SARS-CoV-2-Vaccine-Related Endocrine Disorders: An Updated Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
3. SARS-CoV-2 Vaccination and Thyroid Dysfunction
| Adverse Effects | Study Type (Ref.) | No. of Cases | Age Median (Range) | Sex Female (%) | Vaccine Type | Vaccine Dose | Days from the Last Vaccine Median (Range) | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | Viral | Inactivated | 1st | 2nd | 3rd | |||||||
| Post-Vaccination Subacute Thyroiditis Diagnosis and Outcomes | Subacute Thyroiditis | |||||||||||
| Case reports/small series [15,16,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] | 83 | 41 (26–82) | 61 (73.5) | 51 | 17 | 15 | 40 | 39 | 4 | 10 (1–84) | In 76% of cases, post-vaccination SAT resolved without sequelae. Long-term hypothyroidism developed in 12% of cases. | |
| Retrospective [49] | 23 | 44 (34–49) | 12 (52.2) | 18 | - | 5 | 5 | 16 | 2 | 45 (7–90) | The clinical course of post-vaccination SAT tended to be longer than the classical SAT. | |
| Retrospective [50] | 16 | 46.4 ± 9.9 | 37 (67.3) | 6 | - | 10 | 10 | 6 | - | 6.5 (2–20) | Clinical features of post-vaccination SAT were similar to the classical SAT. | |
| Retrospective [17] | 14 | 43.1 ± 9.3 | 12(86) | 10 | - | 6 | NA | NA | NA | NA (4–11) | Patients with SARS-CoV-2-vaccine-induced SAT had a higher frequency of HLA-B*35 and HLA-C*04 alleles. | |
| Retrospective [48] | 162 | 47 (27–86) | 120 (74) | 130 | 32 | - | 59 | 55 | 1 | 10.5 (1–87) | SAT was reported more frequently for post-mRNA vaccines compared with viral vaccines. | |
| Retrospective [51] | 258 | 42 (36–49) | 187 (82.5) | 199 | - | 54 | 62 | 175 | 16 | 20 (10–40) | Post-vaccination SAT had same clinical course and outcomes as classical one. | |
| Post-Vaccination Graves’ Disease Diagnosis and Outcomes | Graves’ Disease | |||||||||||
| Case reports/small series [52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] | 70 | 43 (22–74) | 51 (73) | 50 | 16 | 1 | 38 | 34 | 1 | 11 (1–63) | 14% of postvaccination cases were relapsed GD. | |
| Retrospective [79] | 20 | 51 (37–64) | 12 | 14 | 6 | - | 5 | 13 | 2 | 9 | Patients with post-vaccination new-onset GD had better initial biochemical and immunologic responses to treatment. | |
| Retrospective [80] | 44 | 48.9 ± 15.6 | 43 | 30 | 14 | - | 23 | 21 | - | 19.9 + 17.6 | Large scale SARS-CoV-2 vaccination may have increased the incidence of GD. | |
| Retrospective [81] | 726 | 40 (30–53) | 541 | 726 | - | - | 191 | 281 | 254 | 275.7 ± 144.4 | No association between COVID-19 vaccination and the incidence of GD. | |
4. Pituitary Gland and SARS-CoV-2 Vaccine
5. Adrenal Glands and SARS-CoV-2 Vaccine
6. SARS-CoV-2 Vaccination and Female Reproductive System
7. SARS-CoV-2 Vaccination and Male Reproductive System
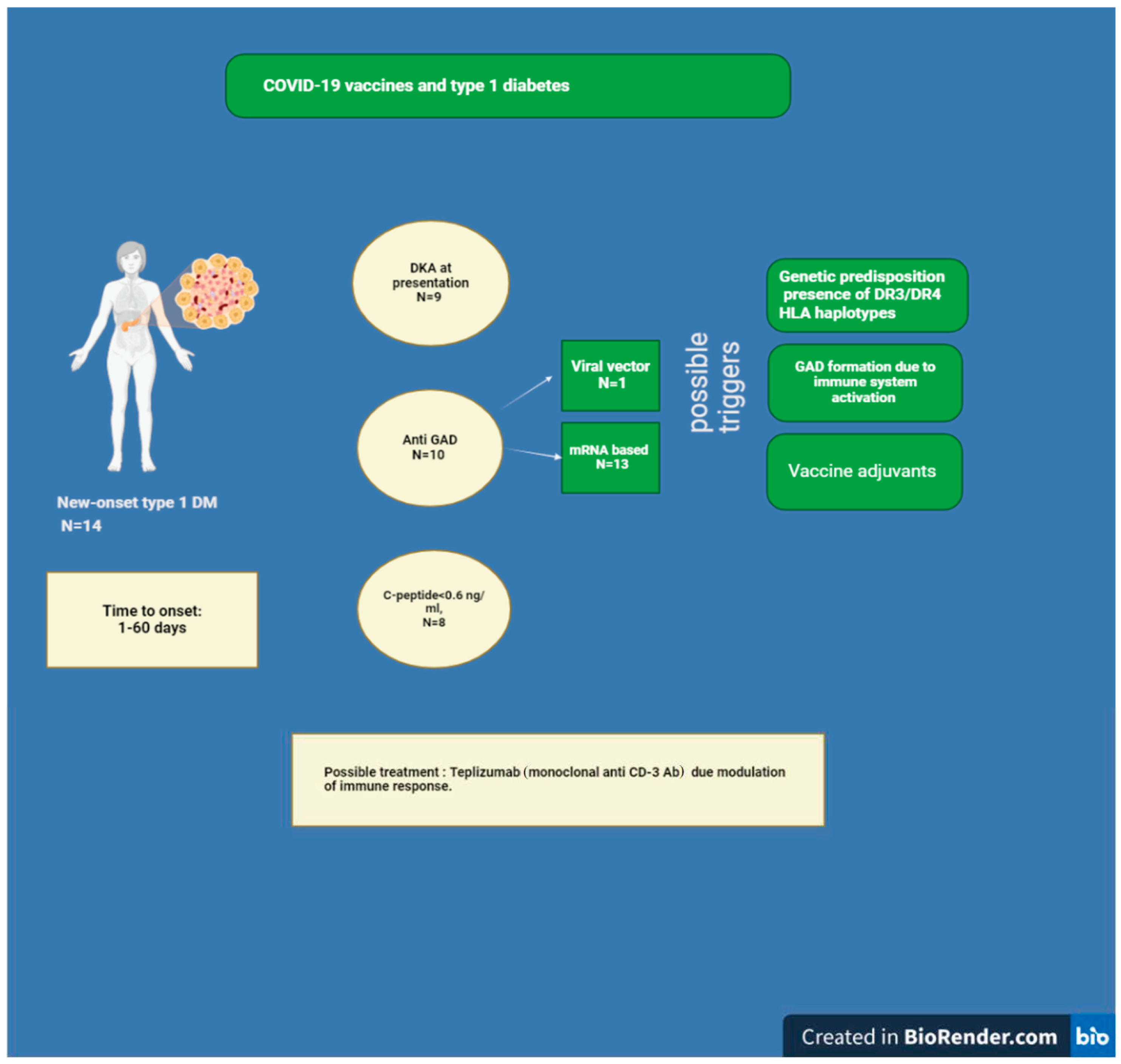
8. Diabetes Mellitus and COVID-19 Vaccine
8.1. Diabetes Mellitus and SARS-CoV-2 Vaccination
8.2. Could the COVID-19 Vaccine Elicit GAD Antibody Formation?
8.3. Relationship between Immune Checkpoint Inhibitors Therapy and the Onset of Type 1 Diabetes Following SARS-CoV-2 Vaccination
8.4. Adjuvants and Type 1 Diabetes
9. Discussion
9.1. Comparative Studies
9.2. Causality
- Temporal relationship: vaccine exposure must precede the event, which is the only criterion essential to establish causality.
- Strength of association, which is based on the statistical analysis of the extensive AEFI database, like the Vaccine Adverse Event Reporting System (VAERS) in the USA [215], or other country-specific systems, like the COVID-19 Vaccine Safety Research Center established in September 2022 at the request of the Korea Disease Control and Prevention Agency [216].
- Dose–response relationship, but in the case of vaccines, these parameters are generally fixed.
- Consistency of evidence.
- Specificity: the vaccine is the only trigger of the adverse event.
- Biological plausibility and coherence: the association between the AEFI and the vaccine should be compatible with the current knowledge of the biology of the vaccine and the AEFI.
9.3. Thyroid Disorders
9.4. Diabetes Mellitus
9.5. Fertility
9.6. Rare Adverse Events and Underreporting
9.7. Limitations of the Reviewed Studies
- Sample Size and Power
- 2.
- Selection and Reporting Bias
- 3.
- Lack of Control Groups
- 4.
- Heterogeneity in Study Designs and Definitions
- 5.
- Temporal Association vs. Causality
- 6.
- Limited Long-Term Follow-Up
- 7.
- Confounding Factors
- 8.
- Variability in Vaccine Types
- 9.
- Potential for Nocebo Effect
- 10.
- Limited Data on Certain Endocrine Disorders
10. Conclusions
- Continue to advocate for COVID-19 vaccination, given the overall benefit in preventing severe disease. Despite a notable reduction in severity and mortality rates in various regions, COVID-19 remains a significant global health concern with continued new cases and societal impacts
- Monitoring patients with preexisting endocrine disorders closely post-vaccination for any changes in their condition.
- Report any suspected adverse events post-vaccination to relevant systems to contribute to safety monitoring and research.
- Consider individual patient risk factors when advising on vaccination and managing post-vaccination symptoms.
- Educate patients about possible adverse events while emphasizing the overall benefits of vaccination.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization Coronavirus (COVID-19) Dashboard. Available online: http://covid19.who.int (accessed on 30 June 2024).
- Kudlay, D.; Svistunov, A. COVID-19 Vaccines: An Overview of Different Platforms. Bioengineering 2022, 9, 72. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Quadeer, A.A.; Ahmed, S.F.; McKay, M.R. Landscape of epitopes targeted by T cells in 852 individuals recovered from COVID-19: Meta-analysis, immunoprevalence, and web platform. Cell Rep. Med. 2021, 2, 100312. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X. Influence of COVID-19 vaccines on endocrine system. Endocrine 2022, 78, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Ishay, A.; Shacham, E.C. Central diabetes insipidus: A late sequela of BNT162b2 SARS-CoV-2 mRNA vaccine? BMC Endocr. Disord. 2023, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Asghar, N.; Mumtaz, H.; Syed, A.A.; Eqbal, F.; Maharjan, R.; Bamboria, A.; Shrestha, M. Safety, efficacy, and immunogenicity of COVID-19 vaccines; a systematic review. Immunol. Med. 2022, 45, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Dhamanti, I.; Suwantika, A.A.; Adlia, A.; Yamani, L.N.; Yakub, F. Adverse Reactions of COVID-19 Vaccines: A Scoping Review of Observational Studies. Int. J. Gen. Med. 2023, 16, 609–618. [Google Scholar] [CrossRef]
- Olivieri, B.; Betterle, C.; Zanoni, G. Vaccinations and autoimmune diseases. Vaccines 2021, 9, 815. [Google Scholar] [CrossRef]
- Jena, A.; Mishra, S.; Deepak, P.; Kumar, M.P.; Sharma, A.; Patel, Y.I.; Kennedy, N.A.; Kim, A.H.; Sharma, V.; Sebastian, S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: Systematic review and meta-analysis. Autoimmun. Rev. 2022, 21, 102927. [Google Scholar] [CrossRef]
- Pezzaioli, L.C.; Gatta, E.; Bambini, F.; Facondo, P.; Gava, M.; Cavadini, M.; Cappelli, C. Endocrine system after 2 years of COVID-19 vaccines: A narrative review of the literature. Front. Endocrinol. 2022, 13, 1027047. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Kharrazian, D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front. Immunol. 2021, 11, 3679. [Google Scholar] [CrossRef] [PubMed]
- Iremli, B.G.; Sendur, S.N.; Onliitlirk, U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 2600–2605. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 45, 1835–1863. [Google Scholar] [CrossRef]
- Stasiak, M.; Lewiński, A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev. Endocr. Metab. Disord. 2021, 22, 1027–1039. [Google Scholar] [CrossRef]
- Şendur, S.N.; Özmen, F.; Oğuz, S.H.; İremli, B.G.; Malkan Ü, Y.; Gürlek, A.; Ünlütürk, U. Association of human leukocyte antigen genotypes with severe acute respiratory syndrome coronavirus 2 vaccine-induced subacute thyroiditis. Thyroid 2022, 32, 640–647. [Google Scholar] [CrossRef]
- Ie, K.; Ishizuka, K.; Sakai, T.; Motohashi, I.; Asai, S.; Okuse, C. Subacute thyroiditis developing within 2 days of vaccination against COVID-19 with BNT162b2 mRNA. Eur. J. Case Rep. Intern. Med. 2023, 10. [Google Scholar] [CrossRef]
- Tomic, A.Z.; Zafirovic, S.S.; Gluvic, Z.M.; Samardzic, V.S.; Macvanin, M.T.; Radunovic, M.L.; Isenovic, E.R. Subacute thyroiditis following COVID-19 vaccination: Case presentation. Antivir. Ther. 2023, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Franquemont, S.; Galvez, J. Subacute Thyroiditis After mRNA vaccine for Covid-19. J. Endocr. Soc. 2021, 5, A956–A957. [Google Scholar] [CrossRef]
- Pujol, A.; Gómez, L.A.; Gallegos, C.; Nicolau, J.; Sanchís, P.; González-Freire, M.; Masmiquel, L. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: From Graves’ disease to silent thyroiditis. J. Endocrinol. Investig. 2022, 45, 875–882. [Google Scholar] [CrossRef]
- Saygili, E.S.; Karakilic, E. Subacute thyroiditis after inactive SARS-Co V-2 vaccine. BMJ Case Rep. 2021, 14, e244711. [Google Scholar] [CrossRef] [PubMed]
- Sahin Tekin, M.; Sayhsoy, S.; Yorulmaz, G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: A case report. Hum. Vaccines Immunother. 2021, 17, 4090–4092. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, C.; Woyk, K.; Bouter, C. Case Report: Two Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccination. Front. Med. 2021, 8, 737142. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Enriquez, L.; Khatiwada, P.; Sanchez-Valenzuela, M.; Sikha, A. A Case Report of Subacute Thyroiditis following mRNA COVID-19 Vaccine. Case Rep. Endocrinol. 2021, 2021, 8952048. [Google Scholar] [CrossRef] [PubMed]
- Siolos, A.; Gartzonika, K.; Tigas, S. Thyroiditis following vaccination against COVID-19: Report of two cases and review of the literature. Metabol. Open 2021, 12, 100136. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.; Alba, E.L.; Chen, A.; Russell, M.; Srinath, R. Thyroiditis and Thyrotoxicosis After the SARS-CoV-2 mRNA Vaccine. Thyroid 2021, 31, 1440. [Google Scholar] [CrossRef]
- Oyibo, S.O. Subacute Thyroiditis After Receiving the Adenovirus-Vectored Vaccine for Coronavirus Disease (COVID-19). Cureus 2021, 13, e16045. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, G.M.; Dworakowska, D.; Grossman, A.B. Can COVID-19 immunization cause subacute thyroiditis? Clin. Endocrinol. 2021, 97, 140–141. [Google Scholar] [CrossRef]
- Chatzi, S.; Karampela, A.; Spiliopoulou, C.; Boutzios, G. Subacute thyroiditis after SARS-CoV-2 vaccination: A report of two sisters and summary of the literature. Hormones 2022, 21, 177–179. [Google Scholar] [CrossRef]
- Jeeyavudeen, M.S.; Patrick, A.W.; Gibb, F.W.; Dover, A.R. COVID-19 vaccine-associated subacute thyroiditis: An unusual suspect for de Quervain’s thyroiditis. BMJ Case Rep. 2021, 14, e246425. [Google Scholar] [CrossRef]
- Kyriacou, A.; Ioakim, S.; Syed, A.A. COVID-19 vaccination and a severe pain in the neck. Eur. J. Intern. Med. 2021, 94, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Soltanpoor, P.; Norouzi, G. Subacute thyroiditis following COVID-19 vaccination. Clin. Case Rep. 2021, 9, e04812. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kim, Y.J.; Jin, H.Y. Thyrotoxicosis after COVID-19 vaccination: Seven case reports and a literature review. Endocrine 2021, 74, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Brassill, M.J. Subacute thyroiditis post-Pfizer-BioNTech mRNA vaccination for COVID-19. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021. [Google Scholar] [CrossRef]
- Leber, H.M.; Sant’Ana, L.; Konichi da Silva, N.R.; Raio, M.C.; Mazzeo TJ, M.M.; Endo, C.M.; de Souza, C.E. Acute Thyroiditis and Bilateral Optic Neuritis following SARS-CoV-2 Vaccination with CoronaVac: A Case Report. Ocul. Immunol. Inflamm. 2021, 29, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Sozen, M.; Topaloglu, Ö.; Çetinarslan, B.; Selek, A.; Cantlirk, Z.; Gezer, E.; Bayraktaroğlu, T. COVID-19 mRNA vaccine may trigger subacute thyroiditis. Hum. Vaccines Immunother. 2021, 17, 5120–5125. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Thota, G.; Wang, X.; Luo, H. Thyroiditis after Coronavirus Disease 2019 (COVID-19) mRNA Vaccine: A Case Series. AACE Clin. Case Rep. 2021, 8, 116–118. [Google Scholar] [CrossRef]
- Sigstad, E.; Grnholt, K.K.; Westerheim, O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr. Nor Laegeforen. 2021, 141. [Google Scholar] [CrossRef]
- Gonzalez Lopez, J.; Martin Nifio, I.; Arana Molina, C. Subacute thyroiditis after SARS-CoV-2 vaccination: Report of two clinical cases. Med. Clin. 2021, 158, e13. [Google Scholar] [CrossRef]
- Rebollar, A.F. Subacute Thyroiditis after Anti SARS-CoV-2 (Ad5-nCoV) Vaccine. Enfermedades Infecc. Microbiol. Clin. 2021, 40, 459–460. [Google Scholar] [CrossRef]
- Ippolito, S.; Gallo, D.; Rossini, A.; Patera, B.; Lanzo, N.; Fazzino, G.F.M.; Tanda, M.L. SARS-CoV-2 vaccine-associated subacute thyroiditis: Insights from a systematic review. J. Endocrinol. Investig. 2022, 45, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Yorulmaz, G.; Sahin Tekin, M. SARS-CoV-2 vaccine-associated subacute thyroiditis. J. Endocrinol. Investig. 2022, 45, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Cunnane, M.E.; Deschler, D.G. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am. J. Otolaryngol. 2022, 43, 103211. [Google Scholar] [CrossRef] [PubMed]
- Pla Peris, B.; Merchante Alfaro, A.; Maravall Royo, F.J.; Abellan Galiana, P.; Perez Naranjo, S.; Gonzalez Boillos, M. Thyrotoxicosis following SARS-COV-2 vaccination: A case series and discussion. J. Endocrinol. Investig. 2022, 45, 1071–1077. [Google Scholar] [CrossRef]
- Bostan, H.; Unsal, I.O.; Kizilgul, M.; Gul, U.; Sencar, M.E.; Ucan, B.; Cakal, E. Two cases of subacute thyroiditis after different types of SARS-CoV-2 vaccination. Arch. Endocrinol. Metab. 2022, 66, 97–103. [Google Scholar] [CrossRef]
- Jhon, M.; Lee, S.H.; Oh, T.H.; Kang, H.C. Subacute Thyroiditis After Receiving the mRNA COVID-19 Vaccine (Modema): The First Case Report and Literature Review in Korea. J. Korean Med. Sci. 2022, 37, e39. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Albizua-Madariaga, I.; Lertxundi, U.; Aguirre, C. Subacute thyroiditis and COVID-19 vaccines: A case/non-case study. Endocrine 2022, 77, 480–485. [Google Scholar] [CrossRef]
- Topaloğlu, Ö.; Tekin, S.; Topaloğlu, S.N.; Bayraktaroglu, T. Differences in clinical aspects between subacute thyroiditis associated with COVID-19 vaccines and classical subacute thyroiditis. Horm. Metab. Res. 2022, 54, 380–388. [Google Scholar] [CrossRef]
- Bostan, H.; Kayihan, S.; Calapkulu, M.; Hepsen, S.; Gul, U.; Ozturk Unsal, I.; Ucan, B. Evaluation of the diagnostic features and clinical course of COVID-19 vaccine-associated subacute thyroiditis. Hormones 2022, 21, 447–455. [Google Scholar] [CrossRef]
- Batman, A.; Yazıcı, D.; Dikbaş, O.; Ağbaht, K.; Saygılı, E.S.; Demirci, I.; Bursa, N.; Ayas, G.; Anıl, C.; Cesur, M.; et al. Subacute THYROiditis Related to SARS-CoV-2 VAccine and Covid-19 (THYROVAC Study): A Multicenter Nationwide Study. J. Clin. Endocrinol. Metab. 2023, 108, e1013–e1026. [Google Scholar] [CrossRef]
- Zettinig, G.; Krebs, M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J. Endocrinol. Investig. 2022, 45, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Vera-Lastra, O.; Ordinola Navarro, A.; Cruz Domiguez, M.P.; Medina, G.; Sanchez Valadez, T.I.; Jara, L.J. Two Cases of Graves’ Disease Following SARS-CoV-2 Vaccination: An Autoimmune/Inflammatory Syndrome Induced by Adjuvants. Thyroid 2021, 31, 1436–1439. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, K.K.; Lee, C.H.; Lee, A.C.H.; Hung, I.F.N.; Tan, K.C.B. Development of Graves’ Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review. Front. Public Health 2021, 9, 778964. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.A.; Ameer, B.; Sinha Gregory, N. Graves Disease Following the SARS-CoV-2 Vaccine: Case Series. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211063356. [Google Scholar] [CrossRef] [PubMed]
- Sriphrapradang, C.; Shantavasinkul, P.C. Graves’ disease following SARS-CoV-2 vaccination. Endocrine 2021, 74, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Pierman, G.; Delgrange, E.; Jonas, C. Recurrence of Graves’ Disease (a Thl-type Cytokine Disease) Following SARS-CoV-2 mRNA Vaccine Administration: A Simple Coincidence? Eur. J. Case Rep. Intern. Med. 2021, 8, 002807. [Google Scholar]
- Patrizio, A.; Ferrari, S.M.; Antonelli, A.; Fallahi, P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J. Autoimmun. 2021, 125, 102738. [Google Scholar] [CrossRef]
- Goblirsch, T.J.; Paulson, A.E.; Tashko, G.; Mekonnen, A.J. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021, 14, e246432. [Google Scholar] [CrossRef]
- Sriphrapradang, C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine 2021, 74, 226–227. [Google Scholar] [CrossRef]
- Rubinstein, T.J. Thyroid Eye Disease Following COVlD-19 Vaccine in a Patient With a History Graves’ Disease: A Case Report. Ophthalmic Plast. Reconstr. Surg. 2021, 37, e221–e223. [Google Scholar] [CrossRef]
- di Filippo, L.; Castellino, L.; Giustina, A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine 2022, 75, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Sawsen, N.; Asma, B.A.; Ghada, S.; Hamza, E.; Yosra, H.; Koussay, A. A rare case of grave’s disease after SARS-CoV-2 vaccine: Is it an adjuvant effect? Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2627–2630. [Google Scholar] [PubMed]
- Chee, Y.J.; Liew, H.; Hoi, W.H.; Lee, Y.; Lim, B.; Chin, H.X.; Dalan, R. SARS-CoV-2 mRNA Vaccination and Graves’ Disease: A report of 12 cases and review of the literature. J. Clin. Endocrinol. Metab. 2022, 107, e2324–e2330. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, A.; Ferrari, S.M.; Antonelli, A.; Fallahi, P. Worsening of Graves’ ophthalmopathy after SARS- CoV-2 mRNA vaccination. Autoimmun. Rev. 2022, 21, 103096. [Google Scholar] [CrossRef]
- Park, K.S.; Fung, S.E.; Ting, M.; Ozzello, D.J.; Yoon, J.S.; Liu, C.Y.; Kikkawa, D.O. Thyroid eye disease reactivation associated with COVID-19 vaccination. Taiwan J. Ophthalmol. 2022, 12, 93–96. [Google Scholar] [PubMed]
- Hamouche, W.; El Soufi, Y.; Alzaraq, S.; Okafor, B.V.; Zhang, F.; Paras, C. A case report of new onset graves’ disease induced by SARS-CoV-2 infection or vaccine? J. Clin. Transl. Endocrinol. Case Rep. 2022, 23, 100104. [Google Scholar] [CrossRef] [PubMed]
- Bostan, H.; Ucan, B.; Kizilgul, M.; Calapkulu, M.; Hepsen, S.; Gul, U.; Cakal, E. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J. Autoimmun. 2022, 128, 102809. [Google Scholar] [CrossRef]
- Singh, G.; Howland, T. Graves’ Disease Following COVID-19 Vaccination. Cureus 2022, 14, e24418. [Google Scholar] [CrossRef]
- Chua, M.W.J. Graves’ disease after COVID-19 vaccination. Ann. Acad. Med. Singap. 2022, 51, 127–128. [Google Scholar] [CrossRef]
- Manta, R.; Martin, C.; Muls, V.; Poppe, K.G. New-onset Graves’ disease following SARS-CoV-2 vaccination: A case report. Eur. Thyroid J. 2022, 11. [Google Scholar] [CrossRef]
- Sakai, M.; Takao, K.; Kato, T.; Ito, K.; Kubota, S.; Hirose, T.; Yabe, D. Graves’ Disease after Administration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in a Type 1 Diabetes Patient. Intern. Med. 2022, 61, 1561–1565. [Google Scholar] [CrossRef]
- Cuenca, D.; Aguilar-Soto, M.; Mercado, M. A Case of Graves’ Disease Following Vaccination with the Oxford-AstraZeneca SARS-CoV-2 Vaccine: Case Report and Review of the Literature. Eur. J. Case Rep. Intern. Med. 2022, 9, 003275. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Giovanellla, L.; Campenni, A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: Real-life experience and review of the literature. J. Endocrinol. Investig. 2022, 45, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Takedani, K.; Notsu, M.; Ishiai, N.; Asami, Y.; Uchida, K.; Kanasaki, K. Graves’ disease after exposure to the SARS-CoV-2 vaccine: A case report and review of the literature. BMC Endocr. Disord. 2023, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Awaya, T.; Ohira, M.; Enomoto, Y.; Moroi, M.; Nakamura, M. Graves’ Disease after mRNA COVID-19 Vaccination, with the Presence of Autoimmune Antibodies Even One Year Later. Vaccines 2023, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.C.; Luo, R.R. Thyrotoxicosis in patients with a history of Graves’ disease after SARS-CoV-2 vaccination (adenovirus vector vaccine): Two case reports. World J. Clin. Cases 2023, 11, 1122. [Google Scholar] [CrossRef]
- Yasuda, S.; Suzuki, S.; Yanagisawa, S.; Morita, H.; Haisa, A.; Satomura, A.; Shimada, A. HLA typing of patients who developed subacute thyroiditis and Graves’ disease after SARS-CoV-2 vaccination: A case report. BMC Endocr. Disord. 2023, 23, 54. [Google Scholar] [CrossRef]
- di Filippo, L.; Castellino, L.; Allora, A.; Frara, S.; Lanzi, R.; Perticone, F.; Valsecchi, F.; Vassallo, A.; Giubbini, R.; Rosen, C.J.; et al. Distinct Clinical Features of Post-COVID-19 Vaccination Early-onset Graves’ Disease. J. Clin. Endocrinol. Metab. 2023, 108, 107–113. [Google Scholar] [CrossRef]
- Barajas Galindo, D.E.; Ramos Bachiller, B.; González Roza, L.; García Ruiz de Morales, J.M.; Sánchez Lasheras, F.; González Arnáiz, E.; Ariadel Cobo, D.; Ballesteros Pomar, M.D.; Rodríguez, I.C. Increased incidence of Graves’ disease during the SARS-CoV2 pandemic. Clin. Endocrinol. 2023, 98, 730–737. [Google Scholar] [CrossRef]
- Gorshtein, A.; Turjeman, A.; Duskin-Bitan, H.; Leibovici, L.; Robenshtok, E. Graves’ Disease Following COVID-19 Vaccination: A Population-based, Matched Case-control Study. J. Clin. Endocrinol. Metab. 2024, 109, e508–e512. [Google Scholar] [CrossRef]
- Peng, K.; Li, X.; Yang, D.; Chan, S.C.; Zhou, J.; Wan, E.Y.; Chui, C.S.; Lai, F.T.; Wong, C.K.; Chan, E.W.; et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: A population-based cohort study. eClinicalMedicine 2023, 63, 102154. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Lui, D.T.; Xiong, X.; Chui, C.S.; Lai, F.T.; Li, X.; Wan, E.Y.; Cheung, C.L.; Lee, C.H.; Woo, Y.C.; et al. Risk of thyroid dysfunction associated with mRNA and inactivated COVID-19 vaccines: A population-based study of 2.3 million vaccine recipients. BMC Med. 2022, 20, 339. [Google Scholar] [CrossRef] [PubMed]
- Abeillon-du Payrat, J.; Grunenwald, S.; Gall, E.; Ladsous, M.; Raingeard, I.; Caron, P. Graves’ orbitopathy post-SARS-CoV-2 vaccines: Report on six patients. J. Endocrinol. Investig. 2023, 46, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Im Teoh, J.H.; Mustafa, N.; Wahab, N. New-onset Thyroid Eye Disease after COVID-19 Vaccination in a Radioactive Iodine-Treated Graves’ Disease Patient: A Case Report and Literature Review. J. ASEAN Fed. Endocr. Soc. 2023, 38, 125–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aliberti, L.; Gagliardi, I.; Rizzo, R.; Bortolotti, D.; Schiuma, G.; Franceschetti, P.; Ambrosio, M.R. Pituitary apoplexy and COVID-19 vaccination: A case report and literature review. Front. Endocrinol. 2022, 13, 1035482. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Manenti, A. Pituitary apoplexy following adenoviral vector-based COVID-19 vaccination. Brain Hemorrhages 2023, 4, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Zainordin, N.A.; Hatta, S.F.W.M.; Ab Mumin, N.; Shah, F.Z.M.; Ghani, R.A. Pituitary apoplexy after COVID-19 vaccination: A case report. J. Clin. Transl. Endocrinol. Case Rep. 2022, 25, 100123. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, S.; Jabbour, S. Abstract #1001394: A Rare Endocrine Complication of the COVID-19 Vaccine. Endocr. Pract. 2021, 27, S116–S117. [Google Scholar]
- Piñar-Gutiérrez, A.; Remón-Ruiz, P.; Soto-Moreno, A. Case report: Pituitary apoplexy after COVID-19 vaccination. Med. Clin. (Engl. Ed.) 2022, 158, 498–499. [Google Scholar] [CrossRef]
- Fernandez, A.; Karavitaki, N.; Wass, J.A.H. Prevalence of pituitary adenomas: A community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 2010, 72, 377–382. [Google Scholar] [CrossRef]
- Briet, C.; Salenave, S.; Bonneville, J.F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- Bujawansa, S.; Thondam, S.K.; Steele, C.; Cuthbertson, D.J.; Gilkes, C.E.; Noonan, C.; Bleaney, C.W.; MacFarlane, I.A.; Javadpour, M.; Daousi, C. Presentation, management and outcomes in acute pituitary apoplexy: A large single-centre experience from the United Kingdom. Clin. Endocrinol. 2014, 80, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Mounira, E.E. Pilot Findings on SARS-CoV-2 Vaccine-Induced Pituitary Diseases: A Mini Review from Diagnosis to Pathophysiology. Vaccines 2022, 10, 2004. [Google Scholar] [CrossRef]
- Taieb, A.; Asma, B.A.; Mounira, E.E. Evidences that SARS-CoV-2 Vaccine-Induced apoplexy may not be solely due to ASIA or VITT syndrome’, Commentary on Pituitary apoplexy and COVID-19 vaccination: A case report and literature review. Front. Endocrinol. 2023, 14, 1111581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prete, A.; Salvatori, R. Hypophysitis. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Bouça, B.; Roldão, M.; Bogalho, P.; Cerqueira, L.; Silva-Nunes, J. Central Diabetes Insipidus Following Immunization with BNT162b2 mRNA COVID-19 Vaccine: A Case Report. Front. Endocrinol. 2022, 13, 889074. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Kammoun, F.; El Fekih, H.; Slama NB, H.; Kahloun, S.; Fredj, F.B.; Ach, K. Central diabetes insipidus revealing a hypophysitis induced by SARS-CoV-2 vaccine. Therapie 2023, 78, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Partenope, C.; Pedranzini, Q.; Petri, A.; Rabbone, I.; Prodam, F.; Bellone, S. AVP deficiency (central diabetes insipidus) following immunization with anti-COVID-19 BNT162b2 Comirnaty vaccine in adolescents: A case report. Front. Endocrinol. 2023, 14, 1166953. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Okubo, K.; Mifune, H.; Imao, T. Bilateral Optic Neuritis and Hypophysitis With Diabetes Insipidus 1 Month After COVID-19 mRNA Vaccine: Case Report and Literature Review. J. Investig. Med. High Impact Case Rep. 2023, 11, 23247096231186046. [Google Scholar] [CrossRef]
- Murvelashvili, N.; Tessnow, A. A Case of Hypophysitis Following Immunization With the mRNA-1273 SARS-CoV-2 Vaccine. J. Investig. Med. High Impact Case Rep. 2021, 9, 23247096211043386. [Google Scholar] [CrossRef]
- Ankireddypalli, A.R.; Chow, L.S.; Radulescu, A.; Kawakami, Y.; Araki, T. A Case of Hypophysitis Associated With SARS-CoV-2 Vaccination. AACE Clin Case Rep. 2022, 8, 204–209. [Google Scholar] [CrossRef]
- Morita, S.; Tsuji, T.; Kishimoto, S.; Uraki, S.; Takeshima, K.; Iwakura, H.; Matsuoka, T.A. Isolated ACTH deficiency following immunization with the BNT162b2 SARS-CoV-2 vaccine: A case report. BMC Endocr. Disord. 2022, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Ryser, B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: Case report. BMC Infect. Dis. 2021, 21, 1000. [Google Scholar] [CrossRef]
- Jara, L.J.; Vera-Lastra, O.; Mahroum, N.; Pineda, C.; Shoenfeld, Y. Autoimmune post-COVID vaccine syndromes: Does the spectrum of autoimmune/inflammatory syndrome expand? Clin. Rheumatol. 2022, 41, 1603–1609. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, B. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F. The spike hypothesis in vaccine-induced adverse effects: Questions and answers. Trends Mol. Med. 2022, 28, 797–799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boschi, C.; Scheim, D.E.; Bancod, A.; Militello, M.; Bideau, M.L.; Colson, P.; Scola, B.L. SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. Int. J. Mol. Sci. 2022, 23, 15480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Guruparan, T.; Siddiqi, S.; Sheth, R.; Jacyna, M.; Naghibi, M.; Vrentzou, E. A case of adrenal infarction in a patient with COVID 19 infection. BJR Case Rep. 2020, 6, 20200075. [Google Scholar] [CrossRef]
- Leyendecker, P.; Ritter, S.; Riou, M.; Wackenthaler, A.; Meziani, F.; Roy, C.; Ohana, M. Acute adrenal infarction as an incidental CT finding and a potential prognosis factor in severe SARS-CoV-2 infection: A retrospective cohort analysis on 219 patients. Eur. Radiol. 2021, 31, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Frankel, M.; Feldman, I.; Levine, M.; Frank, Y.; Bogot, N.R.; Benjaminov, O.; Munter, G. Bilateral Adrenal Hemorrhage in Coronavirus Disease 2019 Patient: A Case Report. J. Clin. Endocrinol. Metab. 2020, 105, dgaa487. [Google Scholar] [CrossRef]
- Álvarez-Troncoso, J.; Larrauri, M.Z.; Vega MD, M.; Vallano, R.G.; Peláez, E.P.; Rojas-Marcos, P.M.; Esteban, E.T. Case Report: COVID-19 with Bilateral Adrenal Hemorrhage. Am. J. Trop. Med. Hyg. 2020, 103, 1156–1157. [Google Scholar] [CrossRef]
- Arlt, W.; Society for Endocrinology Clinical Committee. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr. Connect. 2016, 5, G1–G3. [Google Scholar] [CrossRef] [PubMed]
- Dineen, R.; Thompson, C.J.; Sherlock, M. Adrenal crisis: Prevention and management in adult patients. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819848218. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.; McLaren, D.S.; Rasool, I.; Shah, P.M.; Lynch, J.; Murray, R.D. ChAdOx1 SARS-CoV-2 vaccination: A putative precipitant of adrenal crises. Clin. Endocrinol. 2023, 99, 470–473. [Google Scholar] [CrossRef] [PubMed]
- ADSHG Coronavirus Vaccines and Adrenal Insufficiency. Bristol: Addison’s Disease Self-Help Group. 2021. Available online: https://www.addisonsdisease.org.uk/coronavirus-vaccines (accessed on 30 June 2024).
- Katznelson, L.; Gadelha, M. Glucocorticoid use in patients with adrenal insufficiency following administration of the COVID-19 vaccine: A pituitary society statement. Pituitary 2021, 24, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Pilli, T.; Dalmiglio, C.; Dalmazio, G.; Sagnella, A.; Forleo, R.; Brilli, L.; Castagna, M.G. No need of glucocorticoid dose adjustment in patients with adrenal insufficiency before COVID-19 vaccine. Eur. J. Endocrinol. 2022, 187, K7–K11. [Google Scholar] [CrossRef] [PubMed]
- Haji, N., Jr.; Ali, S.; Wahashi, E.A.; Khalid, M.; Ramamurthi, K. Johnson and Johnson COVID-19 Vaccination Triggering Pheochromocytoma Multisystem Crisis. Cureus 2021, 13, e18196. [Google Scholar] [CrossRef] [PubMed]
- Markovic, N.; Faizan, A.; Boradia, C.; Nambi, S. Adrenal Crisis Secondary to COVID-19 Vaccination in a Patient With Hypopituitarism. AACE Clin Case Rep. 2022, 8, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Allen, L.; Shrikrishnapalasuriyar, N.; Stechman, M.; Rees, A. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage. Clin. Endocrinol. 2022, 97, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Varona, J.F.; García-Isidro, M.; Moeinvaziri, M.; Ramos-López, M.; Fernández- Domínguez, M. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur. J. Intern. Med. 2021, 91, 90–92. [Google Scholar] [CrossRef]
- Tews, H.C.; Driendl, S.M.; Kandulski, M.; Buechler, C.; Heiss, P.; Stöckert, P.; Schmid, S. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia with venous thrombosis, pulmonary embolism, and adrenal haemorrhage: A case report with literature review. Vaccines 2022, 10, 595. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A.M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb. Haemost. 2021, 19, 1771–1775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Grassi, R. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Al Rawahi, B.; BaTaher, H.; Jaffer, Z.; Al-Balushi, A.; Al-Mazrouqi, A.; Al-Balushi, N. Vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine—A case report. Res. Pract. Thromb. Haemost. 2021, 5, e12578. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Armeni, E.; Dickinson, L.; Stubbs, M.; Craven, B.; Srirangalingam, U.; Chung, T.T. Adrenal haemorrhage and infarction in the setting of vaccine-induced immune thrombocytopenia and thrombosis after SARS-CoV-2 (Oxford–AstraZeneca) vaccination. Endocrinol. Diabetes Metab. Case Rep. 2022, 2022. [Google Scholar] [CrossRef]
- Efthymiadis, A.; Khan, D.; Pavord, S.; Pal, A. A case of ChAdOx1 vaccine-induced thrombocytopenia and thrombosis syndrome leading to bilateral adrenal haemorrhage and adrenal insufficiency. Endocrinol. Diabetes Metab. Case Rep. 2022, 2022. [Google Scholar] [CrossRef]
- Tha, T.; Martini, I.; Stefan, E.; Redla, S. Bilateral adrenal haemorrhage with renal infarction after ChAdOx1 nCoV-19 AstraZeneca vaccination. BJR Case Rep. 2022, 8, 20210139. [Google Scholar] [CrossRef] [PubMed]
- Douxfils, J.; Vayne, C.; Pouplard, C.; Lecompte, T.; Favresse, J.; Potier, F.; Mullier, F. Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic thrombocytopenia syndrome after initial successful therapy with intravenous immunoglobulins—A rational for monitoring immunoglobulin G levels. Haematologica 2021, 106, 3249–3252. [Google Scholar] [CrossRef] [PubMed]
- Boyle, L.D.; Morganstein, D.L.; Mitra, I.; Nogueira, E.F. A rare case of multiple thrombi and left adrenal haemorrhage following COVID-19 vaccination. Endocr. Abstr. 2021, 74, NCC4. [Google Scholar] [CrossRef]
- Ahmad, S.; Zaman, N.; Almajali, K.; Muhammadi, A.; Baburaj, R.; Akavarapu, S. A novel case of bilateral adrenal hemorrhage and acute adrenal insufficiency due to VITT (vaccine induced thrombosis and thrombocytopenia) syndrome. Endocr. Abstr. 2021, 74, OC2. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Iqbal, F.; Arlt, W.; Baldeweg, S.E.; Levy, M.; Stewart, P.M.; Ronchi, C.L. COVID-19-related adrenal hemorrhage: Multicentre UK experience and systematic review of the literature. Clin. Endocrinol. 2023, 98, 766–778. [Google Scholar] [CrossRef]
- Elalamy, I.; Gerotziafas, G.; Alamowitch, S.; Laroche, J.P.; Van Dreden, P.; Ageno, W.; Scientific Reviewer Committee. SARS-CoV-2 Vaccine and Thrombosis: An Expert Consensus on Vaccine-Induced Immune Thrombotic Thrombocytopenia. Thromb. Haemost. 2021, 121, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Marchandot, B.; Curtiaud, A.; Trimaille, A.; Sattler, L.; Grunebaum, L.; Morel, O. Vaccine-induced immune thrombotic thrombocytopenia: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. Open 2021, 1, oeab014. [Google Scholar] [CrossRef] [PubMed]
- Harville, E.W. Invited Commentary: Vaccines and Fertility—Why Worry? Am. J. Epidemiol. 2023, 192, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, A.; Stetter, C.; Kunselman, A.R.; Estes, S.J. COVID-19 Vaccination Hesitancy in Women Who Desire Future Fertility/Pregnancy. J. Gynecol. Clin. Obstet. Reprod. Med. 2023, 1, 48–65. [Google Scholar]
- Morris, R.S. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F S Rep. 2021, 2, 253–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coulam, C.B.; Roussev, R.G. Increasing circulating T-cell activation markers are linked to subsequent implantation failure after transfer of in vitro fertilized embryos. Am. J. Reprod. Immunol. 2003, 50, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Moolhuijsen, L.M.; Visser, J.A. Anti-Müllerian hormone and ovarian reserve: Update on assessing ovarian function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Lamanna, B.; Vinciguerra, M.; Tafuri, S.; Stefanizzi, P.; Malvasi, A.; Cascardi, E. SARS-CoV-2 vaccines and adverse effects in gynecology and obstetrics: The first italian retrospective study. Int. J. Environ. Res. Public Health 2022, 19, 13167. [Google Scholar] [CrossRef] [PubMed]
- Kolatorova, L.; Adamcova, K.; Vitku, J.; Horackova, L.; Simkova, M.; Hornova, M.; Duskova, M. COVID-19, vaccination, and female fertility in the Czech Republic. Int. J. Mol. Sci. 2022, 23, 10909. [Google Scholar] [CrossRef]
- Senkaya, A.R.; Çil, Z.; Keskin, Ö.; Güneş, M.E.; Öztekin, D.C. CoronaVac vaccine does not affect ovarian reserve. Ginekol. Pol. 2023, 94, 298–302. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Lin, Y.; Cao, M.; Liang, Z.; Li, L.; Liu, H. Effect of inactivated COVID-19 vaccination on intrauterine insemination cycle success: A retrospective cohort study. Front. Public Health 2022, 10, 966826. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xia, L.; Lin, J.; Liu, B.; Zhao, Y.; Xin, C.; Wu, Q. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: A propensity score-matched study. J. Inflamm. Res. 2022, 15, 839–849. [Google Scholar] [CrossRef]
- Mohr-Sasson, A.; Haas, J.; Abuhasira, S.; Sivan, M.; Doitch Amdurski, H.; Dadon, T.; Rabinovici, J. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum. Reprod. 2022, 37, 534–541. [Google Scholar] [CrossRef]
- Hasdemir, P.S.; Senol Akar, S.; Goker, A.; Kosova, F.; Ucar, D.; Ozalp Ates, F.S.; Akcali, S. The effect of COVID-19 vaccinations on menstrual cycle and serum anti-Mullerian hormone levels in reproductive age women. Hum. Fertil. 2023, 26, 153–161. [Google Scholar] [CrossRef]
- Yildiz, E.; Timur, B.; Guney, G.; Timur, H. Does the SARS-CoV-2 mRNA vaccine damage the ovarian reserve? Medicine 2023, 102, e33824. [Google Scholar] [CrossRef]
- Kumbasar, S.; Salman, S.; Çakmak, G.N.; Gencer, F.K.; Sicakyüz, L.S.; Kumbasar, A.N. Effect of mRNA COVID-19 vaccine on ovarian reserve of women of reproductive age. Ginekol. Pol. 2023, 95, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, T.; Tian, L.; Xia, L.; Xu, D.; Wu, X.; Huang, L.; Chen, M.; Fang, Z.; Xiong, C.; et al. Impact of inactivated COVID-19 vaccination on female ovarian reserve: A propensity score-matched retrospective cohort study. Front. Immunol. 2023, 14, 1198051. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, E.; Mizrachi, Y.; Herman, H.G.; Marcuschamer, E.O.; Shalev, A.; Farhi, J.; Weissman, A. The effect of SARS-CoV-2 mRNA vaccination on AMH concentrations in infertile women. Reprod. Biomed. Online 2022, 45, 779–784. [Google Scholar] [CrossRef]
- Odeh-Natour, R.; Shapira, M.; Estrada, D.; Freimann, S.; Tal, Y.; Atzmon, Y.; Shalom-Paz, E. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am. J. Reprod. Immunol. 2022, 87, e13530. [Google Scholar] [CrossRef]
- Orvieto, R.; Noach-Hirsh, M.; Segev-Zahav, A.; Haas, J.; Nahum, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod. Biol. Endocrinol. 2021, 19, 69. [Google Scholar] [CrossRef]
- Jacobs, E.; Summers, K.; Sparks, A.; Mejia, R. Fresh embryo transfer cycle characteristics and outcomes following in vitro fertilization via intracytoplasmic sperm injection among patients with and without COVID-19 vaccination. JAMA Netw. Open 2022, 5, e228625. [Google Scholar] [CrossRef] [PubMed]
- Requena, A.; Vergara, V.; Gonz’alez-Ravina, C.; Ruiz, M.E.; Cruz, M. The type of SARS-CoV-2 vaccine does not affect ovarian function in assisted reproduction cycle. Fertil. Steril. 2023, 119, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.C.; Nassau, D.E.; Khodamoradi, K.; Ibrahim, E.; Blachman-Braun, R.; Ory, J.; Ramasamy, R. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA 2021, 326, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Barda, S.; Laskov, I.; Grisaru, D.; Lehavi, O.; Kleiman, S.; Wenkert, A.; Michaan, N. The impact of COVID-19 vaccine on sperm quality. Int. J. Gynaecol. Obstet. 2022, 158, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, D.; Haas, J.; Lebovitz, O.; Raviv, G.; Orvieto, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod. BioMed. Online 2022, 44, 145–149. [Google Scholar] [CrossRef]
- Olana, S.; Mazzilli, R.; Salerno, G.; Zamponi, V.; Tarsitano, M.G.; Simmaco, M.; Faggiano, A. 4BNT162b2 mRNA COVID-19 vaccine and semen: What do we know? Andrology 2022, 10, 1023–1029. [Google Scholar] [CrossRef]
- Abd, Z.H.; Muter, S.A.; Saeed, R.A.M.; Ammar, O. Effects of covid-19 vaccination on different semen parameters. Basic Clin. Androl. 2022, 32, 13. [Google Scholar] [CrossRef] [PubMed]
- Gat, I.; Kedem, A.; Dviri, M.; Umanski, A.; Levi, M.; Hourvitz, A.; Baum, M. Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology 2022, 10, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, X.; Zhang, F.; Zhu, Y.; Du, M.R.; Tao, Z.W.; Jiang, F. Evaluation of inactivated COVID-19 vaccine on semen parameters in reproductive-age males: A retrospective cohort study. Asian J. Androl. 2022, 24, 441–444. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Li, Z.; Zhu, Y.; Wei, Z.; He, J.; Cheng, H.; Yang, A.; Chen, F. Effects of inactivated SARS-CoV-2 vaccination on male fertility: A retrospective cohort study. J. Med. Virol. 2023, 95, e28329. [Google Scholar] [CrossRef]
- Reschini, M.; Pagliardini, L.; Boeri, L.; Piazzini, F.; Bandini, V.; Fornelli, G.; Papaleo, E. COVID-19 vaccination does not affect reproductive health parameters in men. Front. Public Health 2022, 10, 839967. [Google Scholar] [CrossRef] [PubMed]
- Safrai, M.; Herzberg, S.; Imbar, T.; Reubinoff, B.; Dior, U.; Ben-Meir, A. The BNT162b2 mRNA COVID-19 vaccine does not impair sperm parameters. Reprod. BioMed. Online 2022, 44, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhao, J.; Hu, Y.; Fang, L.; Wu, S. Investigate the effect of COVID-19 inactivated vaccine on sperm parameters and embryo quality in vitro fertilization. Andrologia 2022, 54, e14483. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Demircan, K.; Weiss, G.; Minich, W.B.; Schenk, M.; Schomburg, L. Detection of antibodies to SARS-CoV-2 after vaccination in seminal plasma and their association to sperm parameters. Int. J. Infect. Dis. 2023, 130, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Yland, J.J.; Wesselink, A.K.; Regan, A.K.; Hatch, E.E.; Rothman, K.J.; Savitz, D.A.; Wang, T.R.; Huybrechts, K.F.; Hernández-Díaz, S.; Eisenberg, M.L.; et al. A prospective cohort study of preconception COVID-19 vaccination and miscarriage. Hum. Reprod. 2023, 38, 2362–2372. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Lewis, S.E. Is sperm evaluation useful in predicting human fertility? Reproduction 2007, 134, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hulme, K.D.; Gallo, L.A.; Short, K.R. Influenza Virus and Glycemic Variability in Diabetes: A Killer Combination? Front. Microbiol. 2017, 8, 861. [Google Scholar] [CrossRef]
- Akamine, C.M.; El Sahly, H.M. Messenger ribonucleic acid vaccines for severe acute respiratory syndrome coronavirus-2—A review. Transl. Res. 2022, 242, 1–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marfella, R.; Sardu, C.; D’Onofrio, N.; Prattichizzo, F.; Scisciola, L.; Messina, V.; Paolisso, G. Glycaemic control is associated with SARS-CoV-2 breakthrough infections in vaccinated patients with type 2 diabetes. Nat. Commun. 2022, 13, 2318. [Google Scholar] [CrossRef]
- Cieślewicz, A.; Dudek, M.; Krela-Kaźmierczak, I.; Jabłecka, A.; Lesiak, M.; Korzeniowska, K. Pancreatic Injury after COVID-19 Vaccine-A Case Report. Vaccines 2021, 9, 576. [Google Scholar] [CrossRef]
- Boskabadi, S.J.; Ala, S.; Heydari, F.; Ebrahimi, M.; Jamnani, A.N. Acute pancreatitis following COVID-19 vaccine: A case report and brief literature review. Heliyon 2023, 9, e12914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cacdac, R.; Jamali, A.; Jamali, R.; Nemovi, K.; Vosoughi, K.; Bayraktutar, Z. Acute pancreatitis as an adverse effect of COVID-19 vaccination. SAGE Open Med. Case Rep. 2022, 10, 2050313X221131169. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, X.; Chen, X.; Li, Q. Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmun. Rev. 2023, 22, 103340. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Ouyang, J.; Hu, X.D.; Wu, N.; Jiang, Z.G.; Bian, N.; Wang, J. Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review. World J. Diabetes 2023, 14, 892–918. [Google Scholar] [CrossRef]
- Bleve, E.; Venditti, V.; Lenzi, A.; Morano, S.; Filardi, T. COVID-19 vaccine and autoimmune diabetes in adults: Report of two cases. J. Endocrinol. Investig. 2022, 45, 1269–1270. [Google Scholar] [CrossRef]
- Moon, H.; Suh, S.; Park, M.K. Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination. J. Korean Med. Sci. 2023, 38, e12. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, B.İ.; Ünlütürk, U.; Cesur, M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine 2022, 78, 42–46. [Google Scholar] [CrossRef]
- Sakurai, K.; Narita, D.; Saito, N.; Ueno, T.; Sato, R.; Niitsuma, S.; Arihara, Z. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J. Diabetes Investig. 2022, 13, 1290–1292. [Google Scholar] [CrossRef]
- Alsudais, A.S.; Alkanani, R.S.; Fathi, A.B.; Almuntashiri, S.S.; Jamjoom, J.N.; Alzhrani, M.A.; Althubaiti, A.; Radi, S. Autoimmune diabetes mellitus after COVID-19 vaccination in adult population: A systematic review of case reports. BMC Endocr. Disord. 2023, 23, 164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, R.; Lin, Y.-W.; Chen, M.-H. Fulminant Type 1 Diabetes Mellitus after SARS-CoV-2 Vaccination: A Case Report. Vaccines 2022, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Morioka, T.; Natsuki, Y.; Sasaki, K.; Kakutani, Y.; Ochi, A.; Yamazaki, Y.; Shoji, T.; Emoto, M. New-onset Type 1 Diabetes after COVID-19 mRNA Vaccination. Intern. Med. 2022, 61, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Morioka, T.; Okada, N.; Natsuki, Y.; Kakutani, Y.; Ochi, A.; Yamazaki, Y.; Shoji, T.; Ohmura, T.; Emoto, M. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. J. Diabetes Investig. 2022, 13, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Kshetree, B.; Lee, J.; Acharya, S. COVID-19 Vaccine-Induced Rapid Progression of Prediabetes to Ketosis-Prone Diabetes Mellitus in an Elderly Male. Cureus 2022, 14, e28830. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, K.; Amagai, R.; Tamabuchi, E.; Kambayashi, Y.; Fujimura, T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J. Dermatol. 2022, 49, e167–e168. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Itoh, A.; Watanabe, Y.; Nakajima, Y.; Saisho, Y.; Irie, J.; Itoh, H. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J. Diabetes Investig. 2022, 13, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Aida, K.; Nishida, Y.; Tanaka, S.; Maruyama, T.; Shimada, A.; Awata, T.; Kobayashi, T. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates beta-cell death in fulminant type 1 diabetes. Diabetes 2011, 60, 884–889. [Google Scholar] [CrossRef]
- Yin, X.; Riva, L.; Pu, Y.; Martin-Sancho, L.; Kanamune, J.; Yamamoto, Y.; Chanda, S.K. MDA5 Governs the Innate Immune Response to SARS-CoV-2 in Lung Epithelial Cells. Cell Rep. 2021, 34, 108628. [Google Scholar] [CrossRef]
- Fiorina, P. GABAergic system in β-cells: From autoimmunity target to regeneration tool. Diabetes 2013, 62, 3674–3676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graus, F.; Saiz, A.; Dalmau, J. GAD antibodies in neurological disorders—Insights and challenges. Nat. Rev. Neurol. 2020, 16, 353–365. [Google Scholar] [CrossRef]
- Tohid, H. Anti-glutamic acid decarboxylase antibody positive neurological syndromes. Neurosciences 2016, 21, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Deniz, Ç.; Altunan, B.; Ünal, A. Anti-GAD Encephalitis Following COVID-19 Vaccination: A Case Report. Noro Psikiyatr. Ars. 2023, 60, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Søgaard, O.S.; Tolstrup, M.; Stærke, N.B.; Lundgren, J.; Østergaard, L.; Hvas, A.M. Inflammation and Platelet Activation After COVID-19 Vaccines—Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front. Immunol. 2021, 12, 779453. [Google Scholar] [CrossRef]
- Chen, X.; Affinati, A.H.; Lee, Y.; Turcu, A.F.; Henry, N.L.; Schiopu, E.; Qin, A.; Othus, M.; Clauw, D.; Ramnath, N.; et al. Immune Checkpoint Inhibitors and Risk of Type 1 Diabetes. Diabetes Care 2022, 45, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruiz, J.I.; Lopez-Olivo, M.A.; Geng, Y.; Suarez-Almazor, M.E. COVID-19 vaccination in patients with cancer receiving immune checkpoint inhibitors: A systematic review and meta-analysis. J. Immunother. Cancer 2023, 11, e006246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, T.; Kodama, S.; Kaneko, K.; Imai, J.; Katagiri, H. Type 1 Diabetes Mellitus Associated with Nivolumab after Second SARS-CoV-2 Vaccination, Japan. Emerg. Infect. Dis. 2022, 28, 1518–1520. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Nakagawa, K.; Yase, E.; Terashima, M.; Murata, T. Diabetic ketoacidosis after the second dose of SARS-CoV-2 mRNA vaccination in a patient with pembrolizumab-induced fulminant type 1 diabetes. Diabetol. Int. 2022, 14, 206–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tervaert JW, C.; Martinez-Lavin, M.; Jara, L.J.; Halpert, G.; Watad, A.; Amital, H.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun. Rev. 2023, 22, 103287. [Google Scholar] [CrossRef]
- Watad, A.; Bragazzi, N.L.; McGonagle, D.; Adawi, M.; Bridgewood, C.; Damiani, G.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin. Immunol. 2019, 203, 1–8. [Google Scholar] [CrossRef]
- Vera-Lastra, O.; Medina, G.; Cruz-Dominguez Mdel, P.; Jara, L.J.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): Clinical and immunological spectrum. Expert Rev. Clin. Immunol. 2013, 9, 361–373. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Hejly, A.; Watad, A.; Adawi, M.; Amital, H.; Shoenfeld, Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101412. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, M.; Lin, J.; Vaghela, S.; Lingohr-Smith, M.; Nguyen, J.L.; Scassellati Sforzolini, T.; Judy, J.; Cane, A.; Moran, M.M. COVID-19 vaccine effectiveness among immunocompromised populations: A targeted literature review of real-world studies. Expert Rev. Vaccines 2022, 21, 435–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirza, S.A.; Sheikh AA, E.; Barbera, M.; Ijaz, Z.; Javaid, M.A.; Shekhar, R.; Sheikh, A.B. COVID-19 and the Endocrine System: A Review of the Current Information and Misinformation. Infect. Dis. Rep. 2022, 14, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Briciu, V.; Topan, A.; Calin, M.; Dobrota, R.; Leucuta, D.C.; Lupse, M. Comparison of COVID-19 Severity in Vaccinated and Unvaccinated Patients during the Delta and Omicron Wave of the Pandemic in a Romanian Tertiary Infectious Diseases Hospital. Healthcare 2023, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Mohr, N.M.; Plumb, I.D.; Harland, K.K.; Pilishvili, T.; Fleming-Dutra, K.E.; Krishnadasan, A.; Talan, D.A. Presence of symptoms 6 weeks after COVID-19 among vaccinated and unvaccinated US healthcare personnel: A prospective cohort study. BMJ Open 2023, 13, e063141. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html (accessed on 30 June 2024).
- Available online: https://www.gov.uk/government/news/ukhsa-review-shows-vaccinated-less-likely-to-have-long-covid-than-unvaccinated (accessed on 30 June 2024).
- Carto, C.; Nackeeran, S.; Ramasamy, R. COVID-19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia 2022, 54, e14281. [Google Scholar] [CrossRef]
- Duskin-Bitan, H.; Robenshtok, E.; Peretz, A.; Beckenstein, T.; Tsur, N.; Netzer, D.; Gorshtein, A. Subacute Thyroiditis Following COVID-19 and COVID-19 Vaccination. Endocr. Pract. 2024. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/publications/i/item/9789241516990 (accessed on 30 June 2024).
- Available online: https://vaers.hhs.gov/data.html (accessed on 30 June 2024).
- Jeong, N.Y.; Park, H.; Oh, S.; Jung, S.E.; Kim, D.H.; Shin, H.S.; Choi, N.K. The COVID-19 Vaccine Safety Research Center: A cornerstone for strengthening safety evidence for COVID-19 vaccination in the Republic of Korea. Osong Public Health Res. Perspect. 2024, 15, 97–106. [Google Scholar] [CrossRef]
- Available online: https://www.nap.edu/catalog/13164/adverse-effects-of-vaccines-evidence-and-causality (accessed on 30 June 2024).
- Endo, M.; Pinto, J.; Roth, M.Y.; Hoofnagle, A.N.; Failor, R.A.; Tylee, T.S. The Incidence of Graves’ Hyperthyroidism Before and After COVID-19 Messenger RNA Vaccination. Endocr. Pract. 2023, 29, 618–622. [Google Scholar] [CrossRef]
- Triantafyllidis, K.K.; Giannos, P.; Stathi, D.; Kechagias, K.S. Graves’ disease following vaccination against SARS-CoV-2: A systematic review of the reported cases. Front. Endocrinol. 2022, 13, 938001. [Google Scholar] [CrossRef] [PubMed]
- Zaçe, D.; La Gatta, E.; Petrella, L.; Di Pietro, M.L. The impact of COVID-19 vaccines on fertility—A systematic review and meta-analysis. Vaccine 2022, 40, 6023–6034. [Google Scholar] [CrossRef] [PubMed]
- Verrienti, M.; Marino Picciola, V.; Ambrosio, M.R.; Zatelli, M.C. Pituitary and COVID-19 vaccination: A systematic review. Pituitary 2024. [Google Scholar] [CrossRef] [PubMed]

| Adverse Ef-fects/Primary Study Point | Study Type (Ref.) | No. of Cases | Vaccine Type | Vaccine Dose | Days from the Last Vaccine | Age Mean ± SD | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | Viral | Inactivated | 1st | 2nd | 3rd | ||||||
| Female Reproductive System | |||||||||||
| Ovarian Reserve Markers | Retrospective [143] | 46 | - | - | 46 | - | 46 | - | 30 | 36.4 ± 4.9 | No effect on AMH level or antral follicle number. |
| Retrospective [144] | 309 | - | - | 309 | 78 | 257 | - | <90 (28%) ≥90 (72%) | 31.2 ± 3.8 | No effect on pregnancy rate in assisted reproduction therapy. | |
| Retrospective [145] | 146 | - | - | 146 | - | 146 | - | 72.4 ± 57.0 | 33.7 ± 5.6 | No effect on pregnancy rate in assisted reproduction therapy. | |
| Prospective [146] | 129 | 129 | - | - | - | 129 | - | 90 | 29 ± 5.23 | No effect on AMH levels. | |
| Prospective [148] | 74 | 74 | - | - | - | 74 | - | 180 | 27.6 ± 5.3 | No effect on AMH levels. | |
| Prospective [149] | 62 | 62 | - | - | - | 62 | - | 90 | 26.3 ± 3.6 | No effect on FSH, LH, E2, AMH, ovarian volume, or number of antral follicles. | |
| Retrospective [150] | 474 | - | - | 474 | - | 474 | - | 508.0 ± 250.2 (mean ± SD) | 30.8 ± 4.95 | No effect on AMH levels. | |
| Prospective [151] | 31 | 31 | - | - | - | 31 | - | 180 (median) | 35.5 ± 4.7 | No effect on AMH levels. | |
| Prospective [152] | 37 | 37 | - | - | - | 37 | - | 14–60 | 33.3 ± 6.1 | No effect on ovarian reserve or pregnancy rate in assisted reproduction therapy. | |
| Retrospective [153] | 36 | 36 | - | - | - | 36 | - | 7–85 | 37.3 ± 4.6 | No effect on ovarian reserve in assisted reproduction therapy. | |
| Retrospective [154] | 142 | 135 | 7 | - | 15 | 127 | - | 93 ± 65 (mean ± SD) | 34 ± 4 | No effect on pregnancy rate in assisted reproduction therapy. | |
| Retrospective [155] | 510 | 441 | 69 | - | - | 510 | - | 60 | 34.8 ± 7.7 | No effect on pregnancy rate in assisted reproduction therapy. | |
| Male Reproductive System | |||||||||||
| Semen Quality Parameters | Prospective [156] | 45 | 45 | - | - | - | 45 | - | 75 (median) | 28 (median) | No deleterious effect on sperm quality. |
| Prospective [157] | 33 | 33 | - | - | - | 33 | - | ≥72 | 27 (median) | No deleterious effect on sperm quality. | |
| Prospective [158] | 75 | 75 | - | - | - | 75 | - | 37 (mean) | 38.6 | No deleterious effect on sperm quality. | |
| Prospective [159] | 47 | 47 | - | - | - | 47 | - | 30 | 29.3 | No deleterious effect on sperm quality. | |
| Prospective [160] | 60 | 60 | - | - | - | 60 | - | ≥90 | 36 (median) | No deleterious effect on sperm quality. | |
| Retrospective [161] | 37 | 37 | - | - | - | 37 | - | >145 | 26.1 | No deleterious effect on sperm quality. | |
| Retrospective [162] | 43 | - | - | 43 | - | 43 | - | 30.1 (mean) | 28.6 | No deleterious effect on sperm quality. | |
| Retrospective [163] | 351 | - | - | 351 | 8 | 183 | 160 | 112.7 (mean) | 35 | No deleterious effect on sperm quality. | |
| Retrospective [164] | 106 | 93 | 11 | - | 22 | 82 | - | 59 (median) | 39 (median) | No deleterious effect on sperm quality and fertilization capacity of men undergoing ART treatments. | |
| Retrospective [165] | 72 | 72 | - | -- | - | 72 | - | 71 (mean) | 35.7 | No deleterious effect on sperm quality. | |
| Retrospective [166] | 105 | - | - | 105 | - | 105 | - | 80.6 (mean) | 33.9 | No significant differences were observed in sperm quality and IVF outcomes. | |
| Observational case–control [167] | 43 | NA | NA | NA | 1 | 17 | 22 | NA | 36 | No association between SARS-CoV-2 vaccination parameters and markers of sperm quality. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishay, A.; Oleinikov, K.; Chertok Shacham, E. SARS-CoV-2-Vaccine-Related Endocrine Disorders: An Updated Narrative Review. Vaccines 2024, 12, 750. https://doi.org/10.3390/vaccines12070750
Ishay A, Oleinikov K, Chertok Shacham E. SARS-CoV-2-Vaccine-Related Endocrine Disorders: An Updated Narrative Review. Vaccines. 2024; 12(7):750. https://doi.org/10.3390/vaccines12070750
Chicago/Turabian StyleIshay, Avraham, Kira Oleinikov, and Elena Chertok Shacham. 2024. "SARS-CoV-2-Vaccine-Related Endocrine Disorders: An Updated Narrative Review" Vaccines 12, no. 7: 750. https://doi.org/10.3390/vaccines12070750







