Determination of Deamidation in Adjuvanted Vaccine Antigens through Isoaspartic Acid Quantification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Formulation
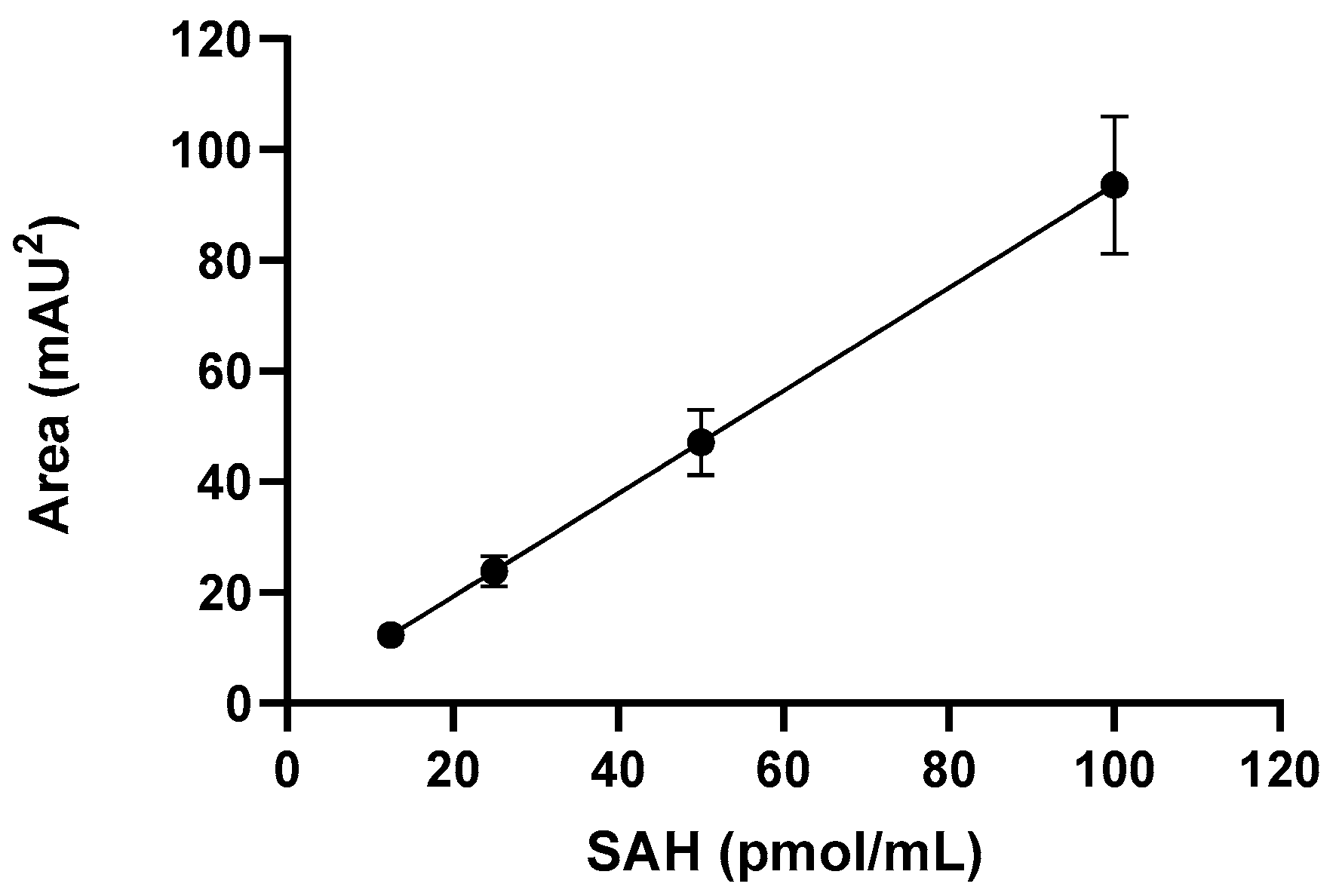
2.2. Quantitation of IsoAsp Residue by Reversed-Phase High-Performance Liquid Chromatography
2.3. PcpA Protein Concentration Quantification by Reversed-Phase High-Performance Liquid Chromatography
2.4. Stability Studies
2.5. Statistical Analyses
3. Results and Discussion
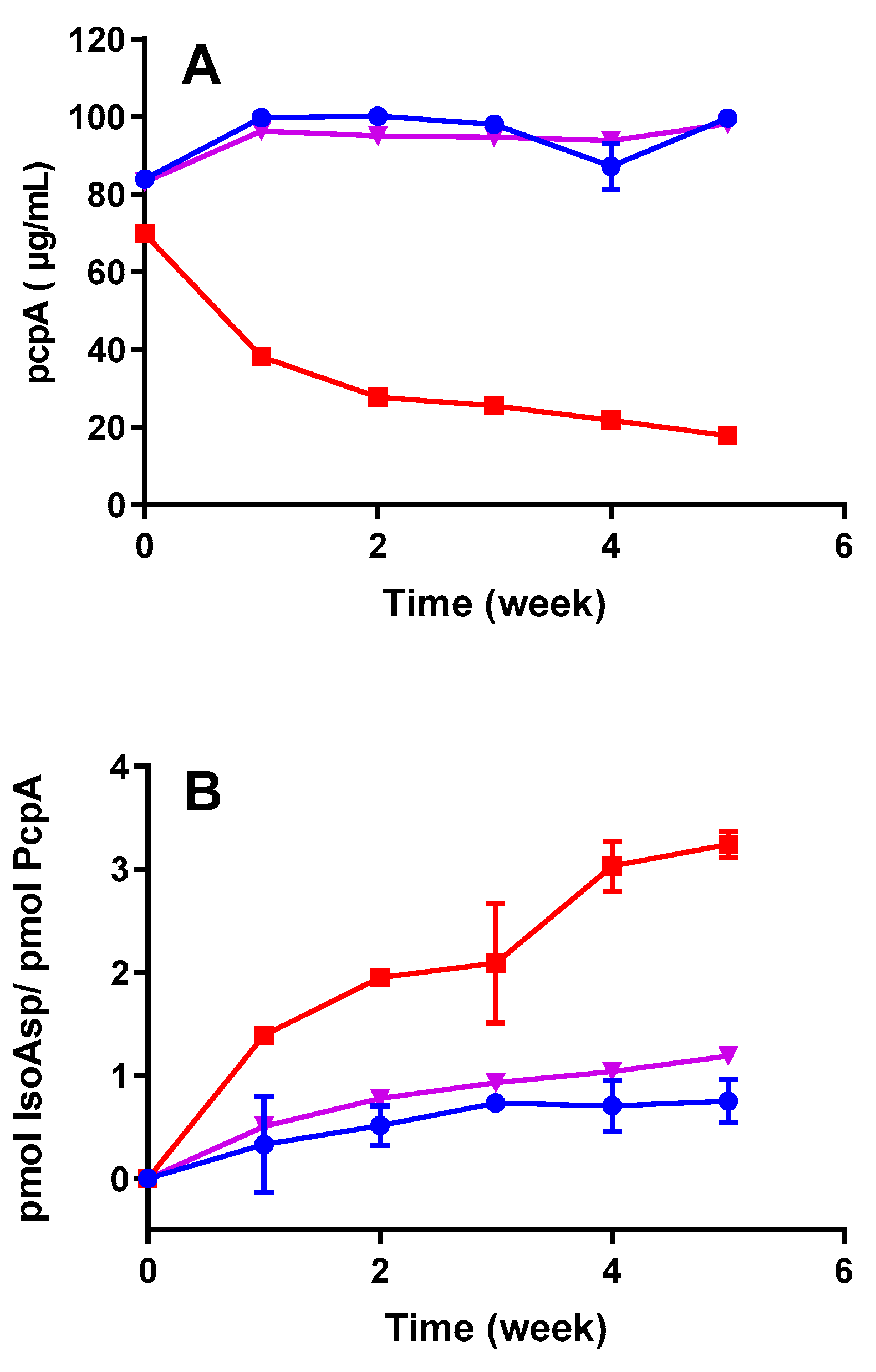
3.1. IsoAsp Levels Are Increased in Thermally Stressed Vaccine Antigens
3.2. IsoAsp Can Be Accurately Detected in Adjuvanted Vaccines
3.3. IsoAsp Levels in Vaccines Are Impacted by pH and Temperature
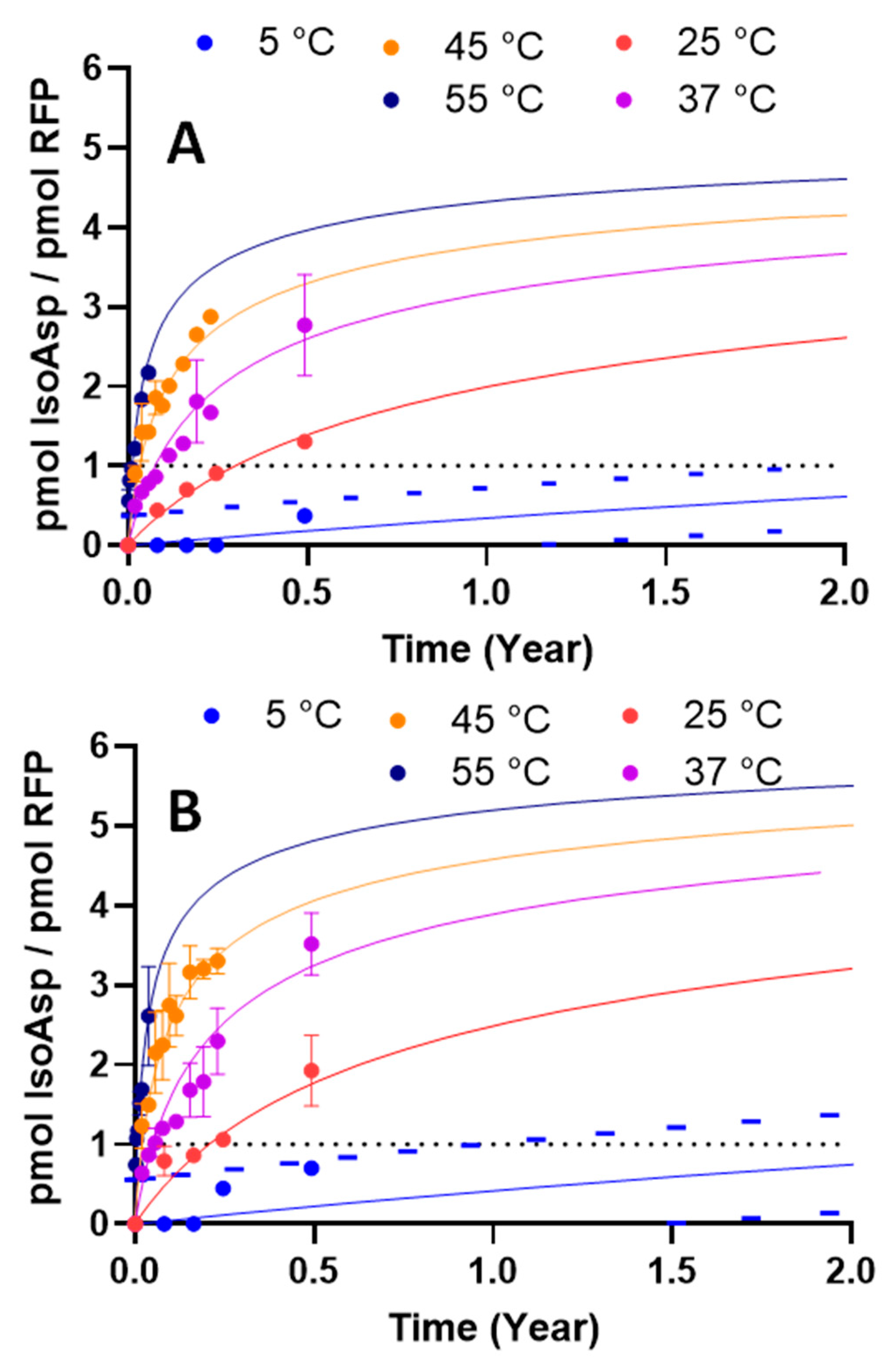
3.4. Thermal Kinetic Modeling Can Predict the Effects of pH and Temperature on IsoAsp Levels during Long-Term Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkin, D.B.; Burke, C.J.; Sanyal, G.; Middaugh, C.R. Analysis of vaccine stability. Dev. Biol. Stand. 1996, 87, 135–142. [Google Scholar]
- Wilson-Welder, J.H.; Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar] [CrossRef]
- Jones, L.S.; Peek, L.J.; Power, J.; Markham, A.; Yazzie, B.; Middaugh, C.R. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J. Biol. Chem. 2005, 280, 13406–13414. [Google Scholar] [CrossRef]
- Hasija, M.; Li, L.; Rahman, N.; Ausar, S.F. Forced degradation studies: An essential tool for the formulation development of vaccines. Vaccine Dev. Ther. 2013, 3, 11–33. [Google Scholar]
- Brandau, D.; Jones, L.T.; Wiethoff, C.; Rexroad, J.; Middaugh, C.R. Thermal Stability of Vaccines. J. Pharm. Sci. 2003, 92, 218–231. [Google Scholar] [CrossRef]
- Estey, T.; Vessely, C.; Randolph, T.W.; Henderson, I.; Braun, L.J.; Nayar, R.; Carpenter, J.F. Evaluation of Chemical Degradation of a Trivalent Recombinant Protein Vaccine Against Botulinum Neurotoxin by LysC Peptide Mapping and MALDI-TOF Mass Spectrometry. J. Pharm. Sci. 2009, 98, 2994–3012. [Google Scholar] [CrossRef]
- Yang, H.; Zubarev, R.A. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis 2010, 31, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Wakankar, A.; Borchardt, R.T. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J. Pharm. Sci. 2006, 95, 2321–2336. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ngundi, M.M.; Burns, D.L. Mechanistic Analysis of the Effect of Deamidation on the Immunogenicity of Anthrax Protective Antigen. Clin. Vaccine Immunol. 2016, 23, 396–402. [Google Scholar] [CrossRef]
- Powell, B.S.; Enama, J.T.; Ribot, W.J.; Webster, W.; Little, S.; Hoover, T.; Adamovicz, J.J.; Andrews, G.P. Multiple asparagine deamidation of Bacillus anthracis protective antigen causes charge isoforms whose complexity correlates with reduced biological activity. Proteins Structuion Funct. Bioinform. 2007, 68, 458–479. [Google Scholar] [CrossRef]
- Jin, Y.; Yi, Y.; Yeung, B. Mass spectrometric analysis of protein deamidation—A focus on top-down and middle-down mass spectrometry. Methods 2022, 200, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Shirokawa, J.M.; Hancock, W.S.; Spellman, M.W.; Basa, L.J.; Aswad, D.W. Formation of isoaspartate at two distinct sites during in vitro aging of human growth hormone. J. Biol. Chem. 1989, 264, 14262–14271. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Aswad, D.W. Optimal conditions for the use of protein L-isoaspartyl methyltransferase in assessing the isoaspartate content of peptides and proteins. Anal. Biochem. 1991, 192, 384–391. [Google Scholar] [CrossRef]
- Aboutorabian, S.; Hakimi, J.; Boudet, F.; Montano, S.; Dookie, A.; Roque, C.; Ausar, S.F.; Rahman, N.; Brookes, R.H. A high ratio of IC31(®) adjuvant to antigen is necessary for H4 TB vaccine immunomodulation. Hum. Vaccines Immunother. 2015, 11, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Klucker, M.F.; Dalençon, F.; Probeck, P.; Haensler, J. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J. Pharm. Sci. 2012, 101, 4490–5000. [Google Scholar] [CrossRef] [PubMed]
- Ljutic, B.; Ochs, M.; Messham, B.; Ming, M.; Dookie, A.; Harper, K.; Ausar, S.F. Formulation, stability and immunogenicity of a trivalent pneumococcal protein vaccine formulated with aluminum salt adjuvants. Vaccine 2012, 30, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Clénet, D.; Imbert, F.; Probeck, P.; Rahman, N.; Ausar, S.F. Advanced Kinetic Analysis as a Tool for Formulation Development and Prediction of Vaccine Stability. J. Pharm. Sci. 2014, 103, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Reissner, K.J.; Aswad, D.W. Deamidation and isoaspartate formation in proteins: Unwanted alterations or surreptitious signals? Cell. Mol. Life Sci. 2003, 60, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Vessely, C.; Estey, T.; Randolph, T.W.; Henderson, I.; Nayar, R.; Carpenter, J.F. Effects of solution conditions and surface chemistry on the adsorption of three recombinant botulinum neurotoxin antigens to aluminum salt adjuvants. J. Pharm. Sci. 2007, 96, 2375–2389. [Google Scholar] [CrossRef]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.A.; Murthy, S.N. Stability of Vaccines. AAPS PharmSciTech 2019, 20, 42. [Google Scholar] [CrossRef]
- Wittayanukulluk, A.; Jiang, D.; Regnier, F.E.; Hem, S.L. Effect of microenvironment pH of aluminum hydroxide adjuvant on the chemical stability of adsorbed antigen. Vaccine 2004, 22, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.L.; Powell, M.F.; Shire, S.J. The development of stable protein formulations: A close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carr. Syst. 1993, 10, 307–377. [Google Scholar]



| Antigen and Stress Condition | Iso-Asparatate (Pmole/µg of Antigen) (Control Stored at −20 °C) | Iso-Asparatate (Pmole/µg of Antigen) (Thermally Stressed *) |
|---|---|---|
| RFP (37 °C) | 2.71 | 12.31 * |
| PcpA antigen (45 °C) | Not detected | 0.49 |
| IPV antigen (45 °C) | Not detected | 27.82 |
| Formulation pH | Kinetic Model | wAIC | wBIC | RSS | ln(A·s) | E (kJ/mol) | N (Reaction Order) | Time to Reach Upper Acceptance Criteria |
|---|---|---|---|---|---|---|---|---|
| pH 6.5 | One step | 71.46 | 71.46 | 2.48 | 18.03 | 87.84 | 4 | 1.90 years |
| pH 7.8 | One step | 61.26 | 61.26 | 5.55 | 19.66 | 91.51 | 4 | 1.03 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasija, M.; Ma, J.; Li, B.; Rahman, N.; Strahlendorf, K.A.; Ausar, S.F. Determination of Deamidation in Adjuvanted Vaccine Antigens through Isoaspartic Acid Quantification. Vaccines 2024, 12, 733. https://doi.org/10.3390/vaccines12070733
Hasija M, Ma J, Li B, Rahman N, Strahlendorf KA, Ausar SF. Determination of Deamidation in Adjuvanted Vaccine Antigens through Isoaspartic Acid Quantification. Vaccines. 2024; 12(7):733. https://doi.org/10.3390/vaccines12070733
Chicago/Turabian StyleHasija, Manvi, Jian Ma, Bing Li, Nausheen Rahman, Kirsten A. Strahlendorf, and Salvador Fernando Ausar. 2024. "Determination of Deamidation in Adjuvanted Vaccine Antigens through Isoaspartic Acid Quantification" Vaccines 12, no. 7: 733. https://doi.org/10.3390/vaccines12070733
APA StyleHasija, M., Ma, J., Li, B., Rahman, N., Strahlendorf, K. A., & Ausar, S. F. (2024). Determination of Deamidation in Adjuvanted Vaccine Antigens through Isoaspartic Acid Quantification. Vaccines, 12(7), 733. https://doi.org/10.3390/vaccines12070733






