A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Chemical Reagents
2.3. Construction of gH-Complementing Cell Line V17gH
2.4. Isolation and Characterization of Keratin 1 (KRT1) Promoters
2.5. Construction of Recombinant Viruses
2.6. Evaluation of the Activity and Heat Regulation of Recombination Plasmids Containing Modified Promoter–Transactivator Cassettes
2.7. Statistical Analyses
2.8. Other Methods
3. Results
3.1. First-Generation RCCVs
3.2. Characterization of KRT1 Promoters
3.3. KRT1 Promoter Control Prevents Viral Replication in the CNS but Does Not Significantly Affect RCCV Vaccine Efficacy
3.4. Engineering out the AP Co-Control from a KRT1 Promoter-Restricted RCCV
3.5. Heat-Only-Activated, KRT1 Promoter-Restricted (Second-Generation) RCCVs Are Stringently Heat-Regulated and Are Broadly Protective Vaccines
3.6. Substitution or Elimination of the Heat Control Is Associated with a Substantial Loss of Vaccine Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Voellmy, R.; Bloom, D.C.; Vilaboa, N. A novel approach for addressing diseases not yielding to effective vaccination? Immunization by replication-competent controlled virus. Expert. Rev. Vaccines 2015, 14, 637–651. [Google Scholar] [CrossRef]
- Voellmy, R.; Bloom, D.C.; Vilaboa, N. Immunization by replication-competent controlled viral pathogen—A novel approach worth exploring for diseases refractory to effective conventional vaccination? Vaccines Vaccin. 2015, 6, 286. [Google Scholar]
- Peng, B.; Wang, L.R.; Gomez-Roman, V.R.; Davis-Warren, A.; Montefiori, D.C.; Kalyanaraman, V.S.; Venzon, D.; Zhao, J.; Kan, E.; Rowell, T.J.; et al. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 2005, 79, 10200–10209. [Google Scholar] [CrossRef]
- Halford, W.P.; Weisend, C.; Grace, J.; Soboleski, M.; Carr, D.J.; Balliet, J.W.; Imai, Y.; Margolis, T.P.; Gebhardt, B.M. ICP0 antagonizes Stat I-dependent repression of herpes simplex virus: Implications for the regulation of viral latency. Virol. J. 2008, 3, 44. [Google Scholar] [CrossRef][Green Version]
- Huang, X.; Lu, B.; Yu, W.; Fang, Q.; Liu, L.; Zhuang, K.; Shen, T.; Wang, H.; Tian, P.; Zhang, L.; et al. A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination. PLoS ONE 2009, 4, e4180. [Google Scholar] [CrossRef]
- Roizman, B. The family Herpesviridae. A brief introduction. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 1996; Volume 2, pp. 2479–2500. [Google Scholar]
- Roizman, B. Herpes simplex viruses and their replication. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 1996; Volume 2, pp. 2501–2602. [Google Scholar]
- Bloom, D.C. HSV vectors for gene therapy. Methods Mol. Med. 1998, 10, 369–386. [Google Scholar]
- Vilaboa, N.; Fenna, M.; Munson, J.; Roberts, S.M.; Voellmy, R. Novel gene switches for targeted and timed expression of proteins of interest. Mol. Ther. 2005, 12, 290–298. [Google Scholar] [CrossRef]
- Bloom, D.C.; Feller, J.; McAnany, P.; Vilaboa, N.; Voellmy, R. Replication-competent controlled herpes simplex virus. J. Virol. 2015, 89, 10668–10679. [Google Scholar] [CrossRef]
- Bloom, D.C.; Tran, R.K.; Feller, J.; Voellmy, R. Immunization by replication-competent controlled herpesvirus vectors. J. Virol. 2018, 92, e00616-8. [Google Scholar] [CrossRef]
- Bloom, D.C.; Lilly, C.; Canty, W.; Vilaboa, N.; Voellmy, R. Very broadly effective hemagglutinin-directed influenza vaccines with anti-herpetic activity. Vaccines 2024, 12, 537. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- DeLuca, N.A.; Schaffer, P.A. Activities of herpes simplex virus type 1 (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987, 15, 4491–4511. [Google Scholar] [CrossRef]
- Chambers, T.M.; Reedy, S.E. Equine influenza culture methods. Methods Mol. Biol. 2014, 1161, 403–410. [Google Scholar] [PubMed]
- Kisseberth, W.C.; Brettingen, N.T.; Lohse, J.K.; Sandgren, E.P. Ubiquitous expression of marker transgenes in mice and rats. Dev. Biol. 1999, 214, 128–138. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z.; Zhang, Y.; Yuan, D.; Ge, W.; Wang, X. The inconsistent regulation of HOXC13 on different keratins and the regulation mechanism on HOXC13 in cashmere goat (Capra hircus). BMC Genom. 2018, 19, 630. [Google Scholar] [CrossRef]
- McKendall, R.R. Efficacy of herpes simplex virus type 1 immunization in protecting against acute and latent infection by herpes simplex type 2 in mice. Infect. Immun. 1977, 16, 717–719. [Google Scholar] [CrossRef]
- Babendure, J.R.; Babendure, J.L.; Ding, J.-H.; Tsien, R.Y. Control of mammalian translation by mRNA structure near caps. RNA 2006, 12, 851–861. [Google Scholar] [CrossRef]
- McLean, C.S.; Erturk, M.; Jennings, R.; Challanain, D.N.; Minson, A.C.; Duncan, I.; Boursnell, M.E.; Inglis, S.C. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J. Infect. Dis. 1994, 170, 1100–1109. [Google Scholar] [CrossRef]
- Wei, C.J.; Boyington, J.C.; McTamney, P.M.; Kong, W.P.; Pearce, M.B.; Xu, L.; Andersen, H.; Rao, S.; Tumpey, T.M.; Yang, Z.-Y.; et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 2010, 329, 1060–1064. [Google Scholar] [CrossRef]
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Kariko, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self- assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Wang, T.T.; Yondola, M.; Gao, Q.; Haye, K.; Garcia-Sastre, A.; Palese, P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio 2010, 1, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.B.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef]
- van der Lubbe, J.E.M.; Huizingh, J.; Verspuij, J.W.A.; Tettero, L.; Schmit-Tillemans, S.P.R.; Mooij, P.; Mortier, D.; Koopman, G.; Rogers, W.M.J.M.; Dekking, L.; et al. Minihemagglutinin vaccination induces cross-reactive antibodies in pre-exposed NHP that protect mice against lethal influenza challenge. NPJ Vaccines 2018, 3, 25. [Google Scholar] [CrossRef]
- Mallajosyula, V.V.A.; Citron, M.; Ferrara, F.; Lu, X.; Callahan, C.; Heidecker, G.J.; Sarma, S.P.; Flynn, J.A.; Temperton, N.J.; Liang, X.; et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc. Natl. Acad. Sci. USA 2014, 111, E2514–E2523. [Google Scholar] [CrossRef]
- Valkenburg, S.A.; Mallajosyula, V.V.A.; Li, O.T.W.; Chin, A.W.H.; Carnell, G.; Temperton, N.; Varadarajan, R.; Poon, L.L.M. Stalking influenza by vaccination with pre-fusion headless HA mini-stem. Sci. Rep. 2016, 6, 22666. [Google Scholar] [CrossRef]
- Krammer, F.; Pica, N.; Hai, R.; Margine, I.; Palese, P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J. Virol. 2013, 87, 6542–6550. [Google Scholar] [CrossRef]
- Margine, I.; Krammer, F.; Hai, R.; Heaton, N.S.; Tan, G.S.; Andrews, S.A.; Runstadler, J.A.; Wilson, P.C.; Albrecht, R.A.; García-Sastre, A.; et al. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J. Virol. 2013, 87, 10435–10446. [Google Scholar] [CrossRef] [PubMed]
- Ermler, M.E.; Kirkpatrick, E.; Sun, W.; Hai, R.; Amanat, F.; Chromikova, V.; Palese, P.; Krammer, F. Chimeric hemagglutinin constructs induce broad protection against influenza B virus challenge in the mouse model. J. Virol. 2017, 91, e00286-17. [Google Scholar] [CrossRef]
- Ibañez, L.I.; Caldevilla, C.A.; Rojas, Y.P.; Mattion, N. Genetic and subunit vaccines based on the stem domain of the equine influenza hemagglutinin provide homosubtypic protection against heterologous strains. Vaccine 2018, 36, 1592–1598. [Google Scholar] [CrossRef]
- Krammer, F.; Palese, P. Universal influenza virus vaccines that target the conserved hemagglutinin stalk and conserved sites in the head domain. J. Infect. Dis. 2019, 219 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef]
- Broecker, F.; Liu, S.T.H.; Suntronwong, N.; Sun, W.; Bailey, M.J.; Nachbagauer, R.; Krammer, F.; Palese, P. A mosaic hemagglutinin-based influenza virus vaccine candidate protects mice from challenge with divergent H3N2 strains. NPJ Vaccines 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hashem, A.M.; Chen, Z.; Li, C.; Doyle, T.; Zhang, Y.; Yi, Y.; Farnsworth, A.; Xu, K.; Li, Z.; et al. Targeting the HA2 subunit of influenza A virus hemagglutinin via CD40L provides universal protection against diverse subtypes. Mucosal Immunol. 2015, 8, 211–220. [Google Scholar] [CrossRef]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U.; et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef]
- Allen, J.D.; Ray, S.; Ross, T.M. Split inactivated COBRA vaccine elicits protective antibodies against H1N1 and H3N2 influenza viruses. PLoS ONE 2018, 13, e0204284. [Google Scholar]
- Park, J.; Fong, S.; Schwartzman, L.M.; Sheng, Z.-M.; Freeman, A.; Matthews, L.; Xiao, Y.; Ramuta, M.D.; Batchenkova, N.A.; Qi, L.; et al. An inactivated multivalent influenza A virus vaccine is broadly protective in mice and ferrets. Sci. Transl. Med. 2022, 14, eabo2167. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Kim, M.; Cheong, Y.; Lee, J.; Lim, J.; Byun, S.; Jang, Y.H.; Seong, B.L. A host-restricted self-attenuated influenza virus provides broad pan-influenza A protection in a mouse model. Front. Immunol. 2021, 12, 779223. [Google Scholar] [CrossRef]
- Voellmy, R.; Zürcher, O.; Zürcher, M.; de Viragh, P.A.; Hall, A.K.; Roberts, S.M. Targeted heat activation of HSP promoters in the skin of mammalian animals and humans. Cell Stress. Chaperones 2018, 23, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Nirschl, C.J.; Anandasabapathy, N. Duality at the gate: Skin dendritic cells as mediators of vaccine immunity and tolerance. Hum. Vaccin. Immunother. 2016, 12, 104–116. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Tian, T.; Zhao, J.; Park, C.D.; Lofftus, S.Y.; Stingley, C.A.; Yan, Y.; Mei, S.; Liu, X.; et al. Epicutaneous immunization with modified Vaccinia Ankara viral vectors generates superior T cell immunity against a respiratory viral challenge. NPJ Vaccines 2021, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-herpesvirus thymidine kinase genes mediate viral virulence and are potential therapeutic targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Field, H.J.; Wildy, P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J. Hyg. 1978, 81, 267–277. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Yao, H.-W.; Wang, L.-C.; Shen, F.-H.; Hsu, S.-M.; Chen, S.-H. Thymidine kinase-negative herpes simplex Virus 1 Can efficiently establish persistent infection in neural tissues of nude mice. J. Virol. 2017, 91, e01979-16. [Google Scholar] [CrossRef]
- Joyce, J.D.; Patel, A.K.; Murphy, B.; Carr, D.J.J.; Gershburg, E.; Bertke, A.S. Assessment of two novel live-attenuated vaccine candidates for herpes simplex virus 2 (HSV-2) in guinea pigs. Vaccines 2021, 9, 258. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Stahl, J.; Chouljenko, V.N.; Subramanian, R.; Charles, A.-S.; Saied, A.A.; Walker, J.D.; Kousoulas, K.G. A single intramuscular vaccination of mice with the HSV-1 VC2 virus with mutations in the glycoprotein K and the membrane protein UL20 confers full protection against lethal intravaginal challenge with virulent HSV-1 and HSV-2 strains. PLoS ONE 2014, 9, e109890. [Google Scholar] [CrossRef]
- Jackson, K.L.; Dayton, R.D.; Deverman, B.E.; Klein, R.L. Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front. Mol. Neurosci. 2016, 9, 116. [Google Scholar]
- Watson, Z.L.; Washington, S.D.; Phelan, D.M.; Lewin, A.S.; Tuli, S.S.; Schultz, G.S.; Neumann, D.M.; Bloom, D.C. In vivo knockdown of the herpes simplex virus 1 latency-associated transcript reduces reactivation from latency. J. Virol. 2018, 92, e00812-8. [Google Scholar] [CrossRef] [PubMed]
- Hanc, P.; Gonzalez, R.J.; Mazo, I.B.; Wang, Y.; Lambet, T.; Ortiz, G.; Miller, E.W.; von Andrian, U.H. Multimodal control of dendritic cell functions by nociceptors. Science 2023, 379, eabm5658. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Knipe, D.M. Herpes simplex virus vectors elicit durable immune responses in the presence of preexisting host immunity. J. Virol. 2002, 76, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Chahlavi, A.; Rabkin, S.; Todo, T.; Sundaresan, P.; Martuza, R. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 1999, 6, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Delman, K.A.; Bennett, J.J.; Zager, J.S.; Burt, B.M.; McAuliffe, P.F.; Petrowsky, H.; Kooby, D.A.; Hawkins, W.G.; Horsburgh, B.C.; Johnson, P.; et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic therapy. Hum. Gene Ther. 2000, 11, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Hocknell, P.K.; Wiley, R.D.; Wang, X.; Evans, T.G.; Bowers, W.J.; Hanke, T.; Federoff, H.J.; Dewhurst, S. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J. Virol. 2002, 76, 5565–5580. [Google Scholar] [CrossRef] [PubMed]
- Lambright, E.S.; Kang, E.H.; Force, S.; Lanuti, M.; Caparrelli, D.; Kaiser, L.R.; Albelda, S.M.; Molnar-Kimber, K.L. Effect of preexisting anti-herpes immunity on the efficacy of herpes simplex viral therapy in a murine intraperitoneal tumor model. Mol. Ther. 2000, 2, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, U.; Kramm, C.M.; Aboody-Guterman, K.S.; Silver, J.S.; Ikeda, K.; Johnston, K.M.; Pechan, P.A.; Barth, R.F.; Finkelstein, D.; Chiocca, E.A.; et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998, 5, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, H.; Ried, C.; Epstein, A.L.; Marconi, P.; Bricker, T. Reduced immune responses after vaccination with a recombinant herpes simplex virus type 1 vector in the presence of antiviral immunity. J. Gen. Virol. 2005, 86, 2401–2410. [Google Scholar] [CrossRef]
- Watanabe, D.; Brockman, M.A.; Ndung’u, T.; Mathews, L.; Lucas, W.T.; Murphy, C.G.; Felber, B.K.; Pavlakis, G.N.; Deluca, N.A.; Knipe, D.M. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology 2007, 357, 186–198. [Google Scholar] [CrossRef]
- Kaugars, K.; Dardick, J.; de Oliveira, A.P.; Weiss, K.A.; Lukose, R.; Kim, J.; Leung, L.; Rajagopalan, S.; Wolin, S.; Akabas, L.; et al. A recombinant herpes virus expressing influenza hemagglutinin confers protection and induces antibody-dependent cellular cytotoxicity. Proc. Natl. Acad. Sci. USA 2021, 118, e2110714118. [Google Scholar] [CrossRef] [PubMed]

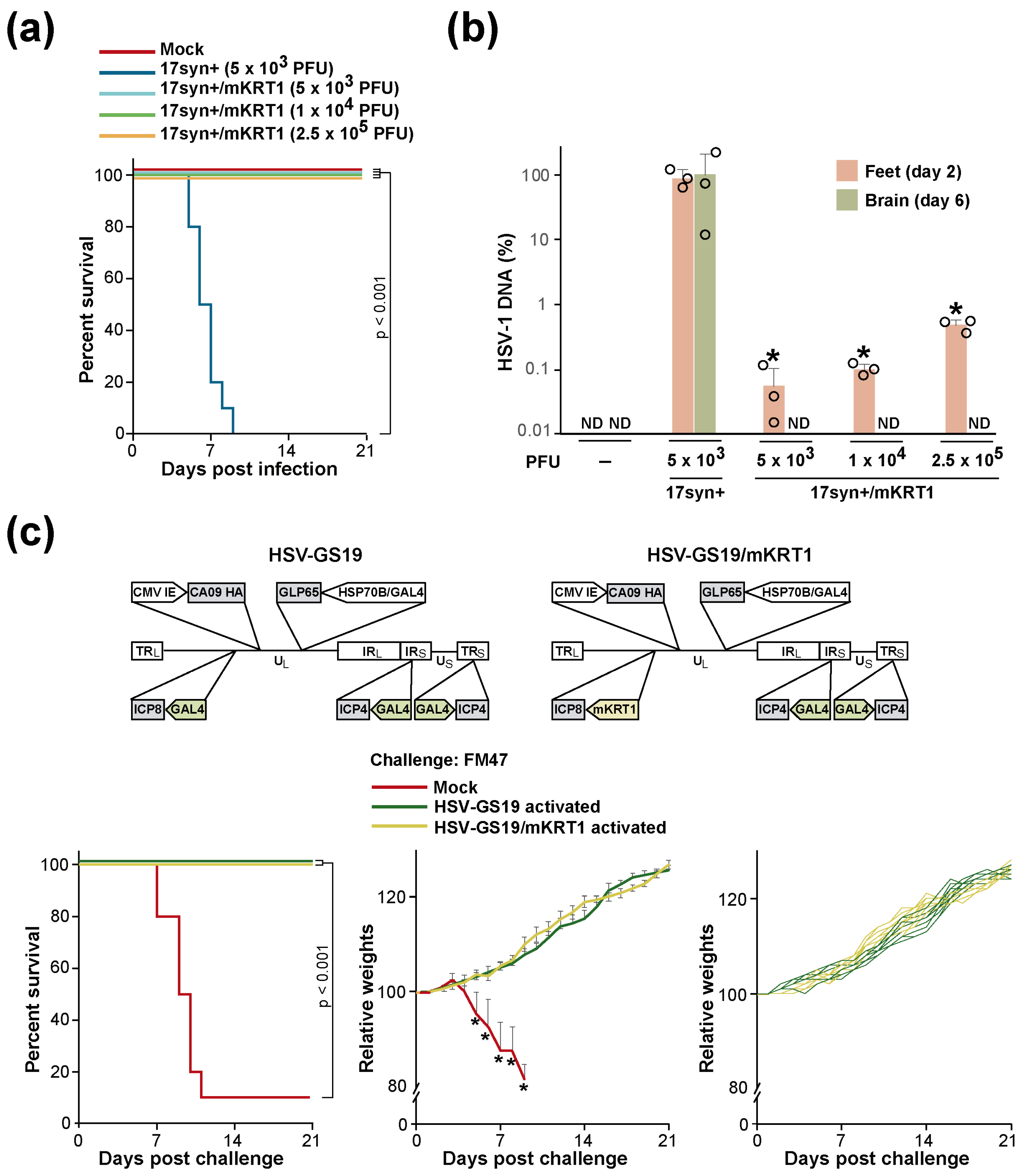
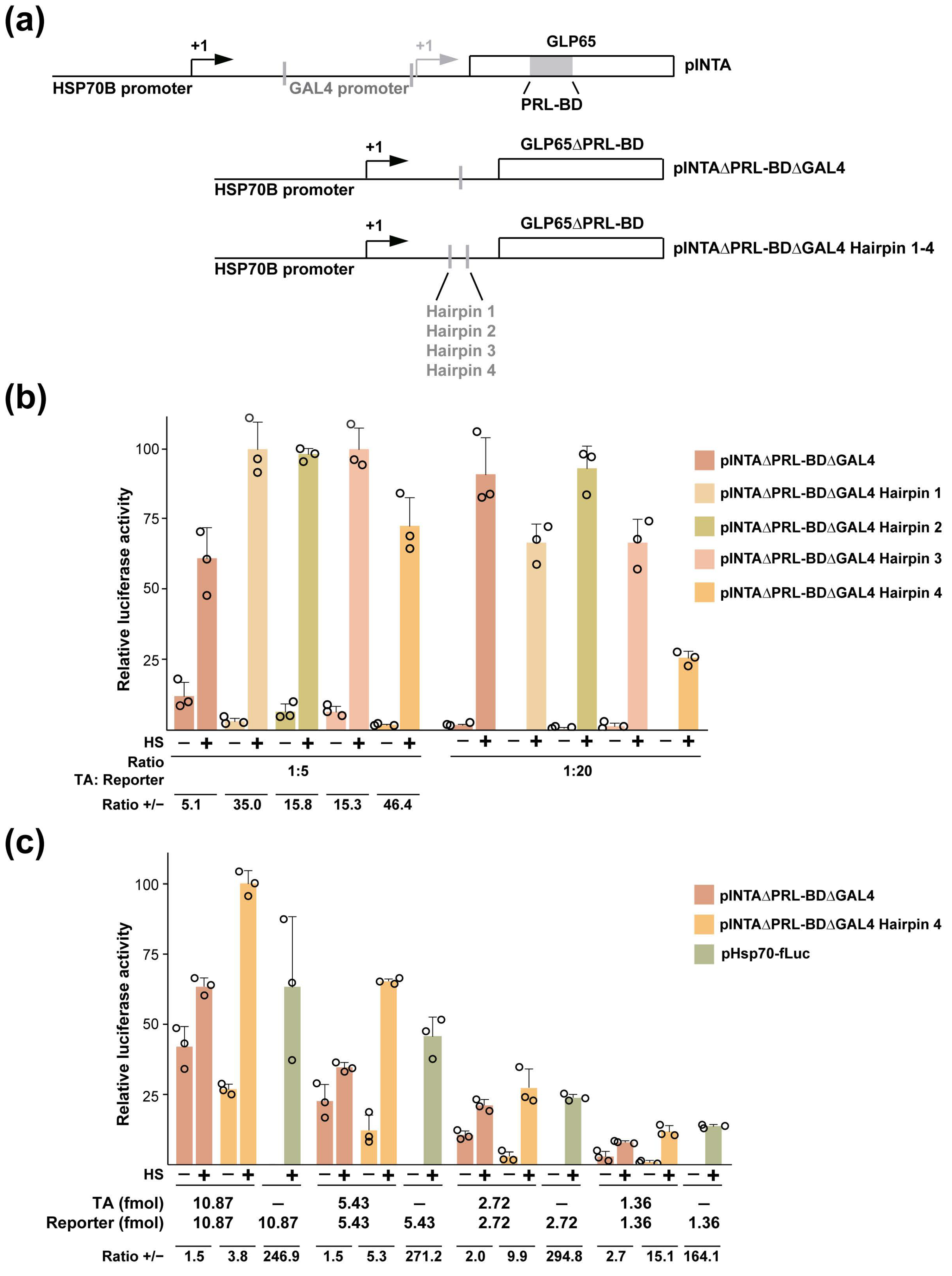
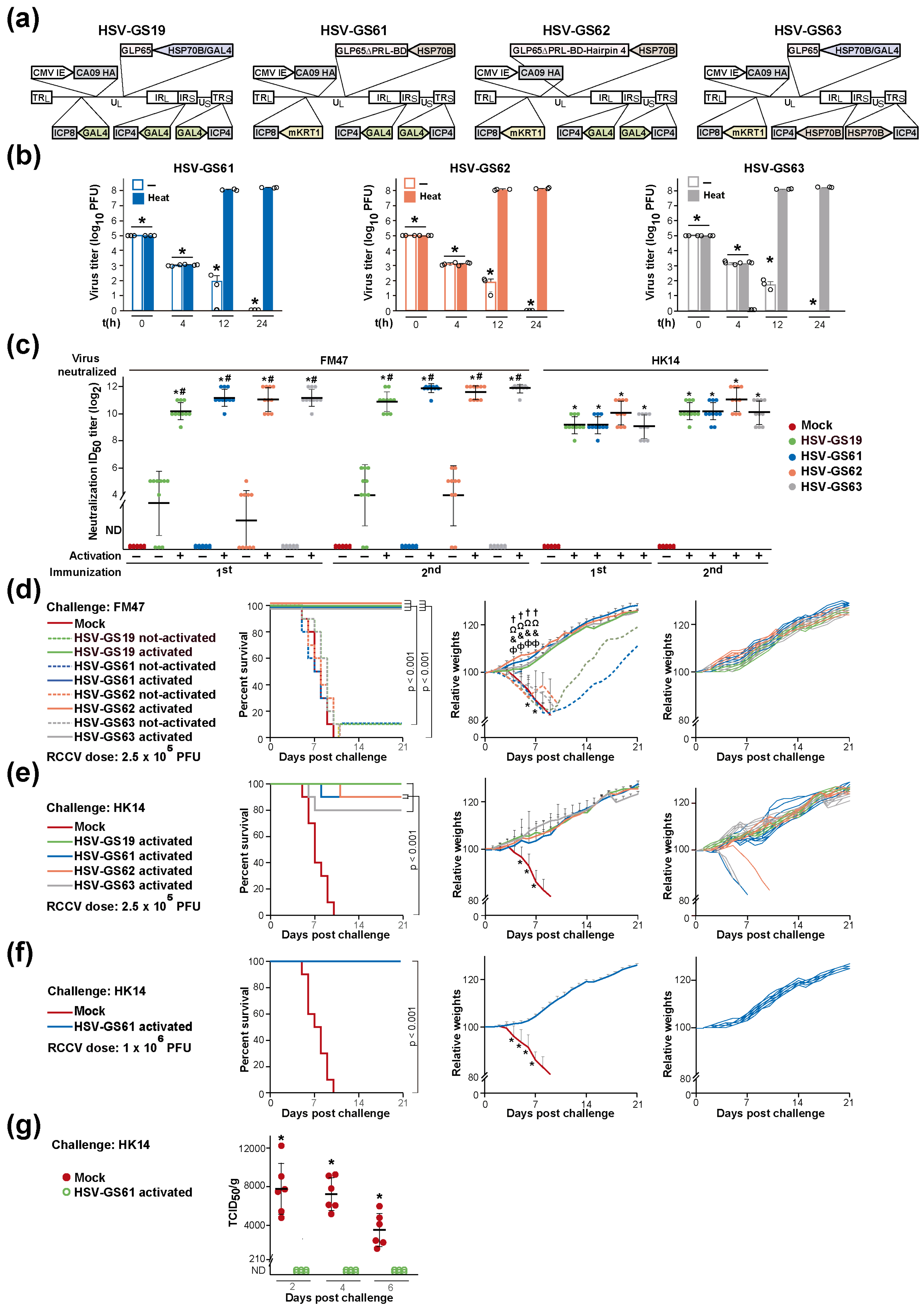

| RCCV | Non-RCCV Recombinant | Description |
|---|---|---|
| HSV-GS19 | ICP4 and ICP8 genes controlled by a heat-activated and antiprogestin-armed gene switch | |
| HSV-GS19/mKRT1 | ICP4 genes controlled by a heat-activated and antiprogestin-armed gene switch; ICP8 gene under the control of a keratin 1 gene promoter | |
| 17syn+/mKRT1 | ICP8 gene under the control of a keratin 1 gene promoter | |
| HSV-GC9 | ICP8 gene under the control of a keratin 1 gene promoter | |
| HSV-GS61 | ICP4 genes controlled by a heat-induced, unregulated transactivator; ICP8 gene under the control of a keratin 1 gene promoter | |
| HSV-GS62 | ICP4 genes controlled by a heat-induced, unregulated transactivator (expressed at a lower level than by HSV-GS61); ICP8 gene under the control of a keratin 1 gene promoter | |
| HSV-GS63 | ICP4 genes controlled by heat shock promoters; ICP8 gene under the control of a keratin 1 gene promoter | |
| HSV-GC2 | Gene for glycoprotein H deleted | |
| HSV-GC3 | Gene for glycoprotein H deleted; ICP8 gene under the control of a keratin 1 gene promoter |
| Vaccine | HSV-GS19 | HSV-GS61 | * HSV-GS62 | HSV-GS63 |
|---|---|---|---|---|
| Not-activated | ND | ND | 44 ± 8 | ND |
| Activated | 2.3 ± 0.4 × 103 | 1.3 ± 0.9 × 103 | 2.1 ± 0.3 × 104 | 4.9 ± 1.0 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaboa, N.; Bloom, D.C.; Canty, W.; Voellmy, R. A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus. Vaccines 2024, 12, 703. https://doi.org/10.3390/vaccines12070703
Vilaboa N, Bloom DC, Canty W, Voellmy R. A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus. Vaccines. 2024; 12(7):703. https://doi.org/10.3390/vaccines12070703
Chicago/Turabian StyleVilaboa, Nuria, David C. Bloom, William Canty, and Richard Voellmy. 2024. "A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus" Vaccines 12, no. 7: 703. https://doi.org/10.3390/vaccines12070703
APA StyleVilaboa, N., Bloom, D. C., Canty, W., & Voellmy, R. (2024). A Broad Influenza Vaccine Based on a Heat-Activated, Tissue-Restricted Replication-Competent Herpesvirus. Vaccines, 12(7), 703. https://doi.org/10.3390/vaccines12070703







