A Flagellin-Adjuvanted Trivalent Mucosal Vaccine Targeting Key Periodontopathic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria and Culture Conditions
2.2. Animal and Ethics Statement
2.3. A Temporary Ligature Plus Oral Infection (LigR + OI) Periodontitis Model Induced by a Combination of a Ligature and Oral Bacterial Gavage
2.4. Intranasal Immunization
2.5. Micro-Computed Tomography (Micro-CT) Analysis
2.6. Hematoxylin and Eosin (H&E) Staining
2.7. Quantitative RT-PCR (qRT-PCR)
2.8. Measurement of Antigen-Specific Antibody Titers by Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Anti-Tf, Anti-Pg, and Anti-Fn Serum Production
2.10. Immunostaining and Confocal Imaging
2.11. Cell Culture
2.12. Bacterial Invasion Assay by Flow Cytometry
2.13. Bacterial Adhesion and Invasion Assay by Confocal Microscopy
2.14. Biofilm Inhibition Assay
2.15. Statistical Analyses
3. Results
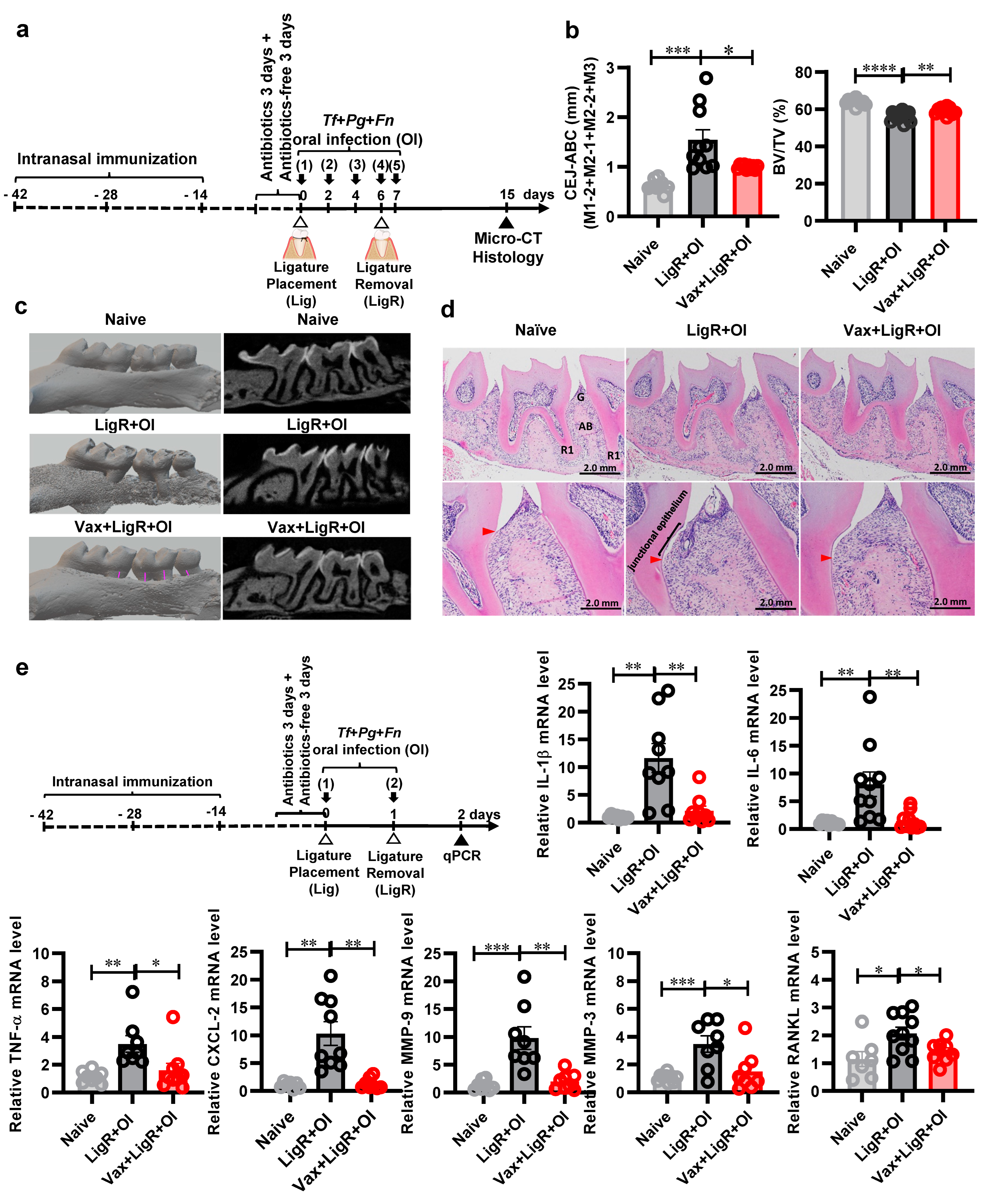
3.1. The Establishment of a Temporary Ligature Plus Oral Infection (LigR + OI) Model
3.2. The Development of a Tannerella forsythia BspA-Specific Mucosal Vaccine with Flagellin as a Built-In Adjuvant
3.3. A Trivalent Mucosal Vaccine (BtB + HB + BtA) Prevents Alveolar Bone Loss Induced by a Mixed Bacterial Infection in the Temporary Ligature Plus Oral Infection Model
3.4. The Trivalent Mucosal Vaccine (BtB + HB + BtA) Inhibits PD-Related Gene Expression Induced by a Mixed PD Pathobiont Infection
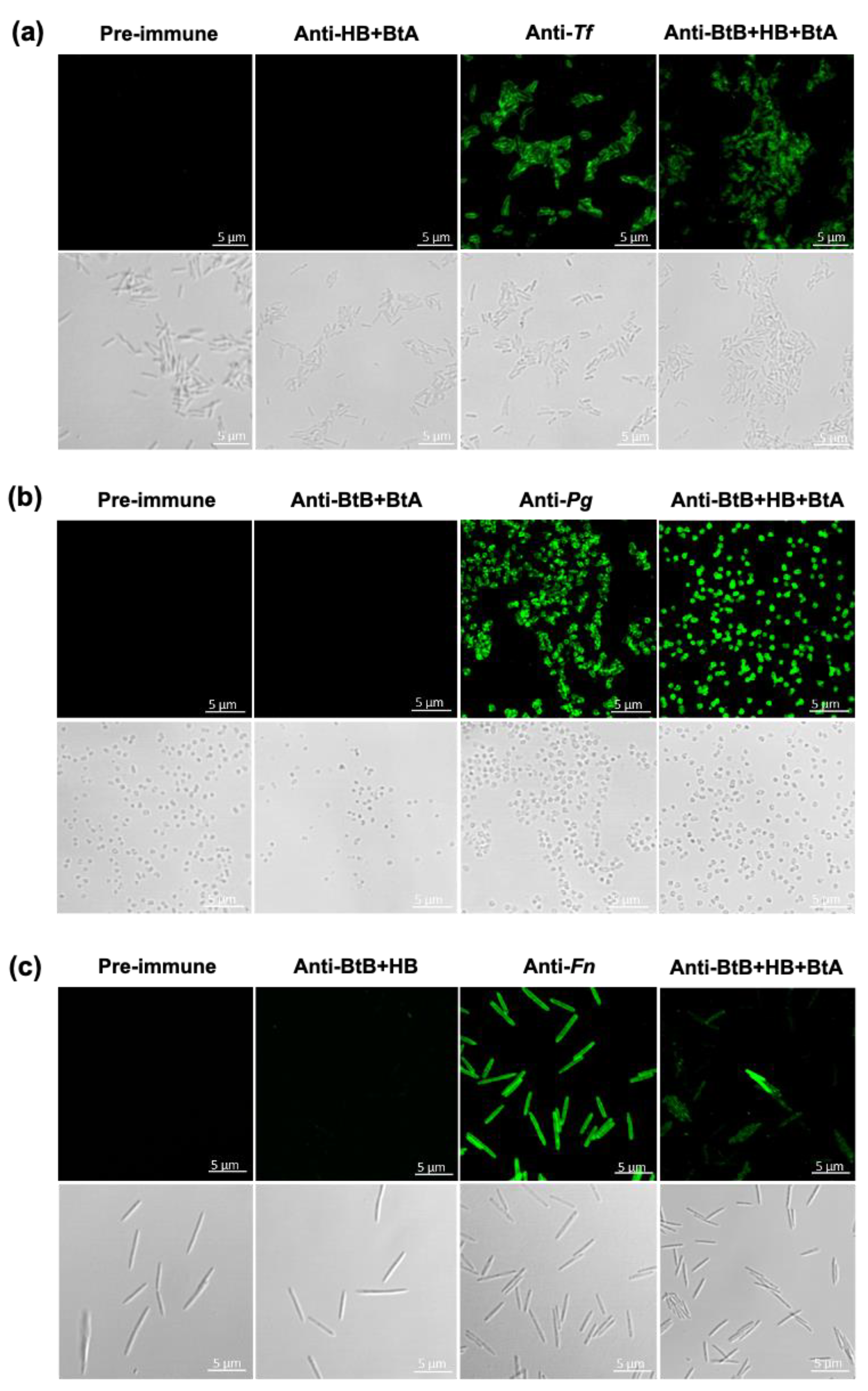
3.5. Intranasal Immunization with the Trivalent Mucosal Vaccine (BtB + HB + BtA) Induced Antigen-Specific Antibody Responses in Both Mucosal and Systemic Immune Compartments
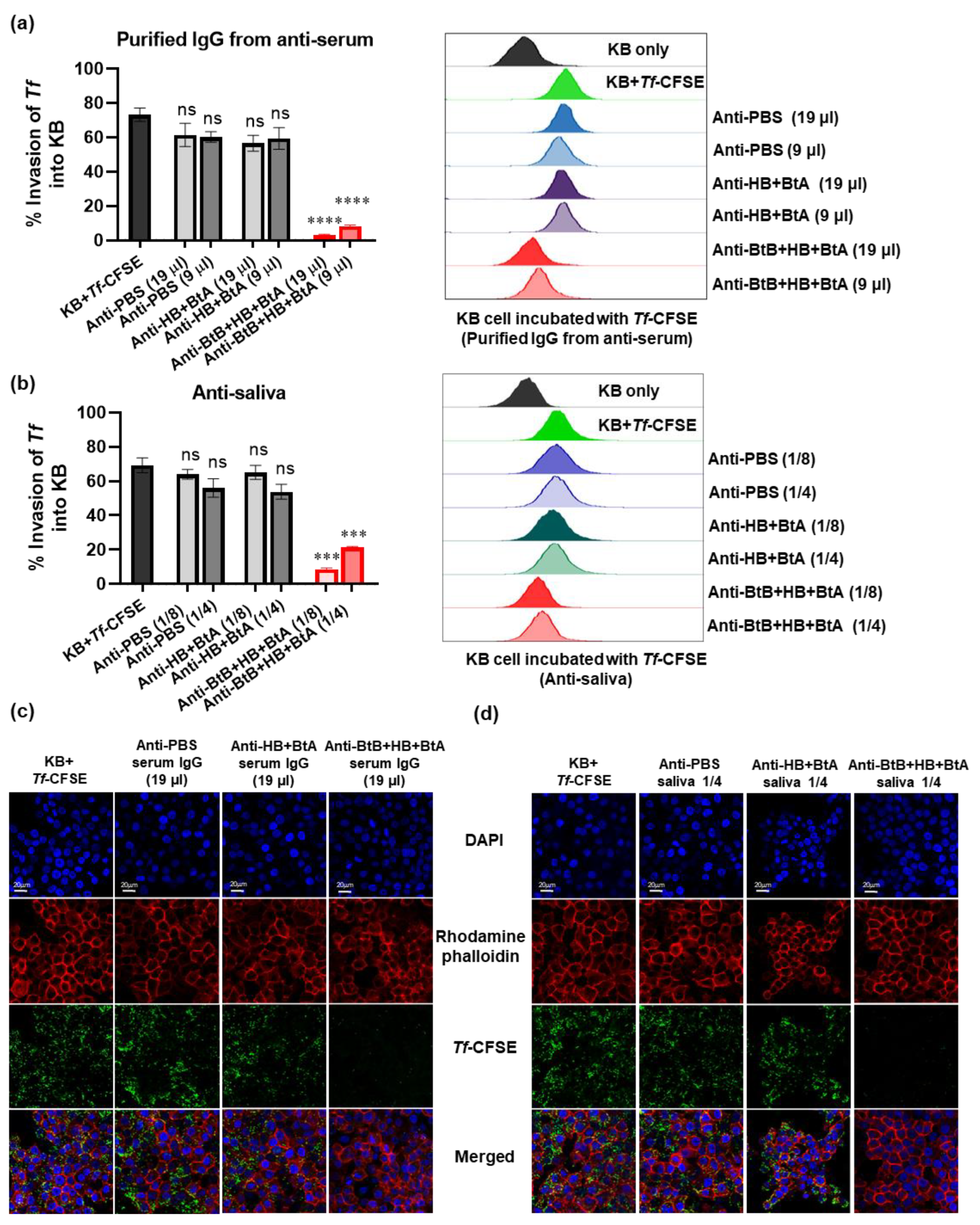
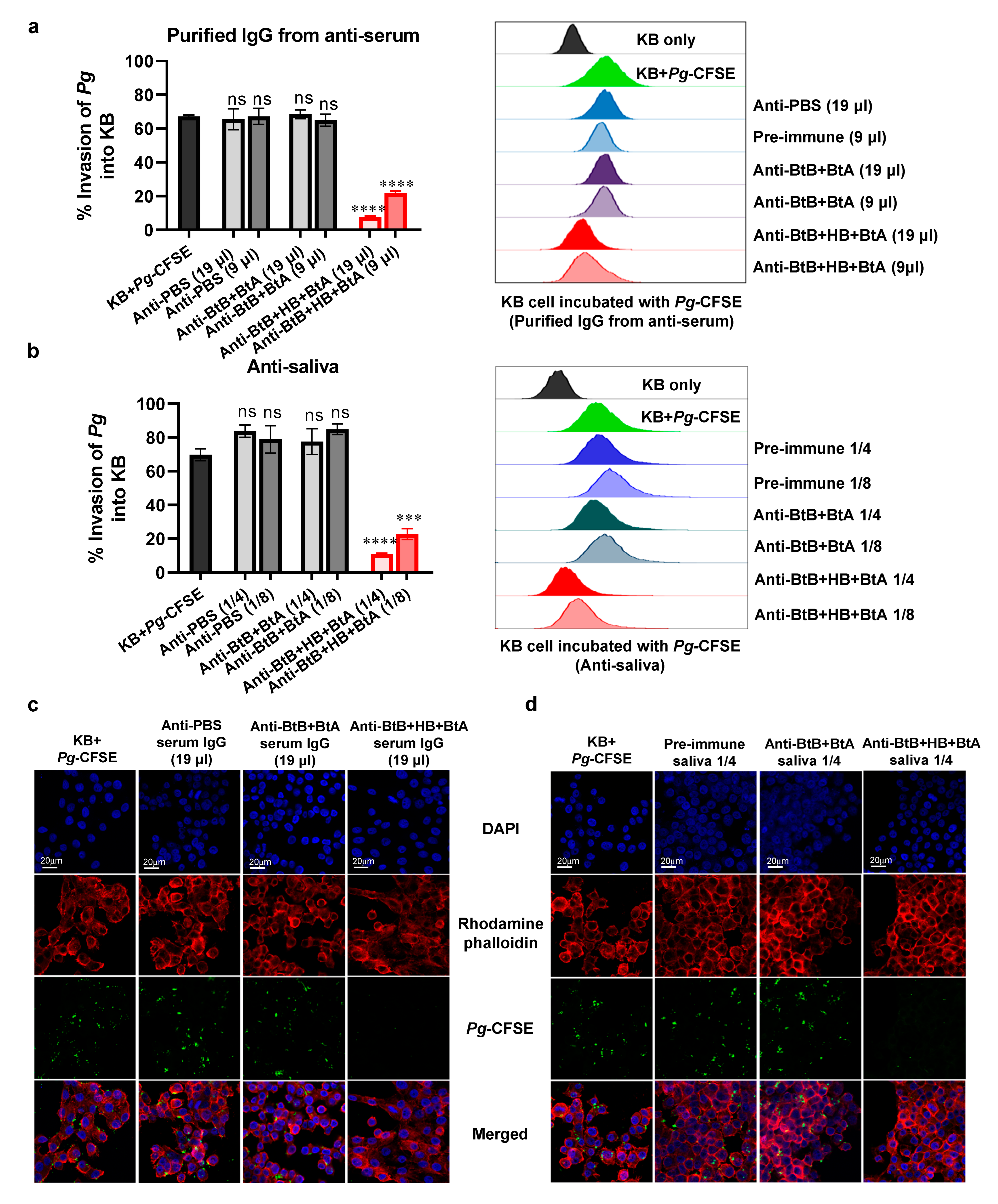
3.6. Anti-Sera and Anti-Saliva Elicited by Intranasal Immunization with the Trivalent Vaccine (BtB + HB + BtA) Inhibited Host–Bacteria Interactions
3.7. Anti-Sera and Anti-Saliva Elicited by Intranasal Immunization with the Trivalent Vaccine (BtB + HB + BtA) Inhibited F. nucleatum-Mediated Biofilm Formation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72. [Google Scholar]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2014, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Yanes, J.; Reynolds, E.; Li, J.; Mariño, E. Microbiome-targeted interventions for the control of oral–gut dysbiosis and chronic systemic inflammation. Trends Mol. Med. 2023, 29, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Kajikawa, T.; Hajishengallis, E.; Maekawa, T.; Reis, E.S.; Mastellos, D.C.; Yancopoulou, D.; Hasturk, H.; Lambris, J.D. Complement-dependent mechanisms and interventions in periodontal disease. Front. Immunol. 2019, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. The polymicrobial synergy and dysbiosis model of periodontal disease pathogenesis. In The Human Microbiota and Chronic Disease: Dysbiosis as a Cause of Human Pathology; Molecular Oral Microbiology: Hoboken, NJ, USA, 2016; pp. 227–242. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial communities in periodontal disease: Their quasi-organismal nature and dialogue with the host. Periodontology 2021, 86, 210–230. [Google Scholar] [CrossRef]
- Mirmohammadsadegh, N.; Mashreghi Mohammadi, N.; Amin, M. Potential Treponema denticola-based periodontal vaccine to resolve a global public health challenge: A narrative literature review. Expert Rev. Vaccines 2022, 21, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Vaernewyck, V.; Arzi, B.; Sanders, N.N.; Cox, E.; Devriendt, B. Mucosal vaccination against periodontal disease: Current status and opportunities. Front. Immunol. 2021, 12, 768397. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 1997, 51, 311–340. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 2022, 167, 124–138. [Google Scholar] [CrossRef]
- Puth, S.; Verma, V.; Hong, S.H.; Tan, W.; Lee, S.E.; Rhee, J.H. An all-in-one adjuvanted therapeutic cancer vaccine targeting dendritic cell cytosol induces long-lived tumor suppression through NLRC4 inflammasome activation. Biomaterials 2022, 286, 121542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, S.Y.; Jeong, B.C.; Kim, Y.R.; Bae, S.J.; Ahn, O.S.; Lee, J.J.; Song, H.C.; Kim, J.M.; Choy, H.E.; et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 2006, 74, 694–702. [Google Scholar] [CrossRef]
- Puth, S.; Hong, S.H.; Na, H.S.; Lee, H.H.; Lee, Y.S.; Kim, S.Y.; Tan, W.; Hwang, H.S.; Sivasamy, S.; Jeong, K.; et al. A built-in adjuvant-engineered mucosal vaccine against dysbiotic periodontal diseases. Mucosal Immunol. 2019, 12, 565–579. [Google Scholar] [CrossRef]
- Khim, K.; Bang, Y.J.; Puth, S.; Choi, Y.; Lee, Y.S.; Jeong, K.; Lee, S.E.; Rhee, J.H. Deimmunization of flagellin for repeated administration as a vaccine adjuvant. NPJ Vaccines 2021, 6, 116. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, S.Y.; Kim, M.S.; Lee, S.E.; Rhee, J.H. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine 2011, 29, 5731–5739. [Google Scholar] [CrossRef]
- Puth, S.; Hong, S.H.; Park, M.J.; Lee, H.H.; Lee, Y.S.; Jeong, K.; Kang, I.C.; Koh, J.T.; Moon, B.; Park, S.C.; et al. Mucosal immunization with a flagellin-adjuvanted Hgp44 vaccine enhances protective immune responses in a murine Porphyromonas gingivalis infection model. Hum. Vaccines Immunother. 2017, 13, 2794. [Google Scholar] [CrossRef] [PubMed]
- Acqua, Y.D.; Hernández, C.; Fogacci, M.; Barbirato, D.; Palioto, D. Local and systemic effects produced in different models of experimental periodontitis in mice: A systematic review. Arch. Oral Biol. 2022, 143, 105528. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nature protocols 2018, 13, 2247–2267. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Chen, B.-Y.; Liu, Y.; Zhang, W.-C.; Duan, S.-Z. A Mouse Periodontitis Model with Humanized Oral Bacterial Community. Front. Cell. Infect. Microbiol. 2022, 12, 842845. [Google Scholar] [CrossRef]
- Chipashvili, O.; Bor, B. Ligature-induced periodontitis mouse model protocol for studying Saccharibacteria. STAR Protoc. 2022, 3, 101167. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Bolstad, A.I.; Jensen, H.B.; Bakken, V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 1996, 9, 55–71. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Cugini, M.A.; Tanner, A.; Pollack, R.P.; Smith, C.; Kent, R.L.; Socransky, S.S. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. J. Clin. Periodontol. 1998, 25, 346–353. [Google Scholar] [CrossRef]
- Mahalakshmi, K.; Krishnan, P.; Chandrasekaran, S.C. Detection of Tannerella forsythia bspA and prtH genotypes among periodontitis patients and healthy subjects—A case—Control study. Arch. Oral Biol. 2018, 96, 178–181. [Google Scholar] [CrossRef]
- Hall, L.M.; Dunford, R.G.; Genco, R.J.; Sharma, A. Levels of serum immunoglobulin G specific to bacterial surface protein A of Tannerella forsythia are related to periodontal status. J. Periodontol. 2012, 83, 228–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanner, A.C.R.; Izard, J. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontology 2006, 42, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Onishi, S.; Kuramitsu, H.K.; Sharma, A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia”. Infect. Immun. 2006, 74, 5023–5028. [Google Scholar] [CrossRef] [PubMed]
- Settem, R.P.; El-Hassan, A.T.; Honma, K.; Stafford, G.P.; Sharma, A. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect. Immun. 2012, 80, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Kurihara, H.; Dahlen, G. Characterization of Bacteroides forsythus Isolates. J. Clin. Microbiol. 1997, 35, 1378–1381. [Google Scholar] [CrossRef]
- Yoneda, M.; Hirofuji, T.; Anan, H.; Matsumoto, A.; Hamachi, T.; Nakayama, K.; Maeda, K. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: Involvement of gingipains in a synergistic effect. J. Periodontal Res. 2001, 36, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Yeon Yoo, J.; Chan Kim, H.; Zhu, W.; Kim, S.-M.; Sabet, M.; Handfield, M.; Hillman, J.; Progulske-Fox, A.; Lee, S.-W. Identi¢cation of Tannerella forsythia antigens speci¢cally expressed in patients with periodontal disease. EMS Microbiol. Lett. 2007, 275, 344–352. [Google Scholar] [CrossRef]
- Sharma, A.; Inagaki, S.; Honma, K.; Sfintescu, C.; Baker, P.J.; Evans, R.T. Tannerella forsythia-induced Alveolar Bone Loss in Mice Involves Leucine-rich-repeat BspA Protein. J. Dent. Res. 2005, 84, 462–467. [Google Scholar] [CrossRef]
- Friedrich, V.; Gruber, C.; Nimeth, I.; Pabinger, S.; Sekot, G.; Posch, G.; Altmann, F.; Messner, P.; Andrukhov, O.; Schäffer, C. Outer membrane vesicles of Tannerella forsythia: Biogenesis, composition, and virulence. Mol. Oral Microbiol. 2015, 30, 451–473. [Google Scholar] [CrossRef]
- Lin, J.; Bi, L.; Yu, X.; Kawai, T.; Taubman, M.A.; Shen, B.; Han, X. Porphyromonas gingivalis Exacerbates Ligature-Induced, RANKL-Dependent Alveolar Bone Resorption via Differential Regulation of Toll-Like Receptor 2 (TLR2) and TLR4. Infect. Immun. 2014, 82, 4127. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, D.-K.; Huh, Y.H.; Lee, G.; Lee, S.-H.; Hong, Y.; Kim, S.-H.; Kook, M.-S.; Koh, J.-T.; Chun, J.-S.; et al. NAMPT enzyme activity regulates catabolic gene expression in gingival fibroblasts during periodontitis. Exp. Mol. Med. 2017, 49, e368. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; He, J.; Tong, Z.; Qian, Y.; Wang, Q.; Jia, D.; Zhu, W.; Zhao, Y.; Cai, B.; Chen, S. Ligature-induced periodontitis drives colorectal cancer: An experimental model in mice. J. Dent. Res. 2023, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Song, J.H.; Oh, S.-H.; Kim, J.-W.; Lee, M.N.; Piao, X.; Yang, J.-W.; Kim, O.-S.; Kim, T.S.; Kim, S.-H. Targeting NLRP3 inflammasome reduces age-related experimental alveolar bone loss. J. Dent. Res. 2020, 99, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Sandros, J.; Papapanou, P.; Dahlén, G. Porphyromonas gingivalis invades oral epithelial cells in vitro. J. Periodontal Res. 1993, 28, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Zhang, S.; Li, Y.; Shi, X.; Liu, D.; Pan, Y. Porphyromonas gingivalis outer membrane vesicles inhibit the invasion of Fusobacterium nucleatum into oral epithelial cells by downregulating FadA and FomA. J. Periodontol. 2022, 93, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Sao, P.; Park, M.-J.; Lee, H.; Kim, S.H.; Rhee, J.H.; Lee, S.E. Development of a novel subunit vaccine targeting fusobacterium nucleatum FomA porin based on in silico analysis. Int. J. Oral Biol. 2017, 42, 63–70. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Thurnheer, T.; Karygianni, L.; Flury, M.; Belibasakis, G.N. Fusobacterium species and subspecies differentially affect the composition and architecture of supra-and subgingival biofilms models. Front. Microbiol. 2019, 10, 1716. [Google Scholar] [CrossRef]
- Nobbs, A.; Jenkinson, H.; Jakubovics, N. Stick to your gums: Mechanisms of oral microbial adherence. J. Dent. Res. 2011, 90, 1271–1278. [Google Scholar] [CrossRef]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community-Rebels with a Cause. Curr. Oral Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Persistence of Tannerella forsythia and Fusobacterium nucleatum in dental plaque: A strategic alliance. Curr. Oral Health Rep. 2020, 7, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Zilm, P.S.; Rogers, A.H. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology 2002, 148, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Àlvarez, G.; Isabal, S.; Teughels, W.; Laleman, I.; Contreras, M.; Isbej, L.; Huapaya, E.; Mendoza, G.; Mor, C. Comparative 16S rRNA gene sequencing study of subgingival microbiota of healthy subjects and patients with periodontitis from four different countries. J. Clin. Periodontol. 2023, 50, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Yanagita, M.; Mori, K.; Hasegawa, S.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. The effects of cigarette smoke condensate and nicotine on periodontal tissue in a periodontitis model mouse. PLoS ONE 2016, 11, e0155594. [Google Scholar] [CrossRef]
- Hoare, A.; Wang, H.; Meethil, A.; Abusleme, L.; Hong, B.-Y.; Moutsopoulos, N.M.; Marsh, P.D.; Hajishengallis, G.; Diaz, P.I. A cross-species interaction with a symbiotic commensal enables cell-density-dependent growth and in vivo virulence of an oral pathogen. ISME J. 2021, 15, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa Jr, C. Animal models to study host-bacteria interactions involved in periodontitis. Periodontal Dis. 2012, 15, 117–132. [Google Scholar]
- Lin, P.; Niimi, H.; Ohsugi, Y.; Tsuchiya, Y.; Shimohira, T.; Komatsu, K.; Liu, A.; Shiba, T.; Aoki, A.; Iwata, T. Application of ligature-induced periodontitis in mice to explore the molecular mechanism of periodontal disease. Int. J. Mol. Sci. 2021, 22, 8900. [Google Scholar] [CrossRef] [PubMed]
- Spolidorio, L.C.; Lucas, P.D.R.; Steffens, J.P.; da Silva, H.A.B.; Alves, V.T.E.; Spolidorio, D.M.P.; Holzhausen, M. Influence of parstatin on experimental periodontal disease and repair in rats. J. Periodontol. 2014, 85, 1266–1274. [Google Scholar] [CrossRef]
- Carvalho, É.B.; Romandini, M.; Sadilina, S.; Sant’Ana, A.C.; Sanz, M. Microbiota associated with peri-implantitis—A systematic review with meta-analyses. Clin. Oral Implant. Res. 2023, 34, 1176–1187. [Google Scholar] [CrossRef]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L. Inflammation and bone loss in periodontal disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Hashizume-Takizawa, T.; Kobayashi, R.; Yamamoto, M. Mucosal Vaccines for Oral Disease. In Mucosal Vaccines: Innovation for Preventing Infectious Diseases; Academic Press: Cambridge, MA, USA, 2020; pp. 649–661. [Google Scholar] [CrossRef]
- Hong, S.H.; Byun, Y.-H.; Nguyen, C.T.; Kim, S.Y.; Seong, B.L.; Park, S.; Woo, G.-J.; Yoon, Y.; Koh, J.T.; Fujihashi, K. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine 2012, 30, 466–474. [Google Scholar] [CrossRef]
- Haque, M.M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontology 2010, 54, 106–116. [Google Scholar] [CrossRef]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.P.; Shi, W.; Lux, R. Identification and characterization of a novel Fusobacterium nucleatum adhesin involved in physical interaction and biofilm formation with Streptococcus gordonii. Microbiologyopen 2017, 6, e00444. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-F.; Shi, W.; Zhu, W.; Smith, J.W.; Hsieh, S.-L.; Gallo, R.L.; Huang, C.-M. Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis. Vaccine 2010, 28, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Badanian, A.; Bueno, L.; Papone, V. Comparative bacterial analysis of chronic and aggressive periodontitis in a sample population from Uruguay. Odontoestomatología 2019, 21, 5–13. [Google Scholar] [CrossRef]
- Yost, S.; Duran-Pinedo, A.E.; Teles, R.; Krishnan, K.; Frias-Lopez, J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015, 7, 27. [Google Scholar] [CrossRef]
- Park, J.C.; Im, S.-H. Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp. Mol. Med. 2020, 52, 1383–1396. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loeurng, V.; Puth, S.; Hong, S.H.; Lee, Y.S.; Radhakrishnan, K.; Koh, J.T.; Kook, J.-K.; Rhee, J.H.; Lee, S.E. A Flagellin-Adjuvanted Trivalent Mucosal Vaccine Targeting Key Periodontopathic Bacteria. Vaccines 2024, 12, 754. https://doi.org/10.3390/vaccines12070754
Loeurng V, Puth S, Hong SH, Lee YS, Radhakrishnan K, Koh JT, Kook J-K, Rhee JH, Lee SE. A Flagellin-Adjuvanted Trivalent Mucosal Vaccine Targeting Key Periodontopathic Bacteria. Vaccines. 2024; 12(7):754. https://doi.org/10.3390/vaccines12070754
Chicago/Turabian StyleLoeurng, Vandara, Sao Puth, Seol Hee Hong, Yun Suhk Lee, Kamalakannan Radhakrishnan, Jeong Tae Koh, Joong-Ki Kook, Joon Haeng Rhee, and Shee Eun Lee. 2024. "A Flagellin-Adjuvanted Trivalent Mucosal Vaccine Targeting Key Periodontopathic Bacteria" Vaccines 12, no. 7: 754. https://doi.org/10.3390/vaccines12070754
APA StyleLoeurng, V., Puth, S., Hong, S. H., Lee, Y. S., Radhakrishnan, K., Koh, J. T., Kook, J.-K., Rhee, J. H., & Lee, S. E. (2024). A Flagellin-Adjuvanted Trivalent Mucosal Vaccine Targeting Key Periodontopathic Bacteria. Vaccines, 12(7), 754. https://doi.org/10.3390/vaccines12070754





