Lot Consistency of a New Plant-Derived Quadrivalent Virus Like Particle Influenza Vaccine in Adults 18–49 Years of Age. Vaccine 2021, 39, 1528–1533. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.A.; Goldenthal, K.L.; Muse, D.; Cox, M.M.J. Randomized Comparison of Immunogenicity and Safety of Quadrivalent Recombinant Versus Inactivated Influenza Vaccine in Healthy Adults 18–49 Years of Age. J. Infect. Dis. 2017, 216, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Rockman, S.; Laurie, K.; Ong, C.; Rajaram, S.; McGovern, I.; Tran, V.; Youhanna, J. Cell-Based Manufacturing Technology Increases Antigenic Match of Influenza Vaccine and Results in Improved Effectiveness. Vaccines 2022, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Suphaphiphat, P.; van Boxmeer, J.; Haag, M.; Leav, B.; Iheanacho, I.; Kistler, K.; Ortiz de Lejarazu, R. Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002–2003 to 2017–2018. Int. J. Environ. Res. Public Health 2020, 17, 5423. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Wojcik, R.; Moore, C.; Ortiz de Lejarazu, R.; de Lusignan, S.; Montomoli, E.; Rossi, A.; Perez-Rubio, A.; Trilla, A.; Baldo, V.; et al. The Impact of Candidate Influenza Virus and Egg-Based Manufacture on Vaccine Effectiveness: Literature Review and Expert Consensus. Vaccine 2020, 38, 6047–6056. [Google Scholar] [CrossRef]




| Type of Influenza Virus | Subtype or Lineage | Type of Protein | Strains * | GenBank and GISAID Accession Number |
|---|---|---|---|---|
| A | H1N1 | HA | A/Hawaii/70/2019 | MT738421.1 |
| NA | A/Hawaii/70/2019 | MT738415.1 | ||
| M1 | A/Hawaii/70/2019 | MT738417.1 | ||
| H3N2 | HA | A/Minnesota/41/2019 | EPI1487157 | |
| NA | A/Minnesota/41/2019 | EPI1487156 | ||
| M1 | A/Minnesota/41/2019 | EPI1487152 | ||
| B | Yamagata | HA | B/Brisbane/09/2014 | EPI539769 |
| NA | B/Brisbane/09/2014 | EPI544258 | ||
| M1 | B/Brisbane/09/2014 | EPI630126 | ||
| Victoria | HA | B/Darwin/7/2019 | EPI1434729 | |
| NA | B/Brisbane/63/2014 | EPI646564 | ||
| M1 | B/Brisbane/63/2014 | EPI630121 |
| Vaccine Name | HA Protein Concentration (µg/mL) | Mean HA Concentration/Strain (µg/mL) | |||
|---|---|---|---|---|---|
| A/H1N1 | A/H3N2 | B/Yamagata | B/Victoria | ||
| Flublok | 198 | 197 | 192 | 173 | 190 |
| Vaxigrip | 60 | 43 | 57 | 53 | 53 |
| Virus Subtype | HA Titer before Purification (HAU/50 µL) | HA Titer after Purification (HAU/50 µL) | HA Content after Purification (µg/mL) | Total Protein Content after Purification (µg/mL) | HA Content/Total Protein (%) | HA Yield (µg) in 40 mL after Purification (mg/L) 1 |
|---|---|---|---|---|---|---|
| A-H1N1 | 128 | 5120 | 220 | 929 | 23.7 | 264 (6.6) |
| A-H3N2 | N/A 2 | N/A 2 | 260 | 592 | 43.9 | 312 (7.8) |
| B-Yam | 512 | 4096 | 80 | 443 | 18.1 | 96 (2.4) |
| B-Vic | 1024 | 16,384 | 353 | 950 | 37.1 | 423.6 (10.6) |
| Name of the VLP | HAU/50 µL | HA Concentration (µg/mL) | ||
|---|---|---|---|---|
| Initial Titer | After 3 Months | Initial Concentration | After 3 Months | |
| 4 °C | 4 °C | |||
| Yamagata-VLPs | 10,240 | 5120 | 77 | 55 |
| Victoria-VLPs | 20,480 | 10,240 | 165 | 110 |
| Name of the VLP | HAU/50 µL | HA Concentration (µg/mL) | ||||
|---|---|---|---|---|---|---|
| Initial Titer 1 | After 12 Months | Initial Concentration | After 12 Months | |||
| 4 °C | −20 °C | 4 °C | −20 °C | |||
| H1N1-VLPs | 5120 | 2560 | 5120 | 94 | 84 | 87 |
| H3N2-VLPs | N/A 2 | N/A | N/A | 262 | 217 | 243 |
| Yamagata-VLPs | 10,240 | 5120 | 5120 | 81 | 73 | N/A 3 |
| Victoria-VLPs | 20,480 | 10,240 | 20,480 | 160 | 138 | 157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badruzzaman, A.T.M.; Cheng, Y.-C.; Sung, W.-C.; Lee, M.-S. Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice. Vaccines 2024, 12, 667. https://doi.org/10.3390/vaccines12060667
Badruzzaman ATM, Cheng Y-C, Sung W-C, Lee M-S. Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice. Vaccines. 2024; 12(6):667. https://doi.org/10.3390/vaccines12060667
Chicago/Turabian StyleBadruzzaman, A. T. M., Yu-Chieh Cheng, Wang-Chou Sung, and Min-Shi Lee. 2024. "Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice" Vaccines 12, no. 6: 667. https://doi.org/10.3390/vaccines12060667
APA StyleBadruzzaman, A. T. M., Cheng, Y.-C., Sung, W.-C., & Lee, M.-S. (2024). Insect Cell-Based Quadrivalent Seasonal Influenza Virus-like Particles Vaccine Elicits Potent Immune Responses in Mice. Vaccines, 12(6), 667. https://doi.org/10.3390/vaccines12060667







