Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Plasma Separation
2.3. Antibody Measurement
2.4. Proteomic Analysis and Data Processing
2.5. Primary Cell Culture and Cytokine Measurement
2.6. Statistical Analysis
3. Results
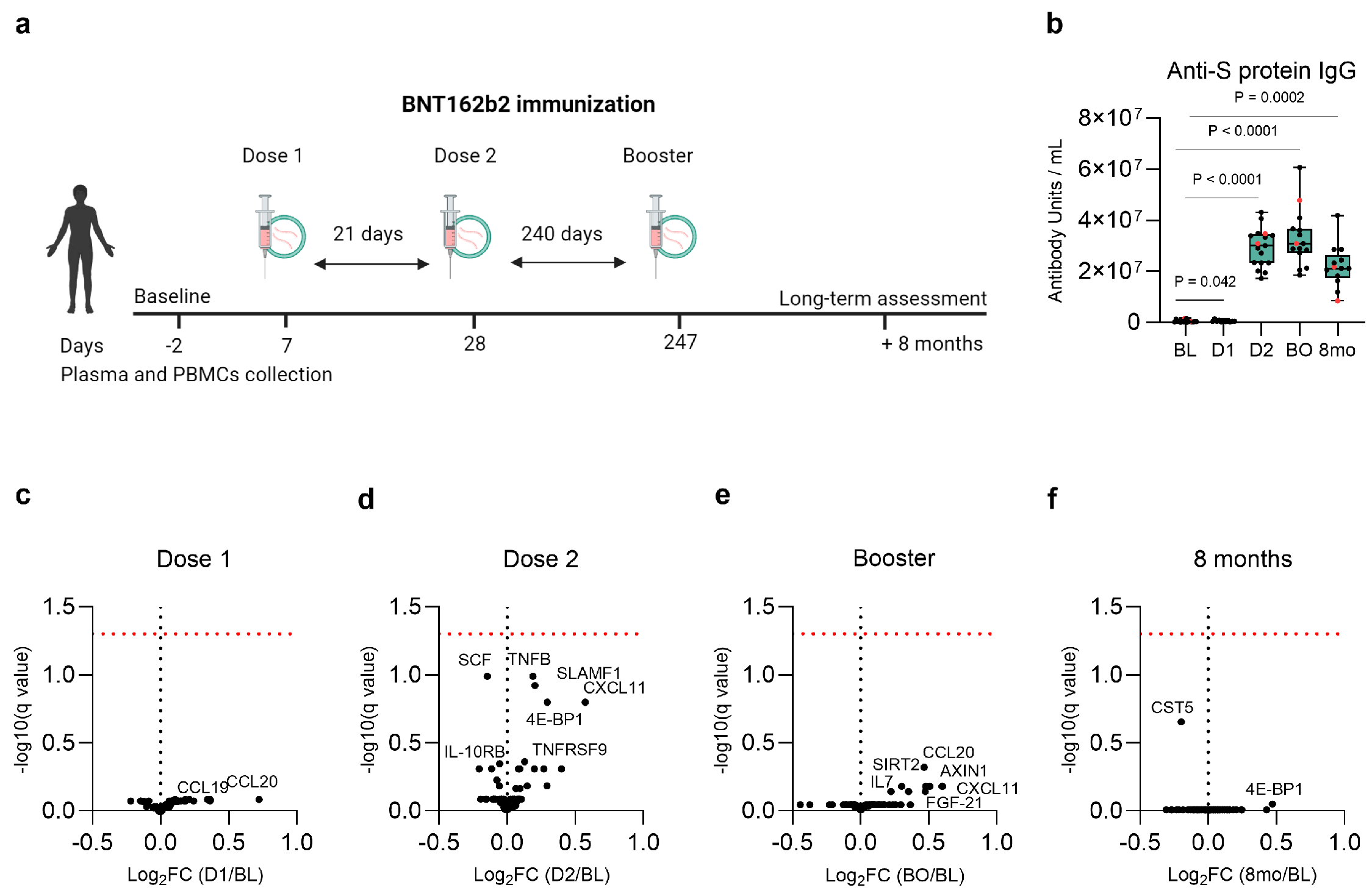
3.1. Study Design and Participants’ Characteristics
3.2. Baseline, Post-Vaccination, and Long-Term Anti-S Protein IgG Antibody Concentration
3.3. No Lasting Changes in the Plasma Inflammatory Proteome Following Vaccination
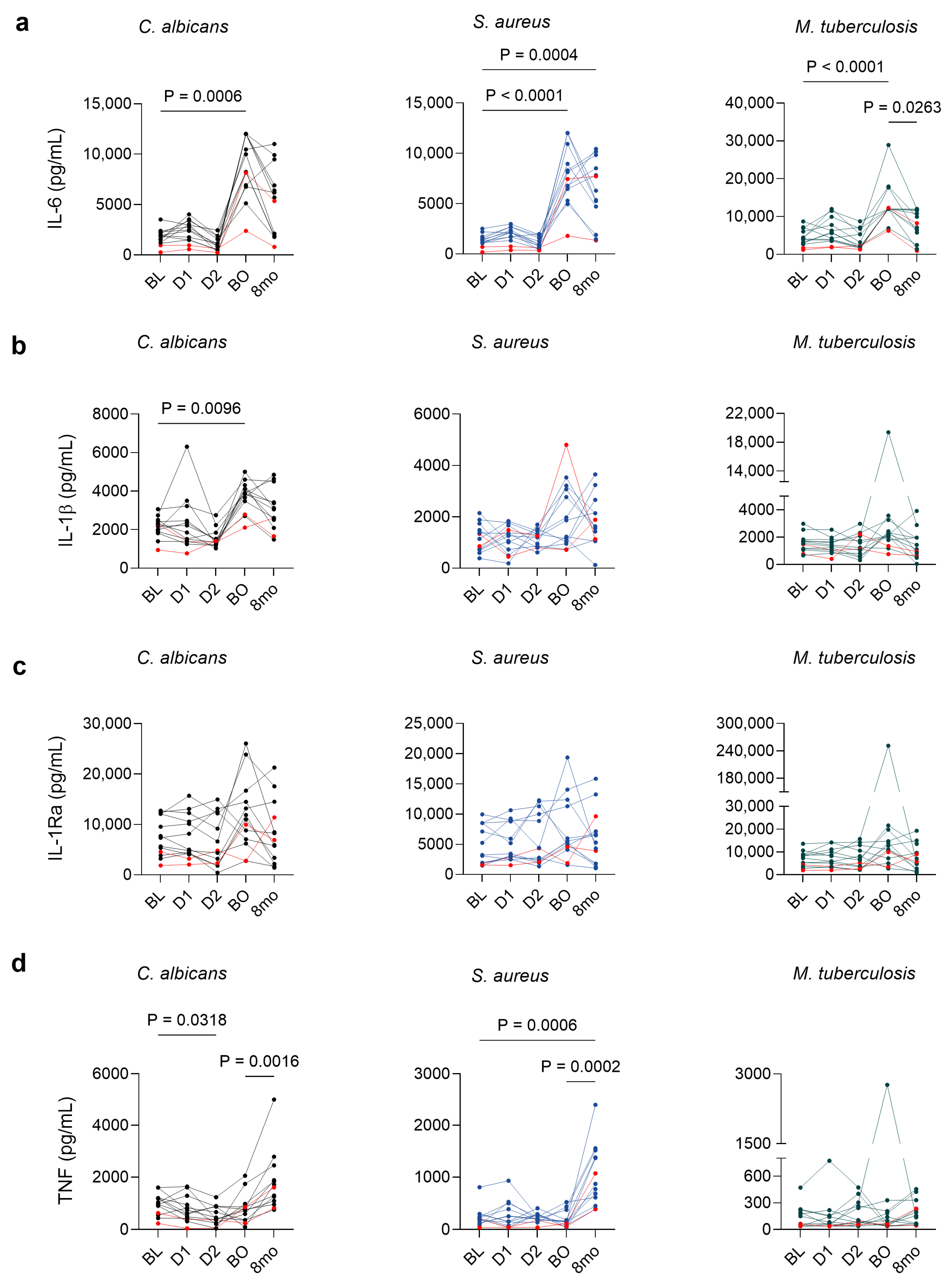
3.4. BNT162b2 Vaccination Is Associated with Persistent Cytokine Alterations in Stimulated PBMCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; Mcneal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Arientová, S.; Matúšková, K.; Bartoš, O.; Holub, M.; Beran, O. Specific Immune Responses after BNT162b2 MRNA Vaccination and COVID-19 Infection. Front. Immunol. 2023, 14, 1271353. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Kahn, B.; Apostolidis, S.A.; Bhatt, V.; Greenplate, A.R.; Kallish, S.; LaCava, A.; Lucas, A.; Meyer, N.J.; Negoianu, D.; Ogdie, A.R.; et al. Multisystem Inflammation and Organ Dysfunction After BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccination. Crit. Care Explor. 2021, 3, e0578. [Google Scholar] [CrossRef]
- Terentes-Printzios, D.; Gardikioti, V.; Solomou, E.; Emmanouil, E.; Gourgouli, I.; Xydis, P.; Christopoulou, G.; Georgakopoulos, C.; Dima, I.; Miliou, A.; et al. The Effect of an MRNA Vaccine against COVID-19 on Endothelial Function and Arterial Stiffness. Hypertens. Res. 2022, 45, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kato, Y.; Edahiro, R.; Søndergaard, J.N.; Murakami, T.; Amiya, S.; Nameki, S.; Yoshimine, Y.; Morita, T.; Takeshima, Y.; et al. Consecutive BNT162b2 MRNA Vaccination Induces Short-Term Epigenetic Memory in Innate Immune Cells. JCI Insight 2022, 7, e163347. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The MRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Xu, S.; Huang, R.; Sy, L.S.; Hong, V.; Glenn, S.C.; Ryan, D.S.; Morrissette, K.; Vazquez-Benitez, G.; Glanz, J.M.; Klein, N.P.; et al. A Safety Study Evaluating Non-COVID-19 Mortality Risk Following COVID-19 Vaccination. Vaccine 2023, 41, 844–854. [Google Scholar] [CrossRef]
- Pálinkás, A.; Sándor, J. Effectiveness of COVID-19 Vaccination in Preventing All-Cause Mortality among Adults during the Third Wave of the Epidemic in Hungary: Nationwide Retrospective Cohort Study. Vaccines 2022, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Cox, D.J.; Connolly, S.A.; Breen, E.P.; Brugman, A.A.I.; Phelan, J.J.; Keane, J.; Basdeo, S.A. Trained Immunity Is Induced in Humans after Immunization with an Adenoviral Vector COVID-19 Vaccine. J. Clin. Investig. 2023, 133, e162581. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of Innate and Adaptive Immunity to the Pfizer-BioNTech BNT162b2 Vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Arunachalam, P. Systems Vaccinology of the BNT162b2 MRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Föhse, K.; Geckin, B.; Zoodsma, M.; Kilic, G.; Liu, Z.; Overheul, G.J.; van de Maat, J.S.; Bulut, O.; Hoogerwerf, J.J.; ten Oever, J.; et al. The Impact of BNT162b2 MRNA Vaccine on Adaptive and Innate Immune Responses. Clin. Immunol. 2023, 255, 109762. [Google Scholar] [CrossRef]
- Baydemir, I.; Dulfer, E.A.; Netea, M.G.; Domínguez-Andrés, J. Trained Immunity-Inducing Vaccines: Harnessing Innate Memory for Vaccine Design and Delivery. Clin. Immunol. 2024, 261, 109930. [Google Scholar] [CrossRef]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Dickens, E.R.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [PubMed]
- Musilova, J.; Mulcahy, M.E.; Kuijk, M.M.; McLoughlin, R.M.; Bowie, A.G. Toll-like Receptor 2-Dependent Endosomal Signaling by Staphylococcus Aureus in Monocytes Induces Type i Interferon and Promotes Intracellular Survival. J. Biol. Chem. 2019, 294, 17031–17042. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Joosten, L.A.B.; van de Veerdonk, F.L.; Savage, N.; van Crevel, R.; Kullberg, B.J.; van der Ven, A.; Ottenhfoff, T.H.M.; Dinarello, C.A.C.A.; van der Meer, J.W.M.; et al. Transcriptional and Inflammasome-Mediated Pathways for the Induction of IL-1beta Production by Mycobacterium Tuberculosis. Eur. J. Immunol. 2009, 39, 1914–1922. [Google Scholar] [CrossRef]
- Singh, S.K.; Girschick, H.J. Toll-like Receptors in Borrelia Burgdorferi-Induced Inflammation. Clin. Microbiol. Infect. 2006, 12, 705–717. [Google Scholar] [CrossRef]
- Gil, M.L.; Murciano, C.; Yanez, A.; Gozalbo, D. Role of Toll-like Receptors in Systemic Candida Albicans Infections. Front. Biosci. Landmark 2016, 21, 278–302. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Rowe, D.C.; Golenbock, D.T. Endotoxin Recognition and Signal Transduction by the TLR4/MD2-Complex. Microbes Infect. 2004, 6, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Unitt, J.; Hornigold, D. Plant Lectins Are Novel Toll-like Receptor Agonists. Biochem. Pharmacol. 2011, 81, 1324–1328. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Homan, P.; Bergamaschi, C.; Karaliota, S.; Ntanasis-Stathopoulos, I.; Devasundaram, S.; Bear, J.; Burns, R.; Bagratuni, T.; et al. Rapid Transient and Longer-Lasting Innate Cytokine Changes Associated with Adaptive Immunity after Repeated SARS-CoV-2 BNT162b2 MRNA Vaccinations. Front. Immunol. 2023, 14, 1292568. [Google Scholar] [CrossRef]
- Hennø, L.T.; Storjord, E.; Christiansen, D.; Bergseth, G.; Ludviksen, J.K.; Fure, H.; Barene, S.; Waage-Nielsen, E.; Mollnes, T.E.; Brekke, O.L. Effect of the Anticoagulant, Storage Time and Temperature of Blood Samples on the Concentrations of 27 Multiplex Assayed Cytokines—Consequences for Defining Reference Values in Healthy Humans. Cytokine 2017, 97, 86–95. [Google Scholar] [CrossRef]
- Prentice, S.; Nassanga, B.; Webb, E.L.; Akello, F.; Kiwudhu, F.; Akurut, H.; Elliott, A.M.; Arts, R.J.W.; Netea, M.G.; Dockrell, H.M.; et al. BCG-Induced Non-Specific Effects on Heterologous Infectious Disease in Ugandan Neonates: An Investigator-Blind Randomised Controlled Trial. Lancet Infect. Dis. 2021, 21, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Netea, M.G. Trained Immunity-Related Vaccines: Innate Immune Memory and Heterologous Protection against Infections. Trends Mol. Med. 2022, 28, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Fanucchi, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Netea, M.G.; Mhlanga, M.M. The Intersection of Epigenetics and Metabolism in Trained Immunity. Immunity 2021, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; Fults, J.B.; Patil, N.K.; Hernandez, A.; Bohannon, J.K. TLR Agonists as Mediators of Trained Immunity: Mechanistic Insight and Immunotherapeutic Potential to Combat Infection. Front. Immunol. 2020, 11, 622614. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.E.; Ryan, F.J.; Messina, N.L.; Blake, S.J.; Norton, T.S.; Germano, S.; James, J.; Eden, G.L.; Tee, Y.C.; Lynn, M.A.; et al. No Evidence of Durable Trained Immunity after Two Doses of Adenovirus-Vectored or MRNA COVID-19 Vaccines. J. Clin. Investig. 2023, 133, e171742. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra Are Key Regulators of the Inflammatory Response to RNA Vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Theobald, S.J.; Simonis, A.; Georgomanolis, T.; Kreer, C.; Zehner, M.; Eisfeld, H.S.; Albert, M.; Chhen, J.; Motameny, S.; Erger, F.; et al. Long-lived Macrophage Reprogramming Drives Spike Protein-mediated Inflammasome Activation in COVID-19. EMBO Mol. Med. 2021, 13, e14150. [Google Scholar] [CrossRef]
- Geckin, B.; Konstantin Föhse, F.; Domínguez-Andrés, J.; Netea, M.G. Trained Immunity: Implications for Vaccination. Curr. Opin. Immunol. 2022, 77, 102190. [Google Scholar] [CrossRef]
- Pine, M.; Arora, G.; Hart, T.M.; Bettini, E.; Gaudette, B.T.; Muramatsu, H.; Tombácz, I.; Kambayashi, T.; Tam, Y.K.; Brisson, D.; et al. Development of an MRNA-Lipid Nanoparticle Vaccine against Lyme Disease. Mol. Ther. 2023, 31, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, L.; Bettencourt, P.J.G. MRNA Vaccines: A New Opportunity for Malaria, Tuberculosis and HIV. Front. Immunol. 2023, 14, 1172691. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabău, G.; Badii, M.; Mirea, A.M.; Gaal, O.I.; van Emst, L.; Popp, R.A.; Crișan, T.O.; Joosten, L.A.B. Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination. Vaccines 2024, 12, 736. https://doi.org/10.3390/vaccines12070736
Cabău G, Badii M, Mirea AM, Gaal OI, van Emst L, Popp RA, Crișan TO, Joosten LAB. Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination. Vaccines. 2024; 12(7):736. https://doi.org/10.3390/vaccines12070736
Chicago/Turabian StyleCabău, Georgiana, Medeea Badii, Andreea M. Mirea, Orsolya I. Gaal, Liesbeth van Emst, Radu A. Popp, Tania O. Crișan, and Leo A. B. Joosten. 2024. "Long-Lasting Enhanced Cytokine Responses Following SARS-CoV-2 BNT162b2 mRNA Vaccination" Vaccines 12, no. 7: 736. https://doi.org/10.3390/vaccines12070736




