Unveiling the Significance of LysE in Survival and Virulence of Mycobacterium tuberculosis: A Review Reveals It as a Potential Drug Target, Diagnostic Marker, and a Vaccine Candidate
Abstract
1. Introduction
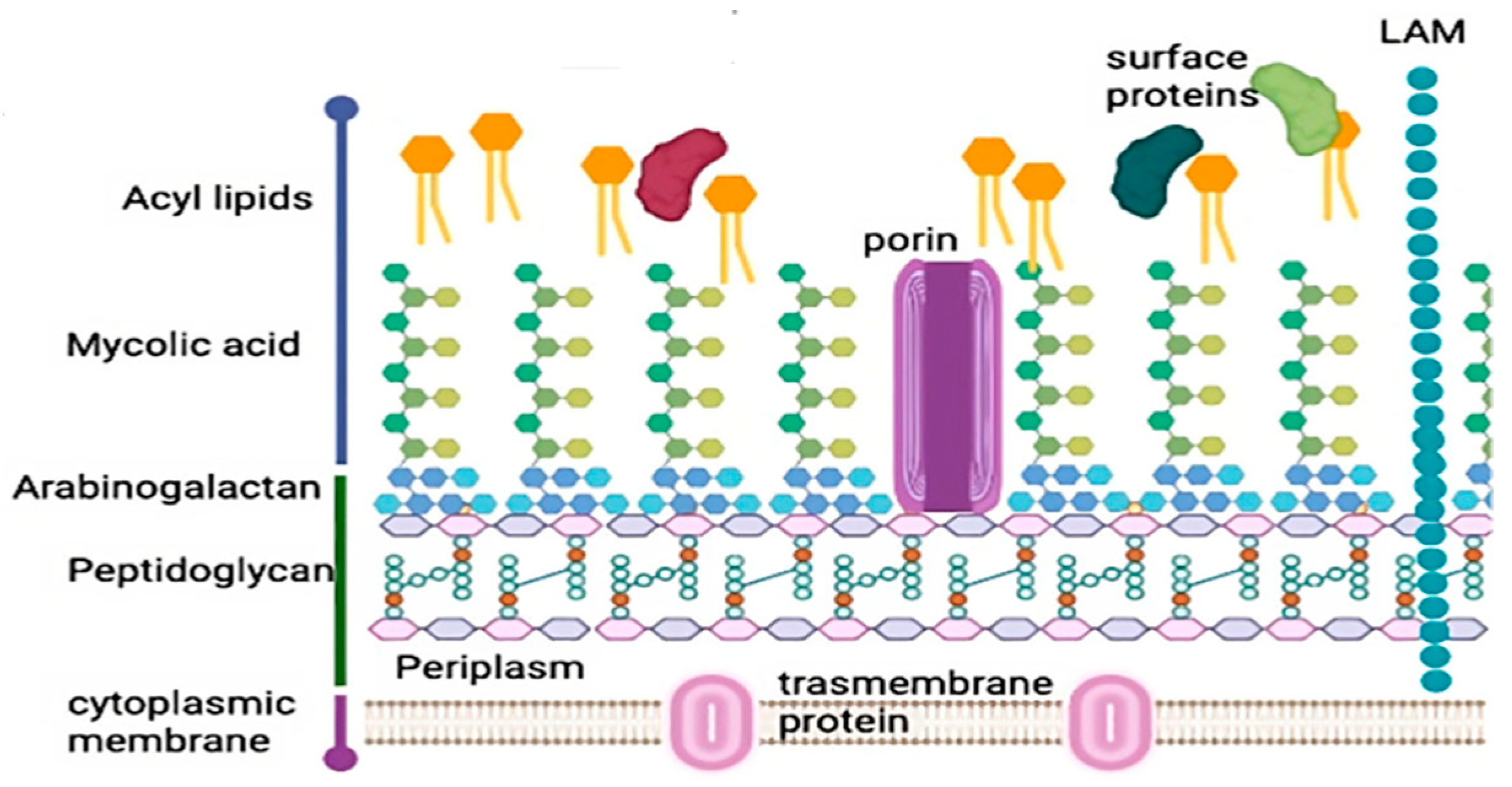
1.1. Overview of Mycobacterium tuberculosis
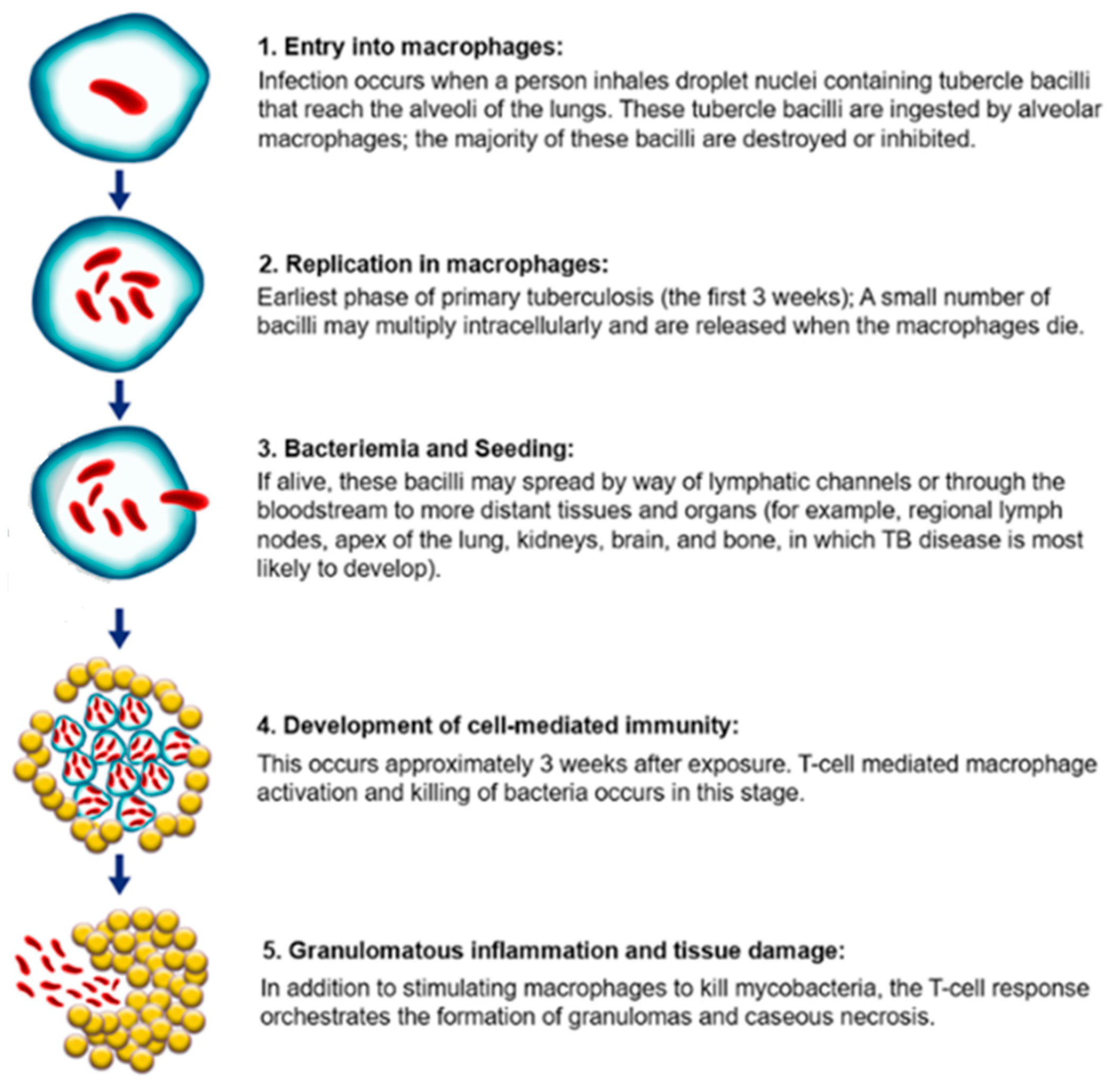
1.2. TB Pathogenesis
1.3. TB Virulence
1.4. LysE, an Amino Acid Transporter Protein
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
2.2.1. Pubmed
2.2.2. Scopus
2.2.3. Web of Science
2.2.4. CENTRAL
| ID | Search | Hits |
| #1 | “Lysine export*” | 0 |
| #2 | “lysine permea*” | 0 |
| #3 | “lysine transport*” | 1 |
| #4 | “lysine efflux” | 1 |
| #5 | LysE | 112 |
| #6 | “amino acid transporter” | 44 |
| #7 | MeSH descriptor: [Lysine] explode all trees and with qualifier(s): [biosynthesis − BI] | 1 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 159 |
| #9 | tubercul* | 9065 |
| #10 | mycobacteri* | 2495 |
| #11 | TB | 7621 |
| #12 | “Mycobacterium tuberculosis” | 1073 |
| #13 | MeSH descriptor: [Mycobacterium tuberculosis] explode all trees | 415 |
| #14 | #9 OR #10 OR #11 OR #12 OR #13 | 14,759 |
| #15 | #8 AND #14 | 0 |
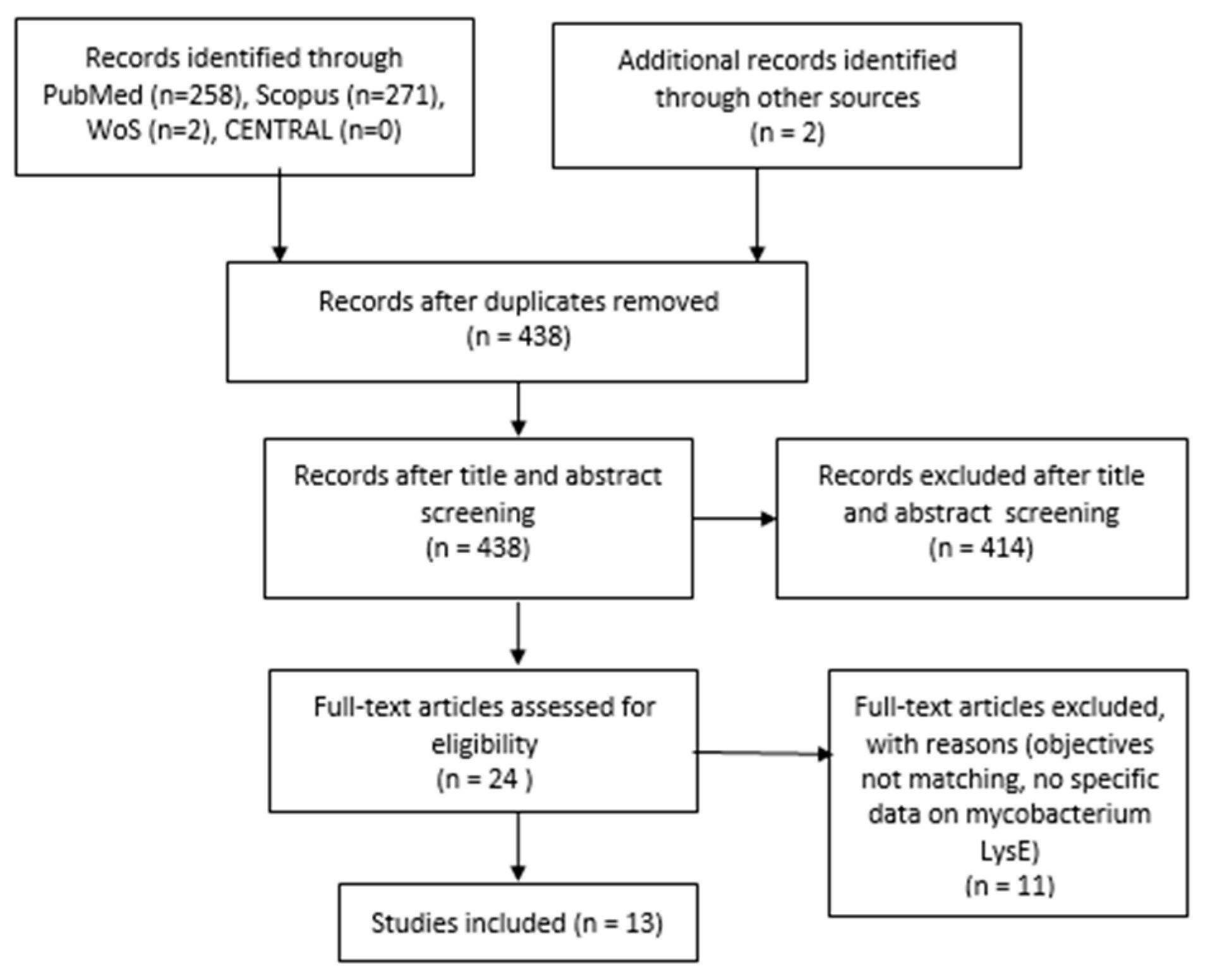
3. Results
Details of Included Studies
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021. 2021. Available online: https://iris.who.int/bitstream/handle/10665/346387/9789240037021-eng.pdf?sequence=1 (accessed on 11 June 2024).
- World Health Organization. Global Tuberculosis Report 2022. 2022. Available online: https://iris.who.int/bitstream/handle/10665/363752/9789240061729-eng.pdf?sequence=1 (accessed on 11 June 2024).
- World Health Organization. Tuberculosis: Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 11 June 2024).
- World Health Organization. Global Tuberculosis Report 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Bu, Q.; Qiang, R.; Fang, L.; Peng, X.; Zhang, H.; Cheng, H. Global trends in the incidence rates of MDR and XDR tuberculosis: Findings from the global burden of disease study 2019. Front. Pharmacol. 2023, 14, 1156249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BCG Vaccine. 2024. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/bcg (accessed on 11 June 2024).
- Pereira, S.M.; Dantas, O.M.S.; Ximenes, R.; Barreto, M.L. Vacina BCG contra tuberculose: Efeito protetor e políticas de vacinação. Rev. Saúde Pública.
- Chen, F.; Shi, T.; Zhu, Q.; Xiao, L. The Role of Mycobacterium tuberculosis Rv1986 and Rv3823c in Stimulating Humoral and Cell-Mediated Immune Responses. Ann. Clin. Immunol. Microbiol. 2018, 1, 6. [Google Scholar]
- Zhang, H.; Liu, M.; Fan, W.; Sun, S.; Fan, X. The impact of Mycobacterium tuberculosis complex in the environment on one health approach. Front. Public Health 2022, 10, 994745. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.V.; Parish, T. Microbe Profile: Mycobacterium tuberculosis: Humanity’s deadly microbial foe. Microbiology 2018, 164, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.; Sali, M.; Fadda, G. The Biology of Mycobacterium Tuberculosis Infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Delgado, Y.M.; Rodríguez-Carlos, A.; Serrano, C.J.; Rivas-Santiago, B. Mycobacterium tuberculosis cell-wall and antimicrobial peptides: A mission impossible? Front. Immunol. 2023, 14, 1194923. [Google Scholar] [CrossRef]
- Marrakchi, H.; Lanéelle, M.-A.; Daffé, M. Mycolic Acids: Structures, Biosynthesis, and Beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Chopra, S.; Dasgupta, A. Drug Discovery Targeting Drug-Resistant Bacteria; Elsevier: Amsterdam, The Netherlands, 2020; Available online: https://linkinghub.elsevier.com/retrieve/pii/C20180034003 (accessed on 1 April 2024).
- Awele Pathogenesis of TB | Knowledge Base for the National TB Elimination Program—NTEP. Available online: https://ntep.in/node/97/KM-pathogenesis-tb (accessed on 8 April 2024).
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef] [PubMed]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; Sabio y García, J.; Morbidoni, H.R.; Santangelo, M.d.l.P.; Cataldi, A.A.; Bigi, F. Virulence factors of the Mycobacterium tuberculosis complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An Adaptable Pathogen Associated with Multiple Human Diseases. Front. Cell. Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef]
- Dwivedi, M.; Mukhopadhyay, S.; Yadav, S.; Dubey, K.D. A multidrug efflux protein in Mycobacterium tuberculosis; tap as a potential drug target for drug repurposing. Comput. Biol. Med. 2022, 146, 105607. [Google Scholar] [CrossRef] [PubMed]
- Sahm, H.; Eggeling, L. New ubiquitous translocators: Amino acid export by Corynebacterium glutamicum and Escherichia coli. Arch. Microbiol. 2003, 180, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Fanouraki, C.; Borbat, P.P. Expression, purification and initial characterization of LysE membrane exporter from Mycobacterium tuberculosis: Towards comprehensive functional and structural study. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Cockle, P.J.; Gordon, S.V.; Lalvani, A.; Buddle, B.M.; Hewinson, R.G.; Vordermeier, H.M. Identification of Novel Mycobacterium tuberculosis Antigens with Potential as Diagnostic Reagents or Subunit Vaccine Candidates by Comparative Genomics. Infect. Immun. 2002, 70, 6996–7003. [Google Scholar] [CrossRef] [PubMed]
- Gideon, H.P.; Wilkinson, K.A.; Rustad, T.R.; Oni, T.; Guio, H.; Kozak, R.A.; Sherman, D.R.; Meintjes, G.; Behr, M.A.; Vordermeier, H.M.; et al. Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines. PLoS Pathog. 2010, 6, e1001237. [Google Scholar] [CrossRef]
- Chen, J.; Ruan, Q.; Shen, Y.; Wang, S.; Shao, L.; Zhang, W. Assessing and screening for T-cell epitopes from Mycobacterium tuberculosis RD2 proteins for the diagnosis of active tuberculosis. Braz. J. Infect. Dis. 2018, 22, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Wang, X.; Xiao, S.; Li, M.; Li, G.; Zhao, L.; Zhao, X.; Dou, X.; Wan, K. Genetic diversity of immune-related antigens in Region of Difference 2 of Mycobacterium tuberculosis strains. Tuberculosis 2017, 104, 1–7. [Google Scholar] [CrossRef]
- Tsu, B.V.; Saier, M.H. The LysE Superfamily of Transport Proteins Involved in Cell Physiology and Pathogenesis. PLoS ONE 2015, 10, e0137184. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.R.; Fanouraki, C.; Borbat, P.P. Purification and Biophysical Characterization of LysE Membrane Exporter from Mycobacterium tuberculosis in Lipodiscs Made of Native E. coli Membranes and Detergent. Biophys. J. 2020, 118, 503a–504a. [Google Scholar] [CrossRef]
- Hasenoehrl, E.J.; Rae Sajorda, D.; Berney-Meyer, L.; Johnson, S.; Tufariello, J.M.; Fuhrer, T.; Cook, G.M.; Jacobs, W.R.; Berney, M. Derailing the aspartate pathway of Mycobacterium tuberculosis to eradicate persistent infection. Nat. Commun. 2019, 10, 4215. [Google Scholar] [CrossRef]
- Vrljic, M.; Garg, J.; Bellmann, A.; Wachi, S.; Freudl, R.; Malecki, M.J.; Sahm, H.; Kozina, V.J.; Eggeling, L.; Saier, M.H.; et al. The LysE superfamily: Topology of the lysine exporter LysE of Corynebacterium glutamicum, a paradyme for a novel superfamily of transmembrane solute translocators. J. Mol. Microbiol. Biotechnol. 1999, 1, 327–336. [Google Scholar]
- Schneefeld, M.; Busche, T.; Geffers, R.; Kalinowski, J.; Bange, F.-C. The transcriptional regulator LysG (Rv1985c) of Mycobacterium tuberculosis activates lysE (Rv1986) in a lysine-dependent manner. PLoS ONE 2017, 12, e0186505. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; García-García, L.; Cruz-Hervert, P.; Guio, H.; Carranza, C.; Ferreyra-Reyes, L.; Canizales, S.; Molina, S.; Ferreira-Guerrero, E.; Téllez, N.; et al. Effect of isoniazid on antigen-specific interferon-γ secretion in latent tuberculosis. Eur. Respir. J. 2015, 45, 473–482. [Google Scholar] [CrossRef]
- Dubey, S.; Majumder, P.; Penmatsa, A.; Sardesai, A.A. Topological analyses of the L-lysine exporter LysO reveal a critical role for a conserved pair of intramembrane solvent-exposed acidic residues. J. Biol. Chem. 2021, 297, 101168. [Google Scholar] [CrossRef]
- Yelamanchi, S.D.; Surolia, A. Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life 2021, 73, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.S.; Jacobs, W.R. Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J. Bacteriol. 1996, 178, 6496–6507. [Google Scholar] [CrossRef]
- Eggeling, L.; Sahm, H. The cell wall barrier of Corynebacterium glutamicum and amino acid efflux. J. Biosci. Bioeng. 2001, 92, 201–213. [Google Scholar] [CrossRef]
- Burkovski, A.; Krämer, R. Bacterial amino acid transport proteins: Occurrence, functions, and significance for biotechnological applications. Appl. Microbiol. Biotechnol. 2002, 58, 265–274. [Google Scholar] [CrossRef]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 2020, 49, D461–D467. [Google Scholar] [CrossRef]
- Kozak, R.A.; Alexander, D.C.; Liao, R.; Sherman, D.R.; Behr, M.A. Region of Difference 2 Contributes to Virulence of Mycobacterium tuberculosis. Infect. Immun. 2011, 79, 59–66. [Google Scholar] [CrossRef]
| Multifaceted Role | Study ID | Findings of the Study | Conclusion |
|---|---|---|---|
| Vaccine Candidate | Georgieva et al. 2020 [21] United States of America (USA) | The study focussed on the M.tb-encoded L-lysine exporter (LysE) that serves as a target for inhibiting bacterial growth because its absence results in the accumulation of toxic levels of L-lysine, suppressing bacterial growth. The most studied H37Rv strain of M.tb encodes two LysEs, but one of them, the Rv1986 protein, is considered the primary exporter. During the early stages of M.tb infection, Rv1986 expression is increased under hypoxia conditions. Thus, the LysE protein can be a probable vaccine candidate. This study developed custom protocols for cloning, expression, purification, and initial characterization of LysE. Protein is purified in lipodiscs made of native E. coli membranes and detergent. | This study represented the initial production of highly pure LysE, providing a platform for in-depth investigations into the functional mechanisms of this protein. There is a need to have detailed knowledge about the less studied M.tb membrane transport system. |
| Chen F. et al. 2018 [8] China | A comprehensive analysis was performed by Chen F. et al. across 12 M. Bovis BCG strains and 200 M. tuberculosis strains. They proposed the presence of proteins, Rv1986, an amino acid transporter, and an integral membrane sulfoglycolipid transporter Rv3823c, responsible for mycolic acid transport in most epidemic strains but absent in BCG. These proteins exhibited a higher number of T-cell and B-cell epitopes. Notably, Rv1986 is found in virulent M. Bovis but not in the BCG strain. The researchers expressed recombinant M.tb proteins Rv1986 and Rv3823c in E. coli, testing their ability to stimulate innate and adaptive immune cell propagation and cytokine release. The results indicated that Rv1986 and Rv3823c can potentially enhance protective immunity through the activation of T cells, plasma cells, and inflammatory cytokines. | The study suggested the possible roles of Rv1986 and Rv3823c in future vaccine development. Additionally, Rv1986 was identified as an immunodominant target for memory T cells, capable of eliciting both humoral and cell-mediated immune responses, highlighting its diagnostic potential. | |
| Jiang et al., 2017 [25] China | Region of Deletion 2 (RD2) plays a role in mycobacterial virulence, and its deletion from M.tb leads to a decrease in bacterial growth. The researchers illustrated that genes within the RD2 antigens exhibit higher variability than other mycobacterial regions, particularly in the T-cell epitopic region. This implies their potential involvement in genetic variation to evade host immunity. The study specifically involved amplifying and comparing the sequences of five genes within the RD2 region, which encompasses both T and B cell epitopic regions. The results indicate that proteins Rv1980c, Rv1985, and Rv1986 in the RD2 region undergo antigenic variation in response to host immune pressure, suggesting their potential role in ongoing immune evasion. | The data confirmed that RD2 regions, aiming to evade host immunity, exhibit unexpected variability, particularly in immune-related antigens and T-cell epitope regions. | |
| Gideon et al. 2010 [23] South Africa | Gideon et al. identified the upregulation of two regions of difference (RD) 11 (Rv2658C and Rv2659c) and one RD2 (Rv1986), absent in widely used BCG strains. Exploring gene response patterns during prolonged hypoxia could unveil novel antigens for potential use in vaccines or diagnostics for tuberculosis. The Rv1986 gene exhibited significant elevation among tuberculosis patients during low oxygen levels. In TB patients, analysis of human immune responses to this protein revealed its strong recognition by cells producing interleukin-2 (IL-2), crucial for long-term immunological memory. These findings established Rv1986 as an immunodominant target for memory T cells. | Further examination of BCG strains with or without Rv1986 is essential, holding promise for potentially enhancing the effectiveness of existing or new vaccines or diagnostics for tuberculosis. | |
| Cockle et al. 2002 [22] United Kingdom | Cockle et al. directed their efforts towards pinpointing more precise reagents that could effectively differentiate between vaccination and infection. Their goal also involved the identification of subunit vaccine candidates to enhance the efficacy of tuberculosis vaccines. This study involved applying comparative genomics and identifying M. Bovis and M. tuberculosis antigens detection in the BCG vaccine. This study carefully selected 13 open reading frames (ORFs) from the RD1, RD2, and RD14 regions of M. tuberculosis. It was investigated in cattle infected with M. Bovis and in BCG-vaccinated and unvaccinated control cattle. This examination revealed eight particularly immunogenic antigens (Rv1983, Rv1986, Rv3872, Rv3873, Rv3878, Rv3879c, Rv1979c, and Rv1769). | The potential of these antigens as diagnostic markers or subunit vaccines merits further exploration and study. | |
| Drug target | Dubey et al. 2021 [32] India | This study focused on LysO, which mediates the export of L-lysine and L-thialysine in E. coli. They studied the hidden aspect of LysO topology and the mechanism it uses to facilitate export. In M.tb, L-Lysine export mediates by LysE, a transmembrane protein. Amino acid exporters are required to arbitrate resistance to toxic analogues of amino acids, which promote translational errors resulting in aberrant protein synthesis. Lack of which can cause bacterial growth retardation | This study presented that amino acid exporters have been characterized primarily at the physiological level. However, there is a notable lack of structural information on this class of membrane proteins, which is crucial for gaining mechanistic insights into the process of amino acid export. |
| Georgieva et al. 2020 [27] United States of America (USA) | Georgieva et al. (2020) involved LysE protein purification in Lipodisc, which comprises native E. coli membranes and detergents and characterized the LysE gene biophysically. LysE deficiency causes increased cellular levels of L-lysine, which inhibits bacterial growth. Consequently, the LysE proteins found in bacterial pathogens emerge as promising drug targets for inhibition. This underscores the importance of understanding these proteins comprehensively, encompassing their structures and the relationships between structure and function. | This study mentioned that it was the first study on highly pure LysE conducted in a controlled environment. It sets the groundwork for more in-depth investigations into how these proteins function. | |
| Hasenoehrl et al., 2019 [28] United States of America (USA) | In M.tb, lysine permease LysE, encoded by Rv1986, catalyses lysine export. A deficiency of LysE contributes to bacteria’s poor growth. This exporter also has a function in non-auxotrophic settings, as indicated by the M.tb lysE mutant’s delayed escape from the lag phase and sluggish development. LysE is a primary target for memory T cells secreting IL-2 and is believed to contribute to human protective immunity. Resting macrophage and lysosomal exposure models revealed that LysE is involved in human latent TB infection. Amino acids, particularly arginine, have been shown to influence human immunometabolism and T-cell responses. These findings highlight the significance of LysE-mediated export. | These results demonstrate that the aspartate pathway depends on a combination of metabolic control mechanisms in M.tb. This pathway is essential for persistence and a promising target for developing anti-tuberculosis drugs. The absence of LysE in eukaryotic cells makes it a probable target for drug discovery. | |
| Tsu and Saier, 2015 [26] China | In 2015, Tsu and Saier [22] explored the LysE superfamily of transmembrane transport proteins, which facilitate the export of amino acids, lipids, and heavy metal ions. This newly identified superfamily comprises three families: L-lysine and L-arginine exporters (LysE), homoserine/threonine resistance proteins (RhtB), and cadmium ion resistance proteins (CadD). While LysE and RhtB proteins are involved in amino acid ex-port, CadD proteins play a more distant role in cadmium (Cd2+) efflux. Members of the LysE Superfamily contribute to ionic homeostasis, protection against cytoplasmic heavy metals/metabolites, cell envelope assembly, and transmembrane electron flow. | As a result, they impact the physiology and pathogenesis of various microbes, serving as potential targets for drug action. However, many family members remain poorly understood regarding their functional and physiological roles. | |
| Vrljic et al. 1999 [29] United States of America (USA) | In C. glutamicum, Vrljic et al. explained that the LysE carrier protein exhibits remarkable functionality in exporting L-lysine. The investigation delves into the membrane topology of LysE, a protein composed of 236 amino acyl residues, by analysing PhoA- and LacZ-fusions. This study explains that LysE is a family of transmembrane proteins found in other bacterial species, such as E. coli, B. subtilis, M. tuberculosis and H. pylori. L-lysine export is required in environments with low concentrations of lysine-containing peptides. | Findings concluded that when the export carrier gene lysE is deleted, exceptionally high cytoplasmic concentrations of L-lysine (>1 M) accumulate, leading to bacteriostasis. Hence, LysE can be a probable drug target. | |
| Diagnostic Marker | Chen J. et al., 2018 [24] China | A study on Region of Deletion 2 (RD2) of M.tb stated that RD2 is responsible for bacterial virulence and showed immunodominancy in infected cattle. Antigens that showed immunogenicity in cattle are Rv1983, Rv1986, Rv1987, and Rv1989c. Out of 87 screened peptides from RD2 proteins, only 10 were recognized by over 10% of active TB patients using a whole blood IFN-γ release assay. The active TB group exhibited significantly elevated IFN-γ release responses to Rv1986-P9, P15, P16, Rv1988-P4, P11, and Rv1987-P11 compared to the control groups (p < 0.05). These findings indicate that the six epitopes originating from the RD2 region of M. tuberculosis hold promise as potential diagnostic markers for tuberculosis. | This study concluded that six epitopes from RD2, including Rv1986, have potential diagnostic value in active TB. In infected cattle, Rv1983, Rv1986, Rv1987, and Rv1989c within RD2 were identified as immunodominant. This study assessed their diagnostic potential in humans. |
| Schneefeld et al., 2017 [30] Germany | Schneefeld et al., 2017 [27] demonstrated that the Rv1985c protein binds to its own and the promoter region of Rv1986. In M. tuberculosis, Rv1985c and Rv1986 share identical genomic organization with lysG and lysE in C. glutamicum, including a promoter region where both genes overlap. The generation of a precisely defined Rv1985c deletion mutant in M. tuberculosis, coupled with gene expression analysis comparing the wild-type strain and the mutant, demonstrated that the Rv1985c protein activates the transcription of Rv1986 in a lysine-dependent manner. Consequently, it serves as an autoregulatory mechanism for its own expression. Furthermore, whole transcriptome expression analysis revealed that, in addition to lysE(Mt), the Rv1985c protein regulated three other genes, ppsB, ppsC, and ppsD, all involved in cell wall metabolism. | This study delineated the regulatory network of Rv1985c in M.tb. Given the resemblance to an orthologous gene pair in C. glutamicum, the study proposed renaming Rv1985c to lysG(Mt) and Rv1986 to lysE(Mt). While the function of the Rv1986 protein remains elusive, studies have demonstrated its regulation and recognition by memory T cells in human TB. | |
| Torres et al. 2015 [31] Mexico | Torres et al. performed a randomized controlled trial including patients with latent tuberculosis infection (LTBI). Patients were randomized into two arms, immediate or delayed isoniazid treatment arms. The research pinpointed the primary inducers of IFN-γ production in individuals with latent tuberculosis infection (LTBI) before isoniazid therapy, identifying Rv0849, Rv1737, and the RD2-encoded Rv1986 (absent in many commonly used BCG strains). Earlier investigations have demonstrated that peptides from Rv1986 exhibit potent stimulation of peripheral cells in both LTBI and active TB patients, inducing notably higher levels of IL2 compared to IFN-γ. | The in vitro IFN-γ responses to these proteins could serve as valuable diagnostic markers for assessing changes linked to the treatment of latent TB infection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyay, S.; Dhok, A.; Kashikar, S.; Quazi, Z.S.; Agarkar, V.B. Unveiling the Significance of LysE in Survival and Virulence of Mycobacterium tuberculosis: A Review Reveals It as a Potential Drug Target, Diagnostic Marker, and a Vaccine Candidate. Vaccines 2024, 12, 779. https://doi.org/10.3390/vaccines12070779
Upadhyay S, Dhok A, Kashikar S, Quazi ZS, Agarkar VB. Unveiling the Significance of LysE in Survival and Virulence of Mycobacterium tuberculosis: A Review Reveals It as a Potential Drug Target, Diagnostic Marker, and a Vaccine Candidate. Vaccines. 2024; 12(7):779. https://doi.org/10.3390/vaccines12070779
Chicago/Turabian StyleUpadhyay, Shilpa, Archana Dhok, Supriya Kashikar, Zahiruddin Syed Quazi, and Vinod B. Agarkar. 2024. "Unveiling the Significance of LysE in Survival and Virulence of Mycobacterium tuberculosis: A Review Reveals It as a Potential Drug Target, Diagnostic Marker, and a Vaccine Candidate" Vaccines 12, no. 7: 779. https://doi.org/10.3390/vaccines12070779
APA StyleUpadhyay, S., Dhok, A., Kashikar, S., Quazi, Z. S., & Agarkar, V. B. (2024). Unveiling the Significance of LysE in Survival and Virulence of Mycobacterium tuberculosis: A Review Reveals It as a Potential Drug Target, Diagnostic Marker, and a Vaccine Candidate. Vaccines, 12(7), 779. https://doi.org/10.3390/vaccines12070779







