Acquired Hemophilia A after SARS-CoV-2 Immunization: A Narrative Review of a Rare Side Effect
Abstract
:1. Introduction
2. Pathophysiology of Aha
3. SARS-CoV-2 Infection, Autoimmunity and Autoimmune Diseases
4. SARS-CoV-2 Vaccination Reactions, Autoimmunity and Autoimmune Diseases
5. Aim
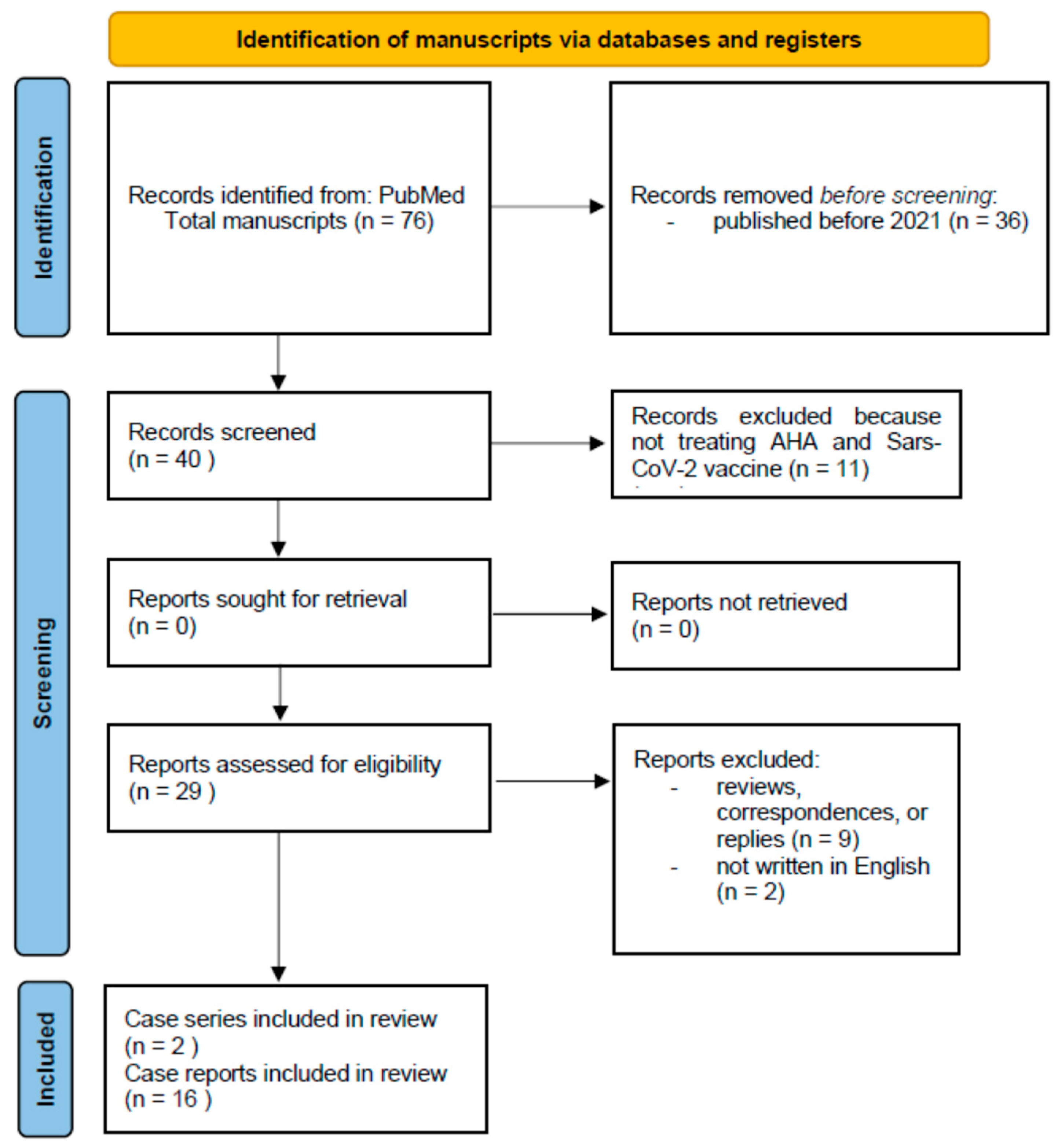
6. Methods
7. Results
8. Discussion
9. Limitations
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delgado, J.; Jimenez-Yuste, V.; Hernandez-Navarro, F.; Villar, A. Acquired hemophilia: Review and meta-analysis focused on therapy and prognostic factors. Br. J. Haematol. 2003, 121, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.E.; Ludlam, C.A. Acquired haemophilia and its management. Br. J. Haematol. 1995, 89, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Camacho, R.; Álvarez Román, M.T.; Butta Coll, N.; Zagrean, D.; Rivas Pollmar, I.; Martín Salces, M.; Gasior Kabat, M.; Jiménez-Yuste, V. Acquired Haemophilia A: A 15-Year Single-Centre Experience of Demography, Clinical Features and Outcome. J. Clin. Med. 2022, 11, 2721. [Google Scholar] [CrossRef] [PubMed]
- Pishko, A.M.; Doshi, B.S. Acquired Hemophilia A: Current Guidance and Experience from Clinical Practice. J. Blood Med. 2022, 13, 255–265. [Google Scholar] [CrossRef]
- Tiede, A.; Zieger, B.; Lisman, T. Acquired bleeding disorders. Haemophilia 2022, 28 (Suppl. S4), 68–76. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.Z.; Anwer, F. Acquired Hemophilia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sallah, S.; Nguyen, N.P.; Abdallah, J.M.; Hanrahan, L.R. Acquired hemophilia in patients with hematologic malignancies. Arch. Pathol. Lab. Med. 2000, 124, 730–734. [Google Scholar] [CrossRef]
- Castelli, R.; Faricciotti, A.; Cicuti, S.; Franceschini, F.; Vismara, A.; Porro, T. Acquired factor VIII inhibitor in association with myelodisplastic syndrome: Report of a new case. Haematologica 2002, 87, ECR02. [Google Scholar] [PubMed]
- Green, D.; Lechner, K. A survey of 215 non-hemophilic patients with inhibitors to Factor VIII. Thromb. Haemost. 1981, 45, 200–203. [Google Scholar] [CrossRef]
- Collins, P.W.; Hirsch, S.; Baglin, T.P.; Dolan, G.; Hanley, J.; Makris, M.; Keeling, D.; Liesner, R.; Brown, S.A.; Hay, C.R.M.; et al. Acquired hemophilia A in the United Kingdom: A 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2006, 109, 1870–1877. [Google Scholar] [CrossRef]
- Knoebl, P.; Marco, P.; Baudo, F.; Collins, P.; Huth-Kühne, A.; Nemes, L.; Pellegrini, F.; Tengborn, L.; Lévesque, H. Demographic and clinical data in acquired hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). J. Thromb. Haemost. 2012, 10, 622–631. [Google Scholar] [CrossRef]
- Prescott, R.; Nakai, H.; Saenko, E.L.; Scharrer, I.; Nilsson, I.M.; Humphries, J.E.; Hurst, D.; Bray, G.; Scandella, D. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood 1997, 89, 3663–3671. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Balog, A.; Constantin, T.; Czirják, L.; Géher, P.; Kovács, L.; Kumánovics, G.; Nagy, G.; Rákóczi, É.; Szamosi, S.; et al. COVID-19: Autoimmunity, multisystemic inflammation and autoimmune rheumatic patients. Expert Rev. Mol. Med. 2022, 24, e13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussein, H.M.; Rahal, E.A. The role of viral infections in the development of autoimmune diseases. Crit. Rev. Microbiol. 2019, 45, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Shoenfeld, Y. On the molecular determinants of the SARS-CoV-2 attack. Clin. Immunol. 2020, 215, 108426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vallianatos, C.N.; Iwase, S. Disrupted intricacy of histone H3K4 methylation in neurodevelopmental disorders. Epigenomics 2015, 7, 503–519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ponchel, F.; Cuthbert, R.J.; Goëb, V. IL-7 and lymphopenia. Clin. Chim. Acta 2011, 412, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Samartin, F.; Salvi, E.; Brambilla, A.M.; Torre, A.; Ingrassia, S.; Gidaro, A. Incidence and outcome of delirium during helmet CPAP treatment in COVID-19 patients. Intern. Emerg. Med. 2022, 17, 307–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl. Sci. 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zinserling, V.A.; Semenova, N.Y.; Markov, A.G.; Rybalchenko, O.V.; Wang, J.; Rodionov, R.N.; Bornstein, S.R. Inflammatory Cell Infiltration of Adrenals in COVID-19. Horm. Metab. Res. 2020, 52, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T., Jr. COVID-19, Myocarditis and Pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patone, M.; Handunnetthi, L.; Saatci, D.; Pan, J.; Katikireddi, S.V.; Razvi, S.; Hunt, D.; Mei, X.W.; Dixon, S.; Zaccardi, F.; et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021, 27, 2144–2153, Erratum in Nat. Med. 2021, 27, 2249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arepally, G.M.; Ortel, T.L. Vaccine-induced immune thrombotic thrombocytopenia: What we know and do not know. Blood 2021, 138, 293–298, Erratum in Blood 2023, 141, 808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.Y.; Sun, M.X.; Hua, M.Q.; Zhang, H.X.; Mu, G.Y.; Zhou, S.; Wang, Z.; Xiang, Q.; Cui, Y.M. New perspectives on the induction and acceleration of immune-associated thrombosis by PF4 and VWF. Front. Immunol. 2023, 14, 1098665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Radwi, M.; Farsi, M. A case report of acquired hemophilia following COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Cittone, M.G.; Battegay, R.; Condoluci, A.; Terzi di Bergamo, L.; Fernandes, E.; Galfetti, E.; Noseda, R.; Leuppi-Taegtmeyer, A.; Drexler, B.; Ceschi, A.; et al. The statistical risk of diagnosing coincidental acquired hemophilia A following anti-SARS-CoV-2 vaccination. J. Thromb. Haemost. 2021, 19, 2360–2362. [Google Scholar] [CrossRef] [PubMed]
- Farley, S.; Ousley, R.; Van Wagoner, N.; Bril, F. Autoimmunity after coronavirus disease 2019 (COVID-19) vaccine: A case of acquired hemophilia A. Thromb. Haemost. 2021, 121, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, C.; Giacobbe, A.G.; Bonifacino, E.; Karapetyan, L.; Seaman, C. A case of acquired haemophilia A in a 70-year-old post COVID-19 vaccine. Haemophilia 2022, 28, e15–e17. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.C.; Canovi, S.; Pilia, A.; Casali, A.; Depietri, L.; Fasano, T.; Colla, R.; Ghirarduzzi, A. Four cases of acquired hemophilia A following immunization with mRNA BNT162b2 SARS-CoV-2 vaccine. Thromb. Res. 2022, 211, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Wong, P.; Gilbar, P.J.; Mangos, H.M. Acquired hemophilia A following Pfizer-BioNTech SARS CoV-2 mRNA vaccine, successfully treated with prednisolone and rituximab. J. Oncol. Pharm. Pract. 2022, 28, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.S.; Al Battah, A.; Al Faridi, D.; Ibrahim, F. Acquired hemophilia A developed post COVID-19 vaccine: An extremely rare complication. J. Med. Cases 2022, 13, 1–4. [Google Scholar] [PubMed]
- Vuen, L.A.; Aun Su-Yin, E.; Naila Kori, A.; Shah, T.M. Case of acquired haemophilia a in Southeast Asia following COVID-19 vaccine. BMJ Case Rep. 2022, 15, e246922. [Google Scholar] [CrossRef] [PubMed]
- Al Hennawi, H.; Al Masri, M.K.; Bakir, M.; Barazi, M.; Jazaeri, F.; Almasri, T.N.; Shoura, S.J.; Barakeh, A.R.R.; Taftafa, A.; Khan, M.K.; et al. Acquired hemophilia A post-COVID-19 vaccination: A case report and review. Cureus 2022, 14, e21909. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.A.; Chen, C.W.; Hsu, Y.T.; Wei, K.C.; Lin, P.C.; Chen, T.Y. A case of acquired hemophilia A and bullous pemphigoid following SARS-CoV-2 mRNA vaccination. J. Formos. Med. Assoc. 2022, 121, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Plüß, M.; Mitteldorf, C.; Szuszies, C.J.; Tampe, B. Case Report: Acquired haemophilia A following mRNA-1273 Booster vaccination against SARS-CoV-2 with concurrent diagnosis of pleomorphic dermal sarcoma. Front. Immunol. 2022, 13, 868133. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Khan, Z.; Alam, J. Acquired hemophilia A with SARS-CoV-2 mRNA vaccine: First case from Pakistan. Scand. J. Clin. Lab. Investig. 2022, 82, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Melmed, A.; Kovoor, A.; Flippo, K. Acquired hemophilia A after vaccination against SARS-CoV-2 with the mRNA-1273 (Moderna) vaccine. Bayl. Univ. Med. Cent. Proc. 2022, 35, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, H.; Tane, M.; Kosako, H.; Ibe, M.; Takeyama, M.; Murata, S.; Mushino, T.; Sonoki, T. Acute-type acquired hemophilia A after COVID-19 mRNA vaccine administration: A new disease entity? J. Autoimmun. 2022, 133, 102915. [Google Scholar] [CrossRef] [PubMed]
- Duminuco, A.; Calagna, M.; Markovic, U.; Esposito, B.; Grasso, S.; Riccobene, C.; Di Raimondo, F.; Giuffrida, G. Acquired hemophilia A following COVID-19 vaccination—The importance of prompt diagnosis: A case report. Transfus. Apher. Sci. 2023, 62, 103577. [Google Scholar] [CrossRef] [PubMed]
- Happaerts, M.; Vanassche, T. Acquired hemophilia following COVID-19 vaccination: Case report and review of literature. Res. Pract. Thromb. Haemost. 2022, 6, e12785. [Google Scholar] [CrossRef] [PubMed]
- Zanon, E.; Pasca, S.; Santoro, C.; Gamba, G.; Siragusa, S.M.; Rocino, A.; Cantori, I.; Federici, A.B.; Mameli, L.; Giuffrida, G.; et al. Activated prothrombin complex concentrate (FEIBA®) in acquired hemophilia A: A large multicentre Italian study—The FAIR registry. Br. J. Haematol. 2019, 184, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Focosi, D. Association between SARS-CoV-2 infection or vaccination and acquired hemophilia A: A case report and literature update. Thromb. Res. 2023, 222, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Emna, B.; Kmira, Z.; Hajer, B.I.; Nadia, S.; Yossra, D.; Amina, B.; Yosra, B.Y.; Haifa, R.; Abderrahim, K. Acquired hemophilia A following COVID-19 vaccine: A case report. J. Med. Case Rep. 2023, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Reding, M.T.; Wu, H.; Krampf, M.; Okita, D.K.; Diethelm-Okita, B.M.; Key, N.S.; Conti-Fine, B.M. CD4+ T response to factor VIII in haemophilia A, acquired haemophilia and Healthy subjects. Thromb. Haemost. 1999, 82, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Mingot-Castellano, M.E.; Núñez, R.; Rodríguez-Martorell, F.J. Acquired haemophilia: Epidemiology, clinical presentation, diagnosis and treatment. Med. Clin. 2017, 148, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef] [PubMed]
- Kruse-Jarres, R.; Kempton, C.L.; Baudo, F.; Collins, P.W.; Knoebl, P.; Leissinger, C.A.; Tiede, A.; Kessler, C.M. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am. J. Hematol. 2017, 92, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Zanon, E.; Mannucci, P.M.; Peyvandi, F. Emicizumab in acquired hemophilia A: Pros and cons of a new approach to the prevention and treatment of bleeding. Blood Transfus. 2023, 21, 549–556. [Google Scholar]
- Baudo, F.; Collins, P.; Huth-Kühne, A.; Lévesque, H.; Marco, P.; Nemes, L.; Pellegrini, F.; Tengborn, L.; Knoebl, P.; EACH2 registry contributors. Management of bleeding in acquired hemophilia A: Results from the European Acquired Haemophilia (EACH2) Registry. Blood 2012, 120, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.W.; Baudo, F.; Knoebl, P.; Lévesque, H.; Nemes, L.; Pellegrini, F.; Marco, P.; Tengborn, L.; Huth-Kühne, A.; EACH2 registry collaborators. Immunosuppression for acquired hemophiliaA: Results of the European Acquired Haemophilia (EACH) registry. Blood 2012, 120, 47–55. [Google Scholar] [CrossRef]
- Marumo, A.; Sugihara, H.; Omori, I.; Morishita, E. Relapse of Acquired Hemophilia A after COVID-19 Infection. J. Nippon. Med. Sch. 2024, 90, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Gidaro, A.; Palmieri, G.; Donadoni, M.; Mameli, L.A.; La Cava, L.; Sanna, G.; Castro, D.; Delitala, A.P.; Manetti, R.; Castelli, R. A diagnostic of acquired hemophilia following PD1/PDL1 inhibitors in advanced melanoma: The experience of two patients and a literature review. Diagnostics 2022, 12, 2559. [Google Scholar] [CrossRef] [PubMed]
| Diseases or Clinical Conditions | Characteristics |
|---|---|
| Oncologic diseases | Multiple myeloma, lymphomas, monoclonal gammopathy of uncertain significance (MGUS), myelofibrosis, myelodysplasia |
| Rheumatic diseases | Rheumatoid arthritis, systemic lupus erythematosus, Sjogren’s syndrome, Goodpasture’s syndrome, temporal arteritis, myasthenia gravis, thyroiditis, multiple sclerosis |
| Infectious diseases | SARS-CoV-2, Epstein Barr virus, hepatitis B/C viruses, Human Immunodeficiency virus |
| Dermatological diseases | Psoriasis, pemphigus |
| Pregnancy or Puerperium | Within 1-4 months of delivery or miscarriage |
| Drugs | Some beta-lactam antibiotics, chloramphenicol, sulfonamides, clopidogrel, nonsteroidal anti-inflammatory drugs (NSAIDs), fludarabine, interferon alpha |
| Other diseases | Asthma, chronic obstructive pulmonary disease, acute hepatitis |
| Ref. | Author(s), Year | Patient(s) (Sex; Age) | Vaccine Type | Dose(s) n | Days after Vaccination | Clinical Manifestations | Concomitant Diseases | Acute Treatments | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| [26] | Radwi & Farsi, 2021 | 1 (M; 69yrs) | BNT162b2 (Pfizer-BioNTech) | 2 | 9 | Spontaneous bruises on arms and legs | Prostate adenocarcinoma, DM2, hypertension | CS | Resolved without sequelae |
| [27] | Cittone et al., 2021 | 1 (M; 85 yrs) 1 (F; 86yrs) 1 (F; 72yrs) | mRNA-1273 (Moderna) mRNA-1273 (Moderna) mRNA-1273 (Moderna | 2 2 1 | Immediately after 2nd dose 21 10 | Multiple hematomas on the right thigh, joint bleeding (knees) Traumatic hemothorax Table Extensive spontaneous cutaneous bruising | Peripheral artery disease, coronary bypass Aortic valve stenosis Arterial disease | rFVIIa, aPCC, CS, RTX rFVIIa, aPCC, CS rFVIIa, TXA, CS, RTX | Active arterial bleeding after gall rupture, death Resolved without sequelae NA |
| [28] | Farley et al., 2021 | 1 (M; 67yrs) | BNT162b2 (Pfizer-BioNTech) | 2 | 21 | Large hematoma posterior left leg | Asymptomatic pulmonary sarcoidosis, hypertension | aPCC, rFVIIa, CS, RTX | Resolved without sequelae |
| [29] | Lemoine et al., 2022 | 1 (M; 70yrs) | mRNA-1273 (Moderna) | 1 | 8 | Extensive right upper limb bruising | Rheumatic polymyalgia, previous HCV infection | aPCC, rFVIIa, CS, Cyc | Resolved without sequelae |
| [30] | Leone et al., 2022 | 1 (M; 86yrs) 1 (F; 73yrs) 1 (M; 67yrs) 1 (M; 77yrs) | BNT162b2 (Pfizer-BioNTech) BNT162b2 (Pfizer-BioNTech) BNT162b2 (Pfizer-BioNTech) BNT162b2 (Pfizer-BioNTech) | 2 2 2 2 | 14 26 49 52 | Disseminated hematomas, severe anemia Spontaneous tongue, jaw, and right knee hematomas Tongue hematoma Hematuria, severe anemia | Rheumatic polymyalgia Rheumatoid arthritis, Sjogren syndrome None Bladder cancer relapse | RBC, CS CS rFVIIa, CS, Cyc rFVIIa, CS, RTX | Resolved without sequelae Resolved without sequelae Resolved without sequelae Sepsis, respiratory complication, death |
| [31] | Murali et al., 2022 | 1 (F; 95yrs) | BNT162b2 (Pfizer-BioNTech) | 2 | 21 | Spontaneous bruising on limbs | Dementia, hypertension, depression, congestive cardiac failure, previous breast cancer | RBC, rFVIII, CS, RTX | Resolved without sequelae |
| [32] | Soliman et al., 2022 | 1 (F; 39yrs) | BNT162b2 (Pfizer-BioNTech) | 1 | 10 | Hematuria | None | CS, RTX | Resolved without sequelae |
| [33] | Vuen et al., 2022 | 1 (M; 80yrs) | BNT162b2 (Pfizer-BioNTech) | 1 | 14 | Ecchymosis at limbs, severe anemia | DM2, hypertension, dyslipidemia, chronic kidney disease, glaucoma in both eyes, ischemic stroke | rFVIIa, TXA, CS | Resolved without sequelae |
| [34] | Al Hennawi et al., 2022 | 1 (M; 75yrs) | BNT162b2 (Pfizer-BioNTech) | 2 | 90 | Soft tissue ecchymoses, compartment syndrome, anemia | Hypertension, dyslipidemia, coronary artery disease | rFVIIA, DDAVP, CS, RTX | Resolved without sequelae |
| [35] | Fu et al., 2022 | 1 (M; 77yrs) | mRNA-1273 (Moderna) | 2 | 21 | Bilateral legs, feet, and ankles ecchymosis | NA | aPCC, rFVIIa, CS, Cyc | Bullous pemphigoid |
| [36] | Plüß et al., 2022 | 1 (M; 72yrs) | BNT162b2 (Pfizer-BioNTech) | Booster | 9 | Arms, left leg, and trunk bruises | BPH, carpal tunnel syndrome | rFVIIa, CS, Cyc, RTX | Pleomorphic dermal sarcoma |
| [37] | Rashid et al., 2022 | 1 (M; 75yrs) | BNT162b2 (Pfizer-BioNTech) | Booster | 6 | Both thighs and back bruises | DM2 | RBC, rFVIIa, CS, Cyc | Resolved without sequelae |
| [38] | Melmed et al., 2022 | 1 (F; 61yrs) | mRNA-1273 (Moderna) | 2 | 14 | Inner thigh bruising, anemia with dyspnea | Rheumatoid arthritis | RBC, FVIII/vWF | Gastrointestinal bleeding |
| [39] | Hosoi et al., 2022 | 1 (F; 45yrs) | mRNA-1273 (Moderna) 1st and 2nd doses Pfizer BioNTech | Booster | 14 | Subcutaneous hemorrhage | None | CS | Resolved without sequelae |
| [40] | Duminuco et al., 2023 | 1 (M; 71yrs) | BNT162b2 (Pfizer-BioNTech) | 2 | 8 | Hemothorax and hemoperitoneum in the abdomen, extending from the pelvic area to the right thigh | None | RBC, rFVIIa, CS, Cyc | Resolved without sequelae |
| [41] | Happaerts & Vanassche, 2022 | 1 (M; 75yrs) | Vaxzevria ChAdOx1 (AstraZeneca) | 1 | 10 | Multiple hematomas, hemorrhagic bullous pemphigoid, gastrointestinal ulcer | Chronic kidney disease, hypertension, DM2, polyneuropathy, chronic foot ulcer | rFVIIa, Emicizumab, RTX, CS | Atrial fibrillation, acute kidney injury, and methicillin-sensitive Staphylococcus aureus sepsis, death |
| [43] | Franchini & Focosi, 2023 | 1 (M; 67yrs) | mRNA-1273 (Moderna) | 1 | 22 | Anemia | Rheumatoid arthritis, pulmonary sarcoidosis | rFVIIa, RTX | Liposarcoma, death |
| [44] | Emna et al., 2023 | 1 (M; 69yrs) | CoronaVac (SinoVac) | 2 | 30 | Left inner thigh ecchymosis | None | RBC, TXA, CS | Resolved without sequelae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelli, R.; Gidaro, A.; Manetti, R.; Castiglia, P.; Delitala, A.P.; Mannucci, P.M.; Pasca, S. Acquired Hemophilia A after SARS-CoV-2 Immunization: A Narrative Review of a Rare Side Effect. Vaccines 2024, 12, 709. https://doi.org/10.3390/vaccines12070709
Castelli R, Gidaro A, Manetti R, Castiglia P, Delitala AP, Mannucci PM, Pasca S. Acquired Hemophilia A after SARS-CoV-2 Immunization: A Narrative Review of a Rare Side Effect. Vaccines. 2024; 12(7):709. https://doi.org/10.3390/vaccines12070709
Chicago/Turabian StyleCastelli, Roberto, Antonio Gidaro, Roberto Manetti, Paolo Castiglia, Alessandro Palmerio Delitala, Pier Mannuccio Mannucci, and Samantha Pasca. 2024. "Acquired Hemophilia A after SARS-CoV-2 Immunization: A Narrative Review of a Rare Side Effect" Vaccines 12, no. 7: 709. https://doi.org/10.3390/vaccines12070709
APA StyleCastelli, R., Gidaro, A., Manetti, R., Castiglia, P., Delitala, A. P., Mannucci, P. M., & Pasca, S. (2024). Acquired Hemophilia A after SARS-CoV-2 Immunization: A Narrative Review of a Rare Side Effect. Vaccines, 12(7), 709. https://doi.org/10.3390/vaccines12070709









