Protection of K18-hACE2 Mice against SARS-CoV-2 Challenge by a Capsid Virus-like Particle-Based Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Characterization of the SARS-CoV-2/Leiden-008 Isolate
2.2. Animal Studies
2.2.1. Ethics Declaration
2.2.2. Infection of K18-ACE2 Mice with SARS-CoV-2
2.2.3. Vaccine and Immunizations
2.2.4. Challenge of K18-hACE2 Mice
2.2.5. Passive Serum Transfer
2.3. Techniques
2.3.1. Lung Virus Titers
2.3.2. Histopathology and Semi-Quantitative Scoring of Lung Pathology
2.3.3. Neutralization Assay with an Authentic SARS-CoV-2 Strain D614G
2.3.4. ELISA Assay
2.3.5. IFN-γ ELISpot Assay
2.4. Statistical Analysis
3. Results
3.1. K18-hACE2 Mice Develop a Dose-Dependent Disease When Challenged with the SARS-CoV-2/Leiden-008 Virus
3.2. Immunogenicity of the RBD-cVLP Vaccine in K18-hACE2 Transgenic Mice
3.3. Protective Efficacy of RBD-cVLP in K18-hACE2 Mice Challenged with a Lethal Dose of SARS-CoV-2
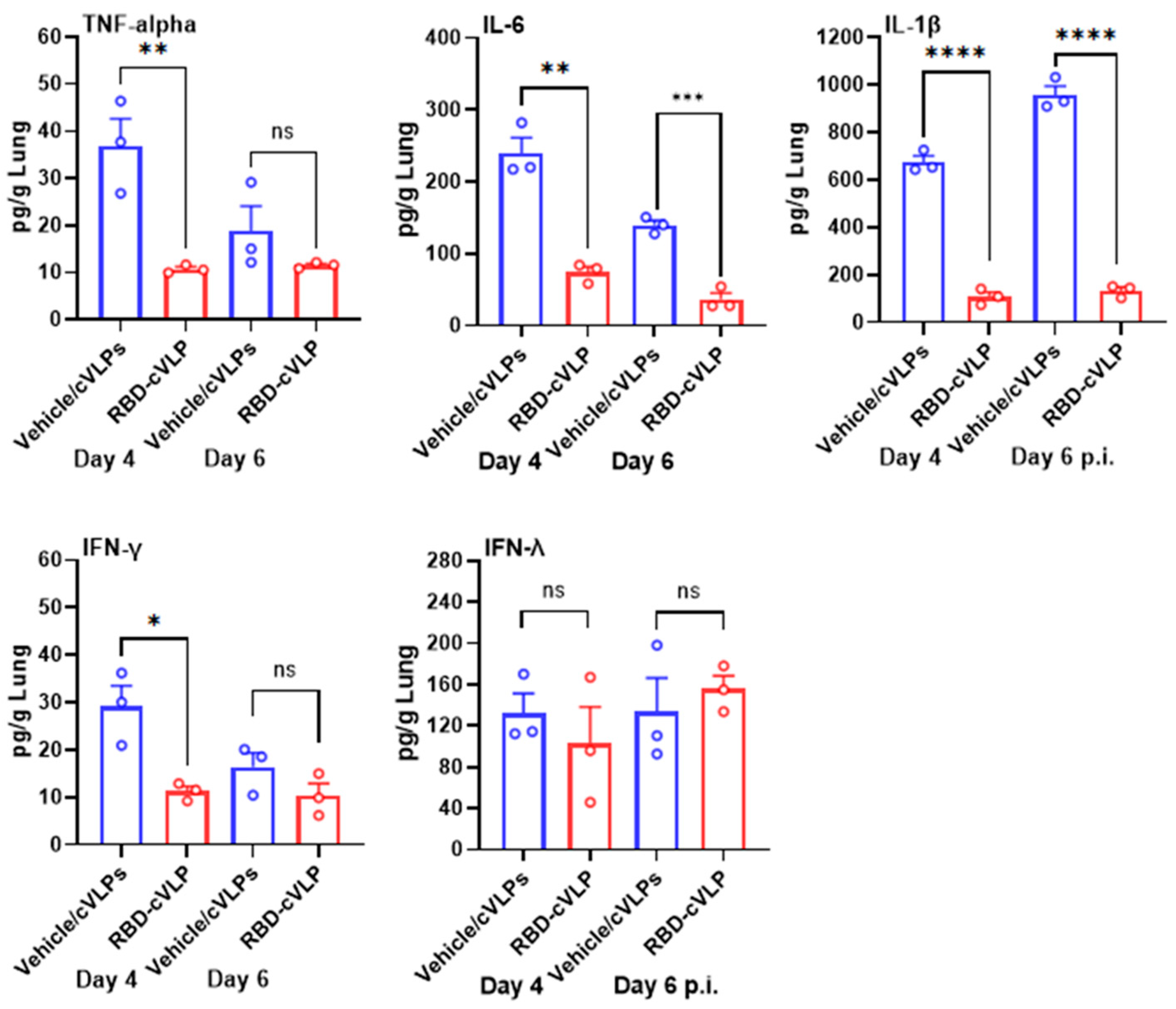
3.4. RBD-cVLP Vaccination Reduces Lung Inflammatory Cytokine Responses to a Lethal Challenge with SARS-CoV-2
3.5. Passive Transfer of Immune Sera from RBD-cVLP-Vaccinated Mice Increases the Survival of Naïve K18-hACE2 Mice from a Lethal SARS-CoV-2 Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 588, E6. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Benjelloun, M.; Lairini, S.; Lahrichi, A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Sci. World J. 2022, 2022, 5578284. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Leighton, T.; Ostrowsky, J.T.; Anderson, C.J.; Danila, R.N.; Ulrich, A.K.; Lackritz, E.M.; Mehr, A.J.; Baric, R.S.; Baylor, N.W.; et al. A research and development (R&D) roadmap for broadly protective coronavirus vaccines: A pandemic preparedness strategy. Vaccine 2023, 41, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Melissa, M.H.; Abu-Raddad, L.J. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and metaregression. Lancet 2022, 399, 924. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Kwan, A.T.; Rodríguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. The next generation of coronavirus vaccines: A graphical guide. Nature 2023, 614, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Thrane, S.; Janitzek, C.M.; Matondo, S.; Resende, M.; Gustavsson, T.; de Jongh, W.A.; Clemmensen, S.; Roeffen, W.; van de Vegte-Bolmer, M.; van Gemert, G.J.; et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnol. 2016, 14, 30. [Google Scholar] [CrossRef]
- Fougeroux, C.; Goksoyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Sogaard, M.; Aves, K.L.; Horsted, E.W.; et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef]
- Smit, M.J.; Sander, A.F.; Ariaans, M.B.P.A.; Fougeroux, C.; Heinzel, C.; Fendel, R.; Esen, M.; Kremsner, P.G.; ter Heine, R.; Wertheim, H.F.; et al. First-in-human use of a modular capsid virus-like vaccine platform: An open-label, non-randomised, phase 1 clinical trial of the SARS-CoV-2 vaccine ABNCoV2. Lancet Microbe 2023, 4, E140–E148. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, A.; Koopman, G.; Mooij, P.; Verschoor, E.J.; Verstrepen, B.E.; Bogers, W.M.J.M.; Idorn, M.; Paludan, S.R.; Vang, S.; Nielsen, M.A.; et al. A Capsid Virus-Like Particle-Based SARS-CoV-2 Vaccine Induces High Levels of Antibodies and Protects Rhesus Macaques. Front. Immunol. 2022, 13, 857440. [Google Scholar] [CrossRef] [PubMed]
- Bavarian Nordic Reports Phase 3 Topline Results for its COVID-19 Booster Vaccine Candidate. Available online: https://www.bavarian-nordic.com/investor/news/news.aspx?news=6807 (accessed on 16 April 2024).
- Bavarian Nordic Provides Update on COVID-19 Booster Vaccine Program. Available online: https://www.bavarian-nordic.com/investor/news/news.aspx?news=6827 (accessed on 16 April 2024).
- McCray, P.B.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Owusu, I.O.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef] [PubMed]
- Clever, S.; Volz, A. Mouse models in COVID-19 research: Analyzing the adaptive immune response. Med. Microbiol. Immun. 2023, 212, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Myeni, S.K.; Bredenbeek, P.J.; Knaap, R.C.M.; Dalebout, T.J.; Morales, S.T.; Sidorov, I.A.; Linger, M.E.; Oreshkova, N.; van Zanen-Gerhardt, S.; Zander, S.A.L.; et al. Engineering potent live attenuated coronavirus vaccines by targeted inactivation of the immune evasive viral deubiquitinase. Nat. Commun. 2023, 14, 1141. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.W.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR (Publication with Expression of Concern). Eurosurveillance 2020, 25, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for Valid Histopathologic Scoring in Research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Park, J.G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allué-Guardia, A.; Olmo-Fontánez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.J.; et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.S.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1470. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e811. [Google Scholar] [CrossRef] [PubMed]
- Tufa, A.; Gebremariam, T.H.; Manyazewal, T.; Getinet, T.; Webb, D.L.; Hellström, P.M.; Genet, S. Inflammatory mediators profile in patients hospitalized with COVID-19: A comparative study. Front. Immunol. 2022, 13, 964179. [Google Scholar] [CrossRef] [PubMed]
- Menegale, F.; Manica, M.; Zardini, A.; Guzzetta, G.; Marziano, V.; d’Andrea, V.; Trentini, F.; Ajelli, M.; Poletti, P.; Merler, S. Evaluation of Waning of SARS-CoV-2 Vaccine-Induced Immunity: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2310650. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827 e19. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133 e124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.D.; Liu, M.Q.; Chen, Y.; Shan, C.; Zhou, Y.W.; Shen, X.R.; Li, Q.; Zhang, L.; Zhu, Y.; Si, H.R.; et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell 2020, 182, 50–58 e58. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Strohmeier, S.; Amanat, F.; Gillespie, V.L.; Krammer, F.; Garcia-Sastre, A.; Coughlan, L.; Schotsaert, M.; Uccellini, M.B. Comparison of transgenic and adenovirus hACE2 mouse models for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2433–2445. [Google Scholar] [CrossRef]
- Moreau, G.B.; Burgess, S.L.; Sturek, J.M.; Donlan, A.N.; Petri, W.A.; Mann, B.J. Evaluation of K18-hACE2 Mice as a Model of SARS-CoV-2 Infection. Am. J. Trop. Med. Hyg. 2020, 103, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Germann, T.; Bongartz, M.; Dlugonska, H.; Hess, H.; Schmitt, E.; Kolbe, L.; Kolsch, E.; Podlaski, F.J.; Gately, M.K.; Rude, E. Interleukin-12 Profoundly up-Regulates the Synthesis of Antigen-Specific Complement-Fixing Igg2a, Igg2b and Igg3 Antibody Subclasses in-Vivo. Eur. J. Immunol. 1995, 25, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Trunova, G.V.; Makarova, O.V.; Diatroptov, M.E.; Bogdanova, I.M.; Mikchailova, L.P.; Abdulaeva, S.O. Morphofunctional Characteristic of the Immune System in BALB/c and C57Bl/6 Mice. Bull. Exp. Biol. Med. 2011, 151, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): A prospective cohort study. Lancet Microbe 2023, 4, E309–E318. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N.; Tan, A.T. SARS-CoV-2-specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 2022, 55, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; St John, A.L. Promises and challenges of mucosal COVID-19 vaccines. Vaccine 2023, 41, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Sampson, J.N.; Porras, C.; Schiller, J.T.; Kemp, T.; Herrero, R.; Wagner, S.; Boland, J.; Schussler, J.; Lowy, D.R.; et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. J. Natl. Cancer Inst. 2020, 112, 1038–1046. [Google Scholar] [CrossRef]
- Aves, K.L.; Janitzek, C.M.; Fougeroux, C.E.; Theander, T.G.; Sander, A.F. Freeze-Drying of a Capsid Virus-like Particle-Based Platform Allows Stable Storage of Vaccines at Ambient Temperature. Pharmaceutics 2022, 14, 1301. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myeni, S.K.; Leijs, A.A.; Bredenbeek, P.J.; Morales, S.T.; Linger, M.E.; Fougeroux, C.; van Zanen-Gerhardt, S.; Zander, S.A.L.; Sander, A.F.; Kikkert, M. Protection of K18-hACE2 Mice against SARS-CoV-2 Challenge by a Capsid Virus-like Particle-Based Vaccine. Vaccines 2024, 12, 766. https://doi.org/10.3390/vaccines12070766
Myeni SK, Leijs AA, Bredenbeek PJ, Morales ST, Linger ME, Fougeroux C, van Zanen-Gerhardt S, Zander SAL, Sander AF, Kikkert M. Protection of K18-hACE2 Mice against SARS-CoV-2 Challenge by a Capsid Virus-like Particle-Based Vaccine. Vaccines. 2024; 12(7):766. https://doi.org/10.3390/vaccines12070766
Chicago/Turabian StyleMyeni, Sebenzile K., Anouk A. Leijs, Peter J. Bredenbeek, Shessy Torres Morales, Marissa E. Linger, Cyrielle Fougeroux, Sophie van Zanen-Gerhardt, Serge A. L. Zander, Adam F. Sander, and Marjolein Kikkert. 2024. "Protection of K18-hACE2 Mice against SARS-CoV-2 Challenge by a Capsid Virus-like Particle-Based Vaccine" Vaccines 12, no. 7: 766. https://doi.org/10.3390/vaccines12070766





