Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Recruitment, and Procedures
2.2. Serological and Immunochemical Assays
2.3. Study Outcomes
- •
- Mucosal vaccine immunogenicity, as determined by levels of IgG and IgA targeting the RBDs of the omicron subvariants XBB.1.5, EG.5.1, and BA.2.86 in saliva before and two to four weeks after vaccination.
- •
- Vaccination-induced adverse events within seven days after vaccination.
2.4. Statistical Analysis
3. Results
3.1. Study Population
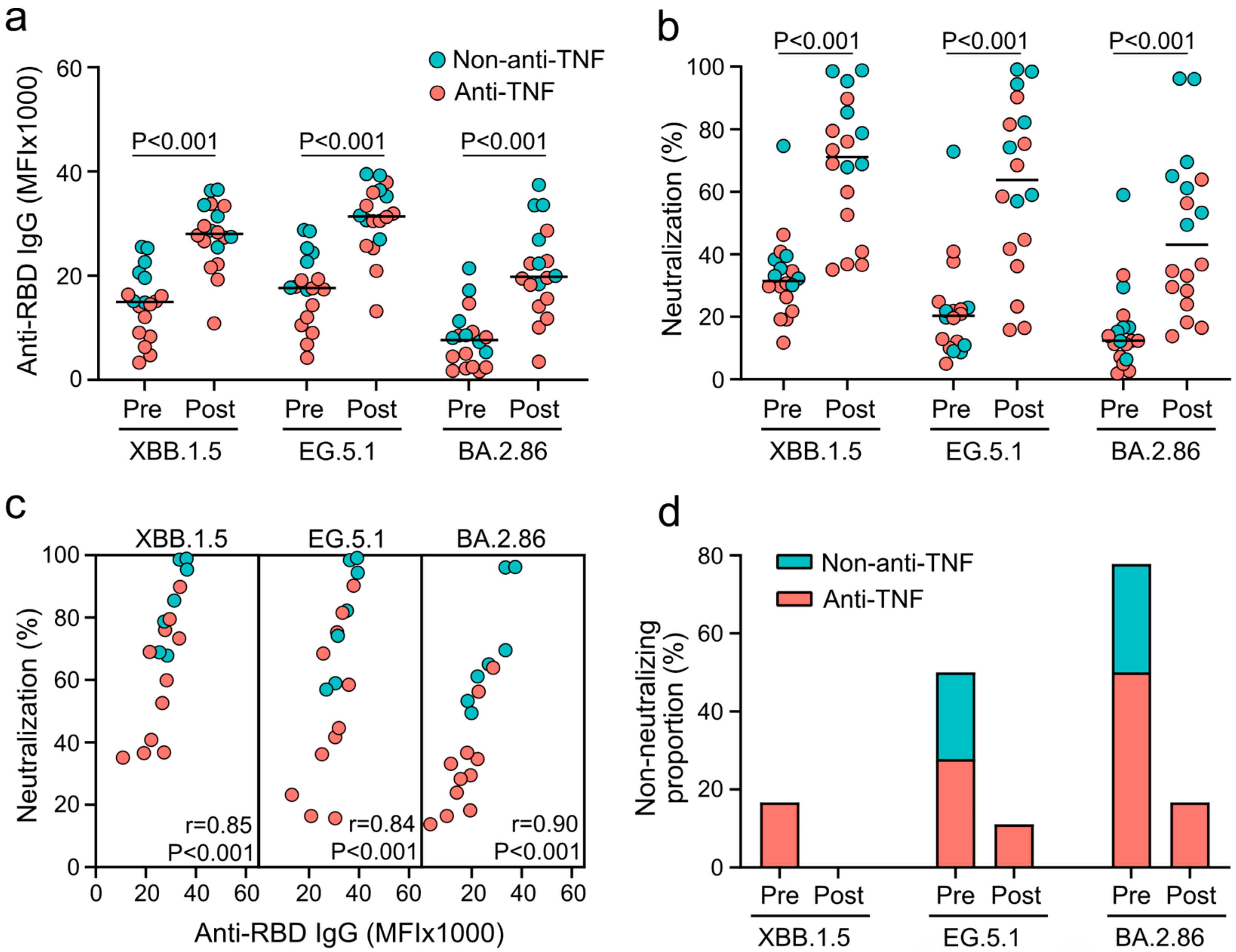
3.2. XBB.1.5-Adapted COVID-19 Vaccines Induce Systemic Humoral and Neutralizing Immunity against Omicron Subvariants in Patients with IBD
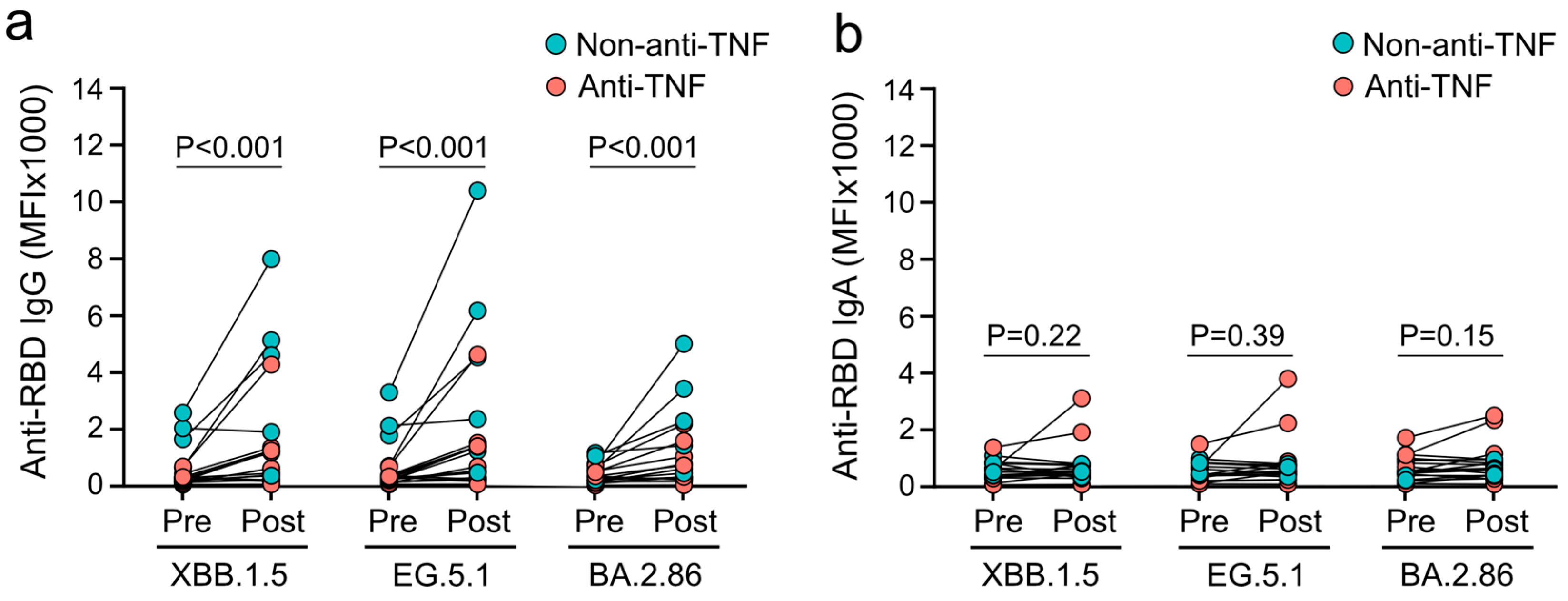
3.3. XBB.1.5-Adapted COVID-19 Vaccines Fail to Induce Mucosal IgA Responses against Omicron Subvariants in Patients with IBD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Lundberg-Morris, L.; Leach, S.; Xu, Y.; Martikainen, J.; Santosa, A.; Gisslén, M.; Li, H.; Nyberg, F.; Bygdell, M. Covid-19 vaccine effectiveness against post-Covid-19 condition among 589 722 individuals in Sweden: Population based cohort study. BMJ 2023, 383, e076990. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.A.; Dube, S.; Lu, Y.; Yates, M.; Arnetorp, S.; Barnes, E.; Bell, S.; Carty, L.; Evans, K.; Graham, S.; et al. Impact of COVID-19 on immunocompromised populations during the omicron era: Insights from the observational population-based INFORM study. Lancet Reg. Health-Eur. 2023, 35, 100747. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Wu, J.C.; Chan, F.K.; Sung, J.J.; Kaplan, G. The worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Edelman-Klapper, H.; Zittan, E.; Bar-Gil Shitrit, A.; Rabinowitz, K.M.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Lower serologic response to COVID-19 mRNA vaccine in patients with inflammatory bowel diseases treated with anti-TNFα. Gastroenterology 2022, 162, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Liu, Z.; Muñoz Sandoval, D.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Anand, N.; Nice, R.; et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef]
- Woelfel, S.; Dütschler, J.; König, M.; Graf, N.; Oikonomou, V.; Krieger, C.; Truniger, S.; Franke, A.; Eckhold, A.; Forsch, K.; et al. Systemic and T cell-associated responses to SARS-CoV-2 immunisation in gut inflammation (STAR SIGN study): Effects of biologics on vaccination efficacy of the third dose of mRNA vaccines against SARS-CoV-2. Aliment. Pharmacol. Ther. 2023, 57, 103–116. [Google Scholar] [CrossRef]
- Liu, Z.; Le, K.; Zhou, X.; Alexander, J.L.; Lin, S.; Bewshea, C.; Chanchlani, N.; Nice, R.; McDonald, T.J.; Lamb, C.A.; et al. Neutralising antibody potency against SARS-CoV-2 wild-type and omicron BA.1 and BA.4/5 variants in patients with inflammatory bowel disease treated with infliximab and vedolizumab after three doses of COVID-19 vaccine (CLARITY IBD): An analysis of a prospective multicentre cohort study. Lancet Gastroenterol. Hepatol. 2023, 8, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Alexander, J.L.; Lin, K.W.; Ahmad, T.; Pollock, K.M.; Powell, N.; Le, K.; Zhou, X.; Ibraheim, H.; Anandabaskaran, S.; et al. Infliximab and tofacitinib attenuate neutralizing antibody responses against SARS-CoV-2 ancestral and omicron variants in inflammatory bowel disease patients after 3 doses of COVID-19 vaccine. Gastroenterology 2023, 164, 300–303.e3. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Dütschler, J.; König, M.; Dulovic, A.; Graf, N.; Junker, D.; Oikonomou, V.; Krieger, C.; Truniger, S.; Franke, A.; et al. STAR SIGN study: Evaluation of COVID-19 vaccine efficacy against the SARS-CoV-2 variants BQ.1.1 and XBB.1.5 in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2023, 58, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced titers of circulating anti-SARS-CoV-2 antibodies and risk of COVID-19 infection in healthcare workers during the nine months after immunization with the BNT162b2 mRNA vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.J.; Weaver, K.N.; Zhang, X.; Kastl, A.J.; Strople, J.A.; Adler, J.; Dubinsky, M.C.; Bousvaros, A.; Watkins, R.; Dai, X.; et al. Long-term effectiveness and durability of COVID-19 vaccination among patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2024, 22, 1475–1486.e4. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Alexander, J.L.; Le, K.; Zhou, X.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Lin, K.W.; McFarlane, L.R.; Constable, L.; et al. Neutralising antibody responses against SARS-CoV-2 omicron BA.4/5 and wild-type virus in patients with inflammatory bowel disease following three doses of COVID-19 vaccine (VIP): A prospective, multicentre, cohort study. eClinicalMedicine 2023, 64, 102249. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Janjua, M.; Chanchlani, N.; Lin, S.; Bewshea, C.; Nice, R.; McDonald, T.J.; Auckland, C.; Harries, L.W.; Davies, M.; et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the omicron wave of the SARS-CoV-2 pandemic. Gut 2023, 72, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Piccoli, L.; Case, J.B.; Park, Y.-J.; Beltramello, M.; Guarino, B.; Dang, H.; De Melo, G.D.; Pinto, D.; Sprouse, K.; et al. Neutralization, effector function and immune imprinting of omicron variants. Nature 2023, 621, 592–601. [Google Scholar] [CrossRef]
- Miller, J.; Hachmann, N.P.; Collier, A.Y.; Lasrado, N.; Mazurek, C.R.; Patio, R.C.; Powers, O.; Surve, N.; Theiler, J.; Korber, B.; et al. Substantial neutralization escape by SARS-CoV-2 omicron variants BQ.1.1 and XBB.1. N. Engl. J. Med. 2023, 388, 662–664. [Google Scholar] [CrossRef]
- Stankov, M.V.; Hoffmann, M.; Gutierrez Jauregui, R.; Cossmann, A.; Morillas Ramos, G.; Graalmann, T.; Winter, E.J.; Friedrichsen, M.; Ravens, I.; Ilievska, T.; et al. Humoral and cellular immune responses following BNT162b2 XBB.1.5 vaccination. Lancet Infect. Dis. 2024, 24, e1–e3. [Google Scholar] [CrossRef]
- Becker, M.; Strengert, M.; Junker, D.; Kaiser, P.D.; Kerrinnes, T.; Traenkle, B.; Dinter, H.; Häring, J.; Ghozzi, S.; Zeck, A.; et al. Exploring beyond clinical routine SARS-CoV-2 serology using MultiCoV-Ab to evaluate endemic coronavirus cross-reactivity. Nat. Commun. 2021, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Junker, D.; Dulovic, A.; Becker, M.; Wagner, T.R.; Kaiser, P.D.; Traenkle, B.; Kienzle, K.; Bunk, S.; Struemper, C.; Haeberle, H.; et al. COVID-19 patient serum less potently inhibits ACE2-RBD binding for various SARS-CoV-2 RBD mutants. Sci. Rep. 2022, 12, 7168. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, H.; Nicols, A.R.; Longet, S.; Liu, C.; Tomic, A.; Angyal, A.; Kronsteiner, B.; Tyerman, J.K.; Tipton, T.; Zhang, P.; et al. Omicron infection following vaccination enhances a broad spectrum of immune responses dependent on infection history. Nat. Commun. 2023, 14, 5065. [Google Scholar] [CrossRef] [PubMed]
- Junker, D.; Becker, M.; Wagner, T.R.; Kaiser, P.D.; Maier, S.; Grimm, T.M.; Griesbaum, J.; Marsall, P.; Gruber, J.; Traenkle, B.; et al. Antibody binding and angiotensin-converting enzyme 2 binding inhibition is significantly reduced for both the BA.1 and BA.2 omicron variants. Clin. Infect. Dis. 2023, 76, e240–e249. [Google Scholar] [CrossRef]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P.; Girardin, R.C.; Rathod, B.; et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.N.; Chokkalingam, N.; Reuschel, E.L.; Purwar, M.; Xu, Z.; Gary, E.N.; Kim, K.Y.; Helble, M.; Schultheis, K.; Walters, J.; et al. SARS-CoV-2 assays to detect functional antibody responses that block ACE2 recognition in vaccinated animals and infected patients. J. Clin. Microbiol. 2020, 58, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Bowen, A.; Mellis, I.A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 2024, 32, 315–321.e3. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 vaccines against omicron subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef]
- Provine, N.M.; Amini, A.; Garner, L.C.; Spencer, A.J.; Dold, C.; Hutchings, C.; Silva Reyes, L.; FitzPatrick, M.E.B.; Chinnakannan, S.; Oguti, B.; et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–526. [Google Scholar] [CrossRef]
- Karim, F.; Riou, C.; Bernstein, M.; Jule, Z.; Lustig, G.; Van Graan, S.; Keeton, R.S.; Upton, J.-L.; Ganga, Y.; Khan, K.; et al. Clearance of persistent SARS-CoV-2 associates with increased neutralizing antibodies in advanced HIV disease post-ART initiation. Nat. Commun. 2024, 15, 2360. [Google Scholar] [CrossRef]
- Raglow, Z.; Surie, D.; Chappell, J.D.; Zhu, Y.; Martin, E.T.; Kwon, J.H.; Frosch, A.E.; Mohamed, A.; Gilbert, J.; Bendall, E.E.; et al. SARS-CoV-2 shedding and evolution in patients who were immunocompromised during the omicron period: A multicentre, prospective analysis. Lancet Microbe 2024, 5, e235–e246. [Google Scholar] [CrossRef]
- Planas, D.; Staropoli, I.; Michel, V.; Lemoine, F.; Donati, F.; Prot, M.; Porrot, F.; Guivel-Benhassine, F.; Jeyarajah, B.; Brisebarre, A.; et al. Distinct evolution of SARS-CoV-2 omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat. Commun. 2024, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Andeweg, S.P.; De Gier, B.; Eggink, D.; Van Den Ende, C.; Van Maarseveen, N.; Ali, L.; Vlaemynck, B.; Schepers, R.; Hahné, S.J.M.; Reusken, C.B.E.M.; et al. Protection of COVID-19 vaccination and previous infection against omicron BA.1, BA.2 and delta SARS-CoV-2 infections. Nat. Commun. 2022, 13, 4738. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Wagstaffe, H.R.; Thwaites, R.S.; Reynaldi, A.; Sidhu, J.K.; McKendry, R.; Ascough, S.; Papargyris, L.; Collins, A.M.; Xu, J.; Lemm, N.-M.; et al. Mucosal and systemic immune correlates of viral control after SARS-CoV-2 infection challenge in seronegative adults. Sci. Immunol. 2024, 9, eadj9285. [Google Scholar] [CrossRef]
- Albatayneh, E.; Alabdallat, Y.; Kreishan, E.; Ayash, H.; Abu-Lubad, M. Humoral immune response to COVID-19 infection or vaccination among celiac disease patients. Cent. Eur. J. Immunol. 2022, 47, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Granito, A.; Zauli, D.; Muratori, P.; Grassi, A.; Bortolotti, R.; Petrolini, N.; Veronesi, L.; Gionchetti, P.; Bianchi, F.B.; Volta, U. Anti-saccharomyces cerevisiae and perinuclear anti-neutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment. Pharmacol. Ther. 2005, 21, 881–887. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Guidi, M.; Lenzi, M.; Bianchi, F.B.; Volta, U. Anti-saccharomyces cerevisiae antibodies (ASCA) in coeliac disease. Gut 2006, 55, 296. [Google Scholar] [PubMed]
- Ying, B.; Darling, T.L.; Desai, P.; Liang, C.-Y.; Dmitriev, I.P.; Soudani, N.; Bricker, T.; Kashentseva, E.A.; Harastani, H.; Raju, S.; et al. Mucosal vaccine-induced cross-reactive CD8+ T cells protect against SARS-CoV-2 XBB.1.5 respiratory tract infection. Nat. Immunol. 2024, 25, 537–551. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 18) | |
|---|---|
| Age, years (SD) | 49.3 (16.8) |
| Sex (%) | |
| Female | 8 (44.4) |
| Male | 10 (55.6) |
| Other | 0 (0.0) |
| BMI, kg/m2 (SD) | 21.9 (4.3) |
| Ethnicity (%) | |
| European | 17 (94.4) |
| Asian | 0 (0.0) |
| African | 0 (0.0) |
| Others | 1 (5.6) |
| Smoking status (%) | |
| Never | 8 (44.4) |
| Former | 7 (38.9) |
| Current | 3 (16.7) |
| Diagnosis (%) | |
| Ulcerative colitis | 10 (55.6) |
| Crohn’s disease | 7 (38.9) |
| Indeterminate colitis | 1 (5.6) |
| Duration of IBD, years (SD) | 15.3 (10.8) |
| PRO2-based disease activity 1 (%) | 3 (16.7) |
| Fecal calprotectin-based disease activity 2 (%) | 8 (44.4) |
| IBD therapy (%) | |
| Infliximab (anti-TNF) | 11 (61.1) |
| Adalimumab (anti-TNF) | 0 (0.0) |
| Certolizumab pegol (anti-TNF) | 0 (0.0) |
| Golimumab (anti-TNF) | 0 (0.0) |
| Vedolizumab (non-anti-TNF) | 5 (27.8) |
| Ustekinumab (non-anti-TNF) | 1 (5.6) |
| Tofacitinib (non-anti-TNF) | 1 (5.6) |
| Systemic steroids | 0 (0.0) |
| Immunomodulators | 0 (0.0) |
| Underlying disease (%) | |
| Cancer | 2 (11.1) |
| Heart disease | 2 (11.1) |
| Hypertension | 3 (16.7) |
| Pulmonary disease | 2 (11.1) |
| Kidney disease | 3 (16.7) |
| Diabetes | 0 (0.0) |
| Arthritis | 3 (16.7) |
| Hyperlipidemia | 1 (5.6) |
| Liver disease | 2 (11.1) |
| SARS-CoV-2 infection since third vaccination (%) | 6 (33.3) |
| Number of SARS-CoV-2 infections ever (%) | |
| 0 | 8 (44.4) |
| 1 | 9 (50.0) |
| 2 | 0 (0.0) |
| 3 | 1 (5.6) |
| Type of fourth-dose vaccine | |
| BNT162b2 XBB.1.5 | 16 (88.9) |
| mRNA-1273.815 | 2 (11.1) |
| Vaccination schedule doses 1–4 (%) | |
| Homologous | 15 (83.3) |
| Heterologous | 3 (16.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woelfel, S.; Dütschler, J.; Junker, D.; König, M.; Leinenkugel, G.; Graf, N.; Krieger, C.; Truniger, S.; Franke, A.; Koller, S.; et al. Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease. Vaccines 2024, 12, 774. https://doi.org/10.3390/vaccines12070774
Woelfel S, Dütschler J, Junker D, König M, Leinenkugel G, Graf N, Krieger C, Truniger S, Franke A, Koller S, et al. Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease. Vaccines. 2024; 12(7):774. https://doi.org/10.3390/vaccines12070774
Chicago/Turabian StyleWoelfel, Simon, Joel Dütschler, Daniel Junker, Marius König, Georg Leinenkugel, Nicole Graf, Claudia Krieger, Samuel Truniger, Annett Franke, Seraina Koller, and et al. 2024. "Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease" Vaccines 12, no. 7: 774. https://doi.org/10.3390/vaccines12070774
APA StyleWoelfel, S., Dütschler, J., Junker, D., König, M., Leinenkugel, G., Graf, N., Krieger, C., Truniger, S., Franke, A., Koller, S., Metzger-Peter, K., Oberholzer, M., Frei, N., Geissler, N., Schaub, P., STAR SIGN Investigators, Albrich, W. C., Friedrich, M., Niess, J. H., ... Brand, S. (2024). Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease. Vaccines, 12(7), 774. https://doi.org/10.3390/vaccines12070774







