Commensal HPVs Have Evolved to Be More Immunogenic Compared with High-Risk α-HPVs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sequences and HLAs
2.2. HPV Protein Homology
2.3. HPV Immunogenic Peptide Predictions
2.4. HPV Immunogenic Epitope Clustering
2.5. Phylogenetic Tree Construction
3. Results
3.1. Early HPV Proteins Have High Amino Acid Sequence Homology at the Species Level
3.2. Early HPV Genes within a Species Generate Multiple Conserved, Immunogenic Peptides
3.3. Commensal HPVs Generate More Immunogenic Peptides Compared with α-HPVs
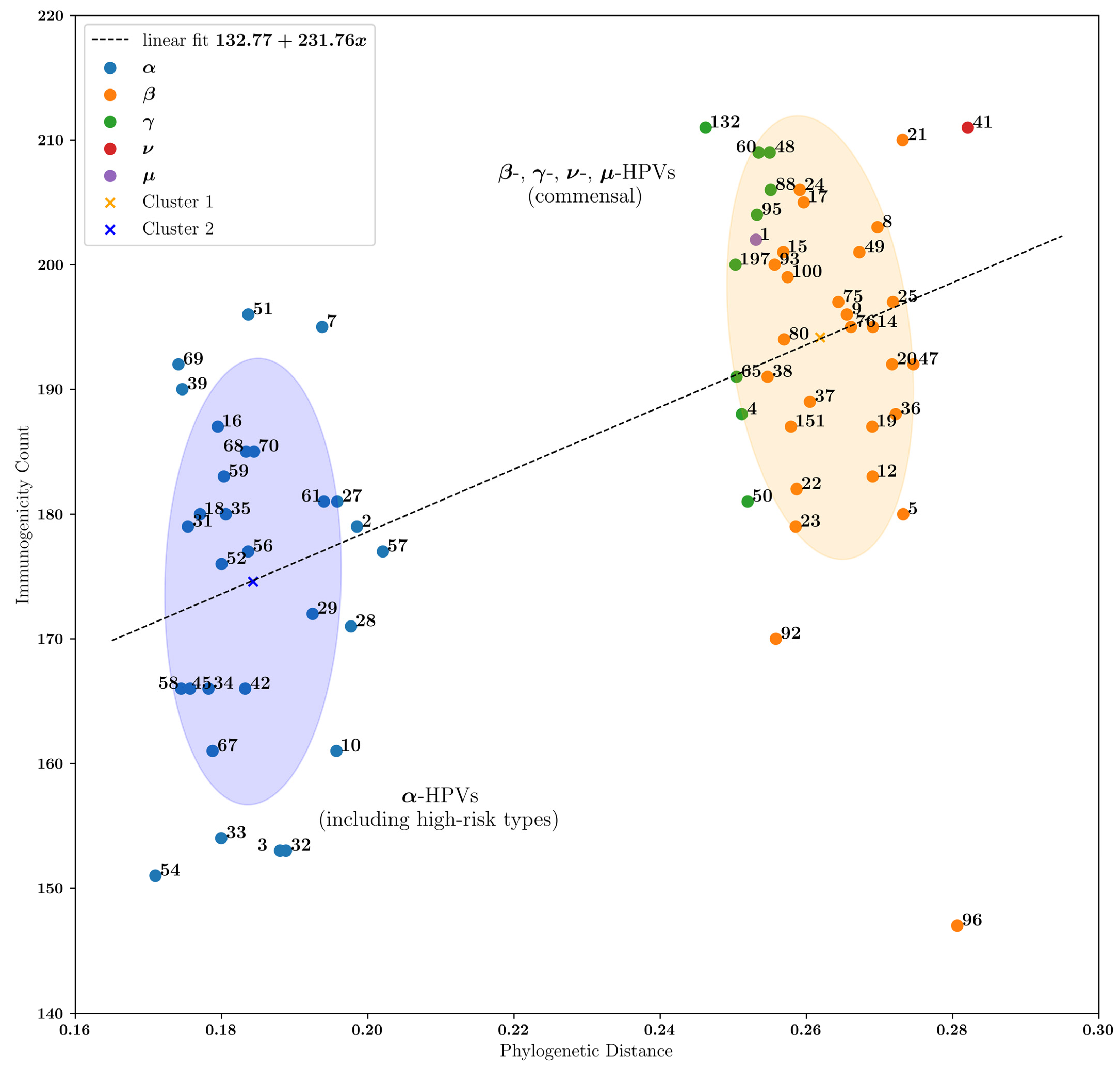
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Arroyo Muhr, L.S.; Eklund, C.; Dillner, J. Misclassifications in human papillomavirus databases. Virology 2021, 558, 57–66. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, E.M. Cross-roads in the classification of papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K. Revisiting Papillomavirus Taxonomy: A Proposal for Updating the Current Classification in Line with Evolutionary Evidence. Viruses 2022, 14, 2308. [Google Scholar] [CrossRef] [PubMed]
- Rollison, D.E.; Viarisio, D.; Amorrortu, R.P.; Gheit, T.; Tommasino, M. An Emerging Issue in Oncogenic Virology: The Role of Beta Human Papillomavirus Types in the Development of Cutaneous Squamous Cell Carcinoma. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 93, e01003-18. [Google Scholar] [CrossRef] [PubMed]
- Foulongne, V.; Sauvage, V.; Hebert, C.; Dereure, O.; Cheval, J.; Gouilh, M.A.; Pariente, K.; Segondy, M.; Burguiere, A.; Manuguerra, J.C.; et al. Human skin microbiota: High diversity of DNA viruses identified on the human skin by high throughput sequencing. PLoS ONE 2012, 7, e38499. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 2015, 6, e01578-15. [Google Scholar] [CrossRef]
- Maor, D.; Brennand, S.; Goh, M.S.Y.; Chong, A.H. Recalcitrant hyperkeratotic verrucae in a renal transplant recipient clearing with cessation of immunosuppression. JAAD Case Rep. 2018, 4, 471–473. [Google Scholar] [CrossRef]
- Geier, C.B.; Ellison, M.; Cruz, R.; Pawar, S.; Leiss-Piller, A.; Zmajkovicova, K.; McNulty, S.M.; Yilmaz, M.; Evans, M.O., 2nd; Gordon, S.; et al. Disease Progression of WHIM Syndrome in an International Cohort of 66 Pediatric and Adult Patients. J. Clin. Immunol. 2022, 42, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Conlan, S.; Deming, C.; Lee-Lin, S.Q.; Huang, X.; Program, N.C.S.; Su, H.C.; Freeman, A.F.; Segre, J.A.; Kong, H.H. Expanded skin virome in DOCK8-deficient patients. Nat. Med. 2018, 24, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Guerin, A.; Moncada-Velez, M.; Jackson, K.; Ogishi, M.; Rosain, J.; Mancini, M.; Langlais, D.; Nunez, A.; Webster, S.; Goyette, J.; et al. Helper T cell immunity in humans with inherited CD4 deficiency. J. Exp. Med. 2024, 221, e20231044. [Google Scholar] [CrossRef]
- Beziat, V.; Rapaport, F.; Hu, J.; Titeux, M.; Bonnet des Claustres, M.; Bourgey, M.; Griffin, H.; Bandet, E.; Ma, C.S.; Sherkat, R.; et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 2021, 184, 3812–3828.e3830. [Google Scholar] [CrossRef] [PubMed]
- Accardi, R.; Gheit, T. Cutaneous HPV and skin cancer. Presse Med. 2014, 43, e435–e443. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Lundegaard, C.; Blicher, T.; Lamberth, K.; Harndahl, M.; Justesen, S.; Roder, G.; Peters, B.; Sette, A.; Lund, O.; et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS ONE 2007, 2, e796. [Google Scholar] [CrossRef]
- Cock, P.J.; Antao, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Altschul, S.F. Amino acid substitution matrices from an information theoretic perspective. J. Mol. Biol. 1991, 219, 555–565. [Google Scholar] [CrossRef]
- Eddy, S.R. Where did the BLOSUM62 alignment score matrix come from? Nat. Biotechnol. 2004, 22, 1035–1036. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 1992, 89, 10915–10919. [Google Scholar] [CrossRef]
- Karlin, S.; Altschul, S.F. Methods for assessing the statistical significance of molecular sequence features by using general scoring schemes. Proc. Natl. Acad. Sci. USA 1990, 87, 2264–2268. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, M.P.; Jensen, K.L.; Rigoutsos, I.; Stephanopoulos, G. BLOSUM62 miscalculations improve search performance. Nat. Biotechnol. 2008, 26, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Conev, A.; Devaurs, D.; Rigo, M.M.; Antunes, D.A.; Kavraki, L.E. 3pHLA-score improves structure-based peptide-HLA binding affinity prediction. Sci. Rep. 2022, 12, 10749. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V.; Faizo, A.A.A. Control of human papillomavirus gene expression by alternative splicing. Virus Res. 2017, 231, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Petrova, G.; Ferrante, A.; Gorski, J. Cross-reactivity of T cells and its role in the immune system. Crit. Rev. Immunol. 2012, 32, 349–372. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Vierthaler, S.L.; Santee, J.A. Review of Gardasil. J. Vaccines Vaccin. 2010, 1, 1000107. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K.; Burk, R.D. Evolution of human papillomavirus carcinogenicity. Adv. Virus Res. 2010, 77, 41–62. [Google Scholar] [CrossRef]
- Ishizuka, J.; Grebe, K.; Shenderov, E.; Peters, B.; Chen, Q.; Peng, Y.; Wang, L.; Dong, T.; Pasquetto, V.; Oseroff, C.; et al. Quantitating T cell cross-reactivity for unrelated peptide antigens. J. Immunol. 2009, 183, 4337–4345. [Google Scholar] [CrossRef]
- Rudolf, M.P.; Man, S.; Melief, C.J.; Sette, A.; Kast, W.M. Human T-cell responses to HLA-A-restricted high binding affinity peptides of human papillomavirus type 18 proteins E6 and E7. Clin. Cancer Res. 2001, 7, 788s–795s. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guennoun, R.; Alyakin, A.; Higuchi, H.; Demehri, S. Commensal HPVs Have Evolved to Be More Immunogenic Compared with High-Risk α-HPVs. Vaccines 2024, 12, 749. https://doi.org/10.3390/vaccines12070749
Guennoun R, Alyakin A, Higuchi H, Demehri S. Commensal HPVs Have Evolved to Be More Immunogenic Compared with High-Risk α-HPVs. Vaccines. 2024; 12(7):749. https://doi.org/10.3390/vaccines12070749
Chicago/Turabian StyleGuennoun, Ranya, Anton Alyakin, Hiroshi Higuchi, and Shadmehr Demehri. 2024. "Commensal HPVs Have Evolved to Be More Immunogenic Compared with High-Risk α-HPVs" Vaccines 12, no. 7: 749. https://doi.org/10.3390/vaccines12070749





