Deciphering the Potential of Probiotics in Vaccines
Abstract
:1. Introduction
2. Brief Background of Probiotics
3. The Impact of Probiotics on Enhancing Vaccine Effectiveness
4. Probiotic-Based Vaccines in Animal Models
4.1. Probiotics in Vaccines
4.2. Probiotic-Based Vaccine Response in Newborns
4.3. Probiotic-Based Vaccine Response in Adults
5. Probiotics and Their Function in Different Vaccine Categories
5.1. Probiotics Improve the Immune System’s Cellular and Humoral Responses
5.2. Probiotics Enhance the Level of Antibodies
5.3. Probiotics Proliferate Immunocytes
5.4. Probiotics Increase the Production of Cytokines
5.5. Probiotics and Cell-Mediated Immunity
5.6. Probiotic-Based Bacterial Ghost Vaccination
5.6.1. DNA Vaccines
5.6.2. Protein Antigen Vaccines
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocourkova, A.; Honegr, J.; Kuca, K.; Danova, J. Vaccine ingredients: Components that influence vaccine efficacy. Mini-Rev. Med. Chem. 2017, 17, 451–466. [Google Scholar] [CrossRef]
- Carpenter, T.E. Evaluation of effectiveness of a vaccination program against an infectious disease at the population level. Am. J. Vet. Res. 2001, 62, 202–205. [Google Scholar] [CrossRef]
- FAO; WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. In Cordoba, Argentina: Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Rønhave Laursen, R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus docu-ment: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appro-priate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Metzger, R.N.; Krug, A.B.; Eisenächer, K. Enteric virome sensing—Its role in intestinal homeostasis and immunity. Viruses 2018, 10, 146. [Google Scholar] [CrossRef]
- Abd El-Gawad, I.A.; El-Sayed, E.M.; El-Zeini, H.M.; Hafez, S.A.; Saleh, F.A. Antibacterial activity of probiotic yoghurt and soy-yoghurt against Escherichia coli and Staphylococcus aureus. J. Nutr. Food Sci. 2014, 4, 1000303. [Google Scholar]
- Bisanz, J.E.; Macklaim, J.M.; Gloor, G.B.; Reid, G. Bacterial metatranscriptome analysis of a probiotic yogurt using an RNA-Seq approach. Int. Dairy J. 2014, 39, 284–292. [Google Scholar] [CrossRef]
- Moineau-Jean, A.; Champagne, C.P.; Roy, D.; Raymond, Y.; LaPointe, G. Effect of Greek-style yoghurt manufacturing processes on starter and probiotic bacteria populations during storage. Int. Dairy J. 2019, 93, 35–44. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics in 2015: Their scope and use. J. Clin. Gastroenterol. 2015, 49, S2–S6. [Google Scholar] [CrossRef]
- Ojha, A.K.; Shah, N.P.; Mishra, V. Conjugal Transfer of Antibiotic Resistances in Lactobacillus spp. Curr. Microbiol. 2021, 78, 2839–2849. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by Lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef]
- Lei, W.T.; Shih, P.C.; Liu, S.J.; Lin, C.Y.; Yeh, T.L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef]
- Makioka, Y.; Tsukahara, T.; Ijichi, T.; Inoue, R. Oral supplementation of Bifidobacterium longum strain BR-108 alters cecal microbiota by stimulating gut immune system in mice irrespectively of viability. Biosci. Biotechnol. Biochem. 2018, 82, 1180–1187. [Google Scholar] [CrossRef]
- Wu, B.-B.; Yang, Y.; Xu, X.; Wang, W.-P. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J. Pediatr. 2016, 12, 177–182. [Google Scholar] [CrossRef]
- Soh, S.E.; Ong, D.Q.R.; Gerez, I.; Zhang, X.; Chollate, P.; Shek, L.P.-C.; Lee, B.W.; Aw, M. Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine 2010, 28, 2577–2579. [Google Scholar] [CrossRef]
- Przemska-Kosicka, A.; Childs, C.E.; Enani, S.; Maidens, C.; Dong, H.; Dayel, I.B.; Bin Dayel, I.; Tuohy, K.; Todd, S.; Gosney, M.A.; et al. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun. Ageing 2016, 13, 6. [Google Scholar] [CrossRef]
- Youngster, I.; Kozer, E.; Lazarovitch, Z.; Broide, E.; Goldman, M. Probiotics and the immunological response to infant vaccinations: A prospective, placebo controlled pilot study. Arch. Dis. Child. 2011, 96, 345–349. [Google Scholar] [CrossRef]
- Arsène, M.M.J.; Davares, A.K.L.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319–328. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Taha-Abdelaziz, K.; Nagy, É.; Bridle, B.; Sharif, S. Probiotic lactobacilli enhance immunogenicity of an inactivated H9N2 influenza virus vaccine in chickens. Viral Immunol. 2021, 34, 86–95. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals 2019, 10, 70. [Google Scholar] [CrossRef]
- Michael, H.; Paim, F.C.; Langel, S.N.; Miyazaki, A.; Fischer, D.D.; Chepngeno, J.; Amimo, J.; Deblais, L.; Rajashekara, G.; Saif, L.J.; et al. Escherichia coli Nissle 1917 enhances innate and adaptive immune responses in a ciprofloxacin-treated defined-microbiota piglet model of human rotavirus infection. mSphere 2021, 6, e00074-21. [Google Scholar] [CrossRef]
- Khor, C.S.; Tsuji, R.; Lee, H.Y.; Nor’e, S.S.; Sahimin, N.; Azman, A.S.; Tiong, V.; Hasandarvish, P.; Teoh, B.T.; Soh, Y.H.; et al. Lactococcus lactis strain plasma intake suppresses the incidence of dengue fever-like symptoms in healthy Malaysians: A randomized, double-blind, placebo-controlled trial. Nutrients 2021, 13, 4507. [Google Scholar] [CrossRef]
- Kazemifard, N.; Dehkohneh, A.; Baradaran Ghavami, S. Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy. Front. Med. 2022, 9, 940454. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ismail, I.H.; Balloch, A.; Mui, M.; Hoe, E.; Lamb, K.; Tang, M.L.K. Maternal supplementation with LGG reduces vaccine-specific immune responses in infants at high-risk of developing allergic disease. Front. Immunol. 2013, 4, 381. [Google Scholar] [CrossRef]
- Jespersen, L.; Tarnow, I.; Eskesen, D.; Morberg, C.M.; Michelsen, B.; Bügel, S.; Dragsted, L.O.; Rijkers, G.T.; Calder, P.C. Effect of Lactobacillus paracasei subsp. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: A randomized, double-blind, placebo-controlled, parallel-group study. Am. J. Clin. Nutr. 2015, 101, 1188–1196. [Google Scholar] [CrossRef]
- Akatsu, H.; Arakawa, K.; Yamamoto, T.; Kanematsu, T.; Matsukawa, N.; Ohara, H.; Maruyama, M. Lactobacillus in Jelly Enhances the Effect of Influenza Vaccination in Elderly Individuals. J. Am. Geriatr. Soc. 2013, 61, 1828–1830. [Google Scholar] [CrossRef]
- Xu, J.; Ren, Z.; Cao, K.; Li, X.; Yang, J.; Luo, X.; Zhu, L.; Wang, X.; Ding, L.; Liang, J.; et al. Boosting vaccine-elicited respiratory mucosal and systemic COVID-19 immunity in mice with the oral Lactobacillus plantarum. Front. Nutr. 2021, 8, 789242. [Google Scholar] [CrossRef]
- Rather, I.A.; Choi, S.B.; Kamli, M.R.; Hakeem, K.R.; Sabir, J.S.M.; Park, Y.H.; Hor, Y.-Y. Potential adjuvant therapeutic effect of Lactobacillus plantarum probio-88 postbiotics against SARS-COV-2. Vaccines 2021, 9, 1067. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Alizadeh, M.; Astill, J.; Alqazlan, N.; Matsuyama-Kato, A.; Shojadoost, B.; Taha-Abdelaziz, K.; Sharif, S. Effects of administration of probiotic lactobacilli on immunity conferred by the herpesvirus of turkeys vaccine against challenge with a very virulent Marek’s disease virus in chickens. Vaccine 2021, 39, 2424–2433. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Hu, S.-Y.; Santos, H.M.; Catulin, G.E.M.; Tayo, L.L.; Chuang, K.P. Probiotic supplementation containing Bacillus velezensis enhances expression of immune regulatory genes against pigeon circovirus in pigeons (Columba livia). J. Appl. Microbiol. 2021, 130, 1695–1704. [Google Scholar] [CrossRef]
- Vasiee, A.; Falah, F.; Sankian, M.; Tabatabaei-Yazdi, F.; Mortazavi, S.A. Oral immunotherapy using probiotic ice cream containing recombinant food-grade Lactococcus lactis which inhibited allergic responses in a BALB/c mouse model. J. Immunol. Res. 2020, 2020, 2635230. [Google Scholar] [CrossRef]
- Lépine, A.F.P.; Konstanti, P.; Borewicz, K.; Resink, J.W.; de Wit, N.J.; de Vos, P.; Smidt, H.; Mes, J.J. Combined dietary supplementation of long chain inulin and Lactobacillus acidophilus W37 supports oral vaccination efficacy against Salmonella Typhimurium in piglets. Sci. Rep. 2019, 9, 18017. [Google Scholar] [CrossRef]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-life intervention using fecal microbiota combined with probiotics promotes gut microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef]
- Santos, F.D.S.; Ferreira, M.R.A.; Maubrigades, L.R.; Gonçalves, V.S.; de Lara, A.P.S.; Moreira, C.; Salvarani, F.M.; Conceição, F.R.; Leivas Leite, F.P. Bacillus toyonensis BCT-7112T transient supplementation improves vaccine efficacy in ewes vaccinated against Clostridium perfringens epsilon toxin. J. Appl. Microbiol. 2021, 130, 699–706. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of regulatory dendritic cells and CD4+ Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef]
- Pinto, M.G.V.; Gómez, M.R.; Seifert, S.; Watzl, B.; Holzapfel, W.H.; Franz, C.M. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2009, 133, 86–93. [Google Scholar] [CrossRef]
- Abreu, M.T.; Fukata, M.; Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 2005, 174, 4453–4460. [Google Scholar] [CrossRef]
- Castillo, N.A.; Perdigón, G.; de Moreno de LeBlanc, A. Oral administration of a probiotic Lactobacillus modulates cytokine production and TLR expression improving the immune response against Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2011, 11, 177. [Google Scholar] [CrossRef]
- Pang, I.K.; Iwasaki, A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 2012, 245, 209–226. [Google Scholar] [CrossRef]
- Parada Venegas, D.; de la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 424615. [Google Scholar]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- McNeil, N.I.; Cummings, J.H.; James, W.P. Short chain fatty acid absorption by the human large intestine. Gut 1978, 19, 819–822. [Google Scholar]
- Trend, S.; Leffler, J.; Jones, A.P.; Cha, L.; Gorman, S.; Brown, D.A.; Breit, S.N.; Kermode, A.G.; French, M.A.; Ward, N.C.; et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci. Rep. 2021, 11, 5244. [Google Scholar] [CrossRef]
- Neish, A.S.; Gewirtz, A.T.; Zeng, H.; Young, A.N.; Hobert, M.E.; Karmali, V.; Rao, A.S.; Madara, J.L. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 2000, 289, 1560–1563. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.-Q.; Jones, D.P.; Neish, A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef]
- Lin, P.W.; Myers, L.E.; Ray, L.; Song, S.C.; Nasr, T.R.; Berardinelli, A.J.; Kundu, K.; Murthy, N.; Hansen, J.M.; Neish, A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free. Radic. Biol. Med. 2009, 47, 1205–1211. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Caicedo, R.; Neu, J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α–induced interleukin-8 production in caco-2 cells. J. Nutr. 2005, 135, 1752–1756. [Google Scholar] [CrossRef]
- Tien, M.-T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.-A.; Coppée, J.-Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 176, 1228–1237. [Google Scholar] [CrossRef]
- Petrof, E.O.; Claud, E.C.; Sun, J.; Abramova, T.; Guo, Y.; Waypa, T.S.; He, S.M.; Nakagawa, Y.; Chang, E.B. Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm. Bowel Dis. 2009, 15, 1537–1547. [Google Scholar] [CrossRef]
- Petrof, E.O.; Kojima, K.; Ropeleski, M.J.; Musch, M.W.; Tao, Y.; De Simone, C.; Chang, E.B. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 2004, 127, 1474–1487. [Google Scholar] [CrossRef]
- Frick, J.S.; Schenk, K.; Quitadamo, M.; Kahl, F.; Köberle, M.; Bohn, E.; Aepfelbacher, M.; Autenrieth, I.B. Lactobacillus fermentum attenuates the proinflammatory effect of Yersinia enterocolitica on human epithelial cells. Inflamm. Bowel Dis. 2007, 13, 83–90. [Google Scholar] [CrossRef]
- Bai, A.-P.; Ouyang, Q.; Zhang, W.; Wang, C.-H.; Li, S.-F. Probiotics inhibit TNF-α-induced interleukin-8 secretion of HT29 cells. World J. Gastroenterol. 2004, 10, 455. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Wu, G.D.; Huang, N.; Wen, X.; Keilbaugh, S.A.; Yang, H. High-level expression of IκB-β in the surface epithelium of the colon: In vitro evidence for an immunomodulatory role. J. Leukoc. Biol. 1999, 66, 1049–1056. [Google Scholar] [CrossRef]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol.-Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar] [CrossRef]
- Kojima, K.; Musch, M.W.; Ren, H.; Boone, D.L.; Hendrickson, B.A.; Ma, A.; Chang, E.B. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 2003, 124, 1395–1407. [Google Scholar] [CrossRef]
- Pant, N.; Marcotte, H.; Brüssow, H.; Svensson, L.; Hammarström, L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007, 7, 86. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Tsitrsikos, P.; Palamidi, I.; Arvaniti, A.; Mohnl, M.; Schatzmayr, G.; Fegeros, K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010, 89, 58–67. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Narbad, A.; Gasson, M.J.; Woodward, M.J. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett. Appl. Microbiol. 2004, 38, 197–205. [Google Scholar] [CrossRef]
- Taras, D.; Vahjen, W.; Macha, M.; Simon, O.; Performance, S.A. Influence of a probiotic Enterococcus faecium strain on development of the immune system of sows and piglets. Vet. Immunol. Immunopathol. 2006, 113, 159–166. [Google Scholar]
- Paineau, D.; Carcano, D.; Leyer, G.; Darquy, S.; Alyanakian, M.-A.; Simoneau, G.; Bergmann, J.-F.; Brassart, D.; Bornet, F.; Ouwehand, A.C. Effects of seven potential probiotic strains on specific immune responses in healthy adults: A double-blind, randomized, controlled trial. FEMS Immunol. Med. Microbiol. 2008, 53, 107–113. [Google Scholar] [CrossRef]
- Luiz, W.B.; Cavalcante, R.C.; Paccez, J.; Souza, R.D.; Sbrogio-Almeida, M.E.; Ferreira, R.C.; Ferreira, L.C. Boosting systemic and secreted antibody responses in mice orally immunized with recombinant Bacillus subtilis strains following parenteral priming with a DNA vaccine encoding the enterotoxigenic Escherichia coli (ETEC) CFA/I fimbriae B subunit. Vaccine 2008, 26, 3998–4005. [Google Scholar] [CrossRef]
- Lee, S.F.; Halperin, S.; Wang, H.; MacArthur, A. Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice. FEMS Microbiol. Lett. 2002, 208, 175–178. [Google Scholar] [CrossRef]
- Perdigon, G.; Alvarez, S.; Rachid, M.; Agüero, G.; Gobbato, N. Immune system stimulation by probiotics. J. Dairy Sci. 1995, 78, 1597–1606. [Google Scholar] [CrossRef]
- Perdigón, G.; Alvarez, S.; de Ruiz Holgado, A.P. Immunoadjuvant activity of oral Lactobacillus casei: Influence of dose on the secretory immune response and protective capacity in intestinal infections. J. Dairy Res. 1991, 58, 485–496. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Yamazaki, R.; Hashimoto, S.; Yokokura, T. The effect of oral feeding of Lactobacillus casei strain Shirota on immunoglobulin E production in mice. J. Dairy Sci. 1998, 81, 48–53. [Google Scholar] [CrossRef]
- Xu, Y.; Yuen, P.-W.; Lam, J.K.-W. Intranasal DNA vaccine for protection against respiratory infectious diseases: The delivery perspectives. Pharmaceutics 2014, 6, 378–415. [Google Scholar] [CrossRef]
- Rubio-Del-Campo, A.; Coll-Marqués, J.M.; Yebra, M.J.; Buesa, J.; Pérez-Martínez, G.; Monedero, V.; Rodríguez-Díaz, J. Noroviral P-particles as an in vitro model to assess the interactions of noroviruses with probiotics. PLoS ONE 2014, 9, e89586. [Google Scholar] [CrossRef]
- Kudela, P.; Paukner, S.; Mayr, U.B.; Cholujova, D.; Schwarczova, Z.; Sedlak, J.; Bizik, J.; Lubitz, W. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J. Immunother. 2005, 28, 136–143. [Google Scholar] [CrossRef]
- Buddenborg, C.; Daudel, D.; Liebrecht, S.; Greune, L.; Humberg, V.; Schmidt, M.A. Development of a tripartite vector system for live oral immunization using a gram-negative probiotic carrier. Int. J. Med. Microbiol. 2008, 298, 105–114. [Google Scholar] [CrossRef]
- Sahoo, A.; Mandal, A.K.; Dwivedi, K.; Kumar, V. A cross talk between the immunization and edible vaccine: Current challenges and future prospects. Life Sci. 2020, 261, 118343. [Google Scholar] [CrossRef]
- Sha, Z.; Shang, H.; Miao, Y.; Huang, J.; Niu, X.; Chen, R.; Hu, L.; Huang, H.; Wei, K.; Zhu, R. Recombinant Lactococcus Lactis expressing M1-HA2 fusion protein provides protective mucosal immunity against H9N2 avian influenza virus in chickens. Front. Vet. Sci. 2020, 7, 153. [Google Scholar] [CrossRef]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]
- Livingston, M.; Loach, D.; Wilson, M.; Tannock, G.W.; Baird, M. Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response. Immunol. Cell Biol. 2010, 88, 99–102. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rath, E.; Hölzlwimmer, G.; Quintanilla-Martinez, L.; Loach, D.; Tannock, G.; Haller, D. Lactobacillus reuteri 100-23 Transiently Activates Intestinal Epithelial Cells of Mice That Have a Complex Microbiota during Early Stages of Colonization13. J. Nutr. 2008, 138, 1684–1691. [Google Scholar] [CrossRef]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4645–4652. [Google Scholar] [CrossRef]
- Andre, F.E.; Booy, R.; Bock, H.L.; Clemens, J.; Datta, S.K.; John, T.J.; Lee, B.W.; Lolekha, S.; Peltola, H.; Ruff, T.A.; et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull. World Health Organ. 2008, 86, 140–146. [Google Scholar] [CrossRef]
- Pulendran, B. Learning immunology from the yellow fever vaccine: Innate immunity to systems vaccinology. Nat. Rev. Immunol. 2009, 9, 741–747. [Google Scholar] [CrossRef]
- Payton, T.; Girgenti, D.; Frenck, R.W.; Patterson, S.; Love, J.; Razmpour, A.; Sidhu, M.S.; Emini, E.A.; Gruber, W.C.; Scott, D.A. Immunogenicity, safety and tolerability of 3 lots of 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in the United States. Pediatr. Infect. Dis. J. 2013, 32, 871–880. [Google Scholar] [CrossRef]
- Dye, C. Making wider use of the world’s most widely used vaccine: Bacille Calmette–Guérin revaccination reconsidered. J. R. Soc. Interface 2013, 10, 20130365. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Antunes, L.C.M.; Finlay, B.B. Should the human microbiome be considered when developing vaccines? PLoS Pathog. 2010, 6, e1001190. [Google Scholar] [CrossRef]
- Jamieson, A.M. Influence of the microbiome on response to vaccination. Hum. Vaccines Immunother. 2015, 11, 2329–2331. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.-P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Land, M.H.; Rouster-Stevens, K.; Woods, C.R.; Cannon, M.L.; Cnota, J.; Shetty, A.K. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005, 115, 178–181. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Prabu, S.M.; et al. Probiotics: Versatile bioactive components in promoting human health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Nicaise, P.; Gleizes, A.; Forestier, F.; Quéro, A.M.; Labarre, C. Influence of intestinal bacterial flora on cytokine (IL-1, IL-6 and TNF-alpha) production by mouse peritoneal macrophages. Eur. Cytokine Netw. 1993, 4, 133–138. [Google Scholar]
- Stein, E.; Inic-Kanada, A.; Belij, S.; Montanaro, J.; Bintner, N.; Schlacher, S.; Mayr, U.B.; Lubitz, W.; Stojanovic, M.; Najdenski, H.; et al. In vitro and in vivo uptake study of Escherichia coli Nissle 1917 bacterial ghosts: Cell-based delivery system to target ocular surface diseases. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6326–6333. [Google Scholar] [CrossRef]
- Walcher, P.; Mayr, U.B.; Azimpour-Tabrizi, C.; Eko, F.O.; Jechlinger, W.; Mayrhofer, P.; Alefantis, T.; Mujer, C.V.; DelVecchio, V.G.; Lubitz, W. Antigen discovery and delivery of subunit vaccines by nonliving bacterial ghost vectors. Expert Rev. Vaccines 2004, 3, 681–691. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, H.; Chen, S.; Bai, Q.; Chen, X.; Chen, L.; Zeng, X.; Liu, L.; Chen, L. MOMP and MIP DNA-loaded bacterial ghosts reduce the severity of lung lesions in mice after Chlamydia psittaci respiratory tract infection. Immunobiology 2019, 224, 739–746. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Zheng, W.; Zhang, Q.; Zhao, D.; Li, G. Enhancement of immune responses by co-administration of bacterial ghosts-mediated Neisseria gonorrhoeae DNA vaccines. J. Appl. Microbiol. 2021, 130, 1770–1777. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Zhao, D.; Chen, J.; Zhang, Q.; Liang, J.; Yin, Y.; Kong, G.; Li, G. Design and immune characterization of a novel Neisseria gonorrhoeae DNA vaccine using bacterial ghosts as vector and adjuvant. Vaccine 2018, 36, 4532–4539. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.-C.; Liu, X.-Y.; Yuan, K.; Zhang, J.-J.; Gao, H.-H.; Li, J.-N. An oral double-targeted DNA vaccine induces systemic and intestinal mucosal immune responses and confers high protection against Vibrio mimicus in grass carps. Aquaculture 2019, 504, 248–259. [Google Scholar] [CrossRef]
- Tuntufye, H.N.; Ons, E.; Pham, A.D.N.; Luyten, T.; Van Gerven, N.; Bleyen, N.; Goddeeris, B.M. Escherichia coli ghosts or live E. coli expressing the ferri-siderophore receptors FepA, FhuE, IroN and IutA do not protect broiler chickens against avian pathogenic E. coli (APEC). Vet. Microbiol. 2012, 159, 470–478. [Google Scholar] [CrossRef]
- Gong, S.; Nan, N.; Sun, Y.; He, Z.; Li, J.; Chen, F.; Li, T.; Ning, N.; Wang, J.; Li, Z.; et al. Protective immunity elicited by VP1 chimeric antigens of bacterial ghosts against hand-foot-and-mouth disease virus. Vaccines 2020, 8, 61. [Google Scholar] [CrossRef]
- Riedmann, E.M.; Lubitz, W.; McGrath, J.; Kyd, J.M.; Cripps, A.W. Effectiveness of engineering the nontypeable Haemophilus influenzae antigen Omp26 as an S-layer fusion in bacterial ghosts as a mucosal vaccine delivery. Hum. Vaccines 2011, 7 (Suppl. S1), 99–107. [Google Scholar] [CrossRef]
- Sührer, I.; Langemann, T.; Lubitz, W.; Weuster-Botz, D.; Castiglione, K. A novel one-step expression and immobilization method for the production of biocatalytic preparations. Microb. Cell Factories 2015, 14, 180. [Google Scholar] [CrossRef]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The bacterial ghost platform system: Production and applications. Bioeng. Bugs 2010, 1, 326–336. [Google Scholar] [CrossRef]
- Paukner, S.; Kohl, G.; Jalava, K.; Lubitz, W. Sealed bacterial ghosts—Novel targeting vehicles for advanced drug delivery of water-soluble substances. J. Drug Target. 2003, 11, 151–161. [Google Scholar]
- Singh, K.; Kallali, B.; Kumar, A.; Thaker, V. Probiotics: A review. Asian Pac. J. Trop. Biomed. 2011, 1, S287–S290. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef]
- Boyle, R.J.; Robins-Browne, R.M.; Tang, M.L. Probiotic use in clinical practice: What are the risks? Am. J. Clin. Nutr. 2006, 83, 1256–1264. [Google Scholar] [CrossRef]
- Arora, M.; Sharma, S.; Baldi, A. Comparative insight of regulatory guidelines for probiotics in USA, India and Malaysia: A critical review. Int. J. Biotechnol. Wellness Ind. 2013, 2, 51. [Google Scholar]
- Jones, K. Probiotics: Preventing Antibiotic-Associated Diarrhea. J. Spec. Pediatr. Nurs. 2010, 15, 160–162. [Google Scholar] [CrossRef]
- Li, H.; Limenitakis, J.P.; Fuhrer, T.; Geuking, M.B.; Lawson, M.A.; Wyss, M.; Brugiroux, S.; Keller, I.; Macpherson, J.A.; Rupp, S.; et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 2015, 6, 8292. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Kharrat, P.; Chatel, J.-M.; Langella, P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Factories 2013, 12, S4. [Google Scholar] [CrossRef]
- Levine, M.M.; Robins-Browne, R.M.; Bavari, S. Progress in Vaccines for Enteric Diseases. Safety and Immunogenicity of Live Oral Cholera Vaccine Strain CVD 103-HgR in Streptomycin-Dependent Rabbits. Infect. Immun. 2016, 84, 252–257. [Google Scholar]
- Lavelle, E.C.; Ward, R.W.; Mestecky, J. Perspective: Novel strategies for mucosal vaccination. Immunol. Rev. 2010, 239, 102–118. [Google Scholar]
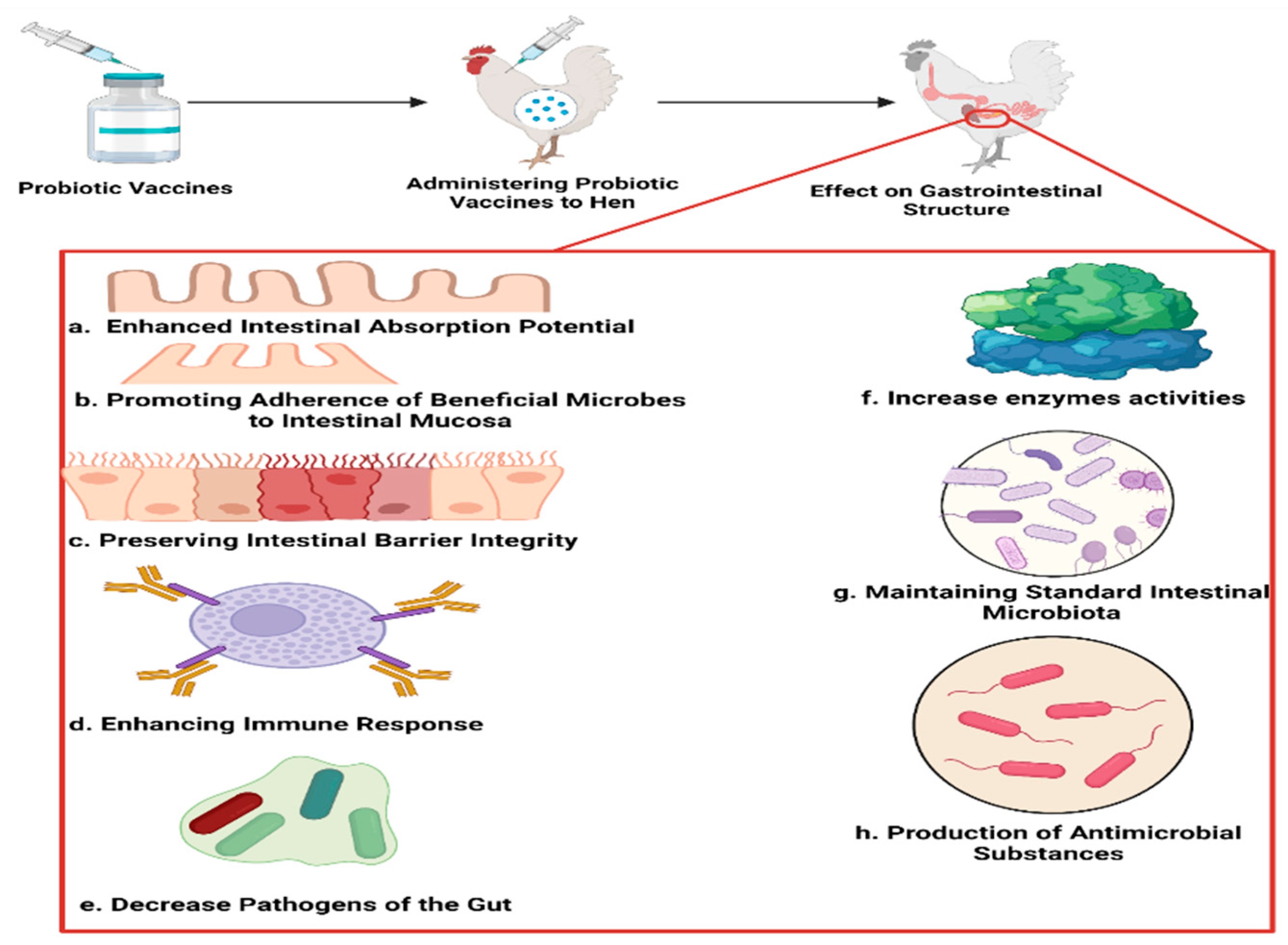
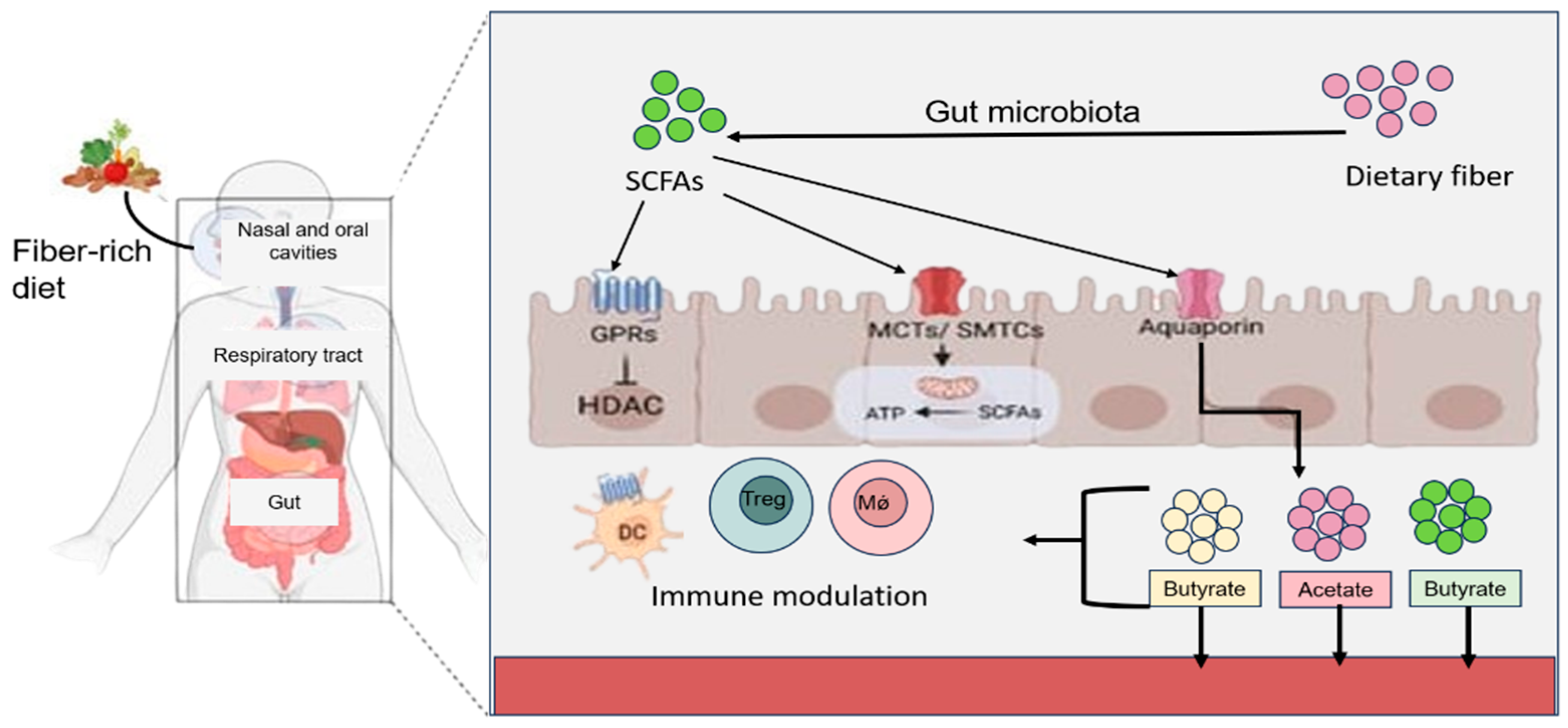

| Study | Probiotic Bacteria | Role | Vaccine Type | Key Findings |
|---|---|---|---|---|
| Lei et al. [17] | Lactobacillus casei, Bifidobacterium longum | Adjuvant | Influenza | Enhanced seroconversion and seroprotection rates in vaccinated individuals. |
| Soh et al. [20] | Bifidobacterium longum BL999, Lactobacillus rhamnosus | Adjuvant | Hepatitis B | Augmented antibody responses post-vaccination. |
| Przemska-Kosicka et al. [21] | Bifidobacterium longum infantis, Lactobacillus paracasei | Adjuvant | Seasonal influenza | Increased total antibody titers and seroprotection rates. |
| Makioka et al. [18] | Bifidobacterium species | Adjuvant | General | Stimulation of oral and systemic immune responses. |
| Wu et al. [19] | Bifidobacterium longum BB536 | Candidate | General | Increased proportion of IFN-γ-secreting cells relative to IL-4. |
| Probiotic Strain | Animal | Vaccine | Probiotics and Their Effects on the Response to Vaccines | Reference |
|---|---|---|---|---|
| L. plantarum GUANKE (LPG) | Mice | SARS-CoV-2 vaccine | Increased neutralization of SARS-CoV-2 antibodies within hours. SARS-CoV-2 vaccine increased specific neutralizing antibodies within 24 h. | [32] |
| Lactobacillus plantarum Probio-88 | In vitro and in silico study | SARS-CoV-2 infection | In the spleen, MHC II expression on macrophages and B cells is elevated, the number of CD4+CD25+ T regulatory cells is reduced, IFN-α levels are higher at 21 dpi, and TGF-β4 expression is decreased. | [33] |
| Lactobacillus | Chickens | Herpes virus vaccine from turkeys | The findings reveal an upregulation of MHC II expression on macrophages and B cells within the spleen, accompanied by a decrease in the number of CD4+CD25+ T regulatory cells. Moreover, there is heightened expression of IFN-α at 21 days post-infection (dpi), coupled with a reduction in TGF-β4 expression. | [34] |
| Bacillus velezensis | Pigeons | Pigeon circovirus | There is a significant reduction in PiCV viral load in the feces and spleens of pigeons, along with up-regulation of IFN-γ, Mx1, STAT1, TLR2, and TLR4 gene expression. | [35] |
| Lactococcus lactis NZ1330 | BALB/c Mouse Model | Allergy to Amaranthus retroflexus pollens | In addition to reducing serum IgE levels, enhanced Th1 and Treg responses are the best ways to improve allergies. | [36] |
| L. acidophilus; L. plantarum; B. subtilis; B. licheniformis | Broiler chickens | Salmonella Enteritidis vaccine | The detrimental impacts of the live vaccine on growth performance are mitigated, leading to a decrease in mortality rate, fecal shedding, and re-isolation of Salmonella Enteritidis (SE) from vital organs such as the liver, spleen, heart, and cecum. | [25] |
| L.acidophilus W37 | Piglets | Salmonella Typhimurium strains | Vaccination efficacy doubled, correlating with a higher relative abundance of Prevotellaceae and a lower relative abundance of Lactobacillaceae in fecal samples. Additionally, an increase in the relative abundance of fecal lactobacilli was associated with firmer fecal consistency. | [37] |
| Fecal microbiome+ Clostridium butyricum and Saccharomyces boulardii | Gn piglets | - | The observed effects include increased plasma concentrations of IL-23, IL-17, and IL-22, alongside elevated levels of anti-M.hyo and anti-PCV2 antibodies. Moreover, there are reductions in inflammation and oxidative-stress-induced damage, coupled with enhancements in intestinal barrier function. | [38] |
| B. toyonensis BCT-7112T | Ewes of the Corriedale sheep | Recombinant Clostridium perfringens epsilon toxin | Several cytokines and transcription factors have been increased, including total IgG anti-rETX and isotypes IgG1 and IgG2, as well as Bcl6 mRNA. | [39] |
| Saccharomyces boulardii | Sheep | Clostridium chauvoei vaccine | There were 24- and 14-fold increases in total IgG levels, as well as specific IgG, IgG1, and IgG2 titers. Further transcription of IFNs, ILs, and Bcl6 mRNAs was observed. | [39] |
| Probacteria | Species | Effect | References |
|---|---|---|---|
| Lactobacillus casei Shirota, oral (heat-killed) | Rodent | Inhibited splenocyte immunoglobulin (Ig)E production in vitro and reduced serum IgE levels | [71] |
| L. casei, oral (live) | Rodent | Increased secretory IgA (sIgA) levels and reduced incidence of enteric infections | [72] |
| L. acidophilus + Peptostreptococcus, oral (live) | Rodent | Reduced translocation and elevated levels of anti-E. coli IgM and IgE | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Aqib, A.I.; Fatima, M.; Muneer, S.; Zaheer, T.; Peng, S.; Ibrahim, E.H.; Li, K. Deciphering the Potential of Probiotics in Vaccines. Vaccines 2024, 12, 711. https://doi.org/10.3390/vaccines12070711
Xu C, Aqib AI, Fatima M, Muneer S, Zaheer T, Peng S, Ibrahim EH, Li K. Deciphering the Potential of Probiotics in Vaccines. Vaccines. 2024; 12(7):711. https://doi.org/10.3390/vaccines12070711
Chicago/Turabian StyleXu, Chang, Amjad Islam Aqib, Mahreen Fatima, Sadia Muneer, Tean Zaheer, Song Peng, Essam H. Ibrahim, and Kun Li. 2024. "Deciphering the Potential of Probiotics in Vaccines" Vaccines 12, no. 7: 711. https://doi.org/10.3390/vaccines12070711







