Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Review Strategy
2.3. Data Extraction and Analytical Strategy
2.4. Statistical Analysis
3. Ethics
4. Results
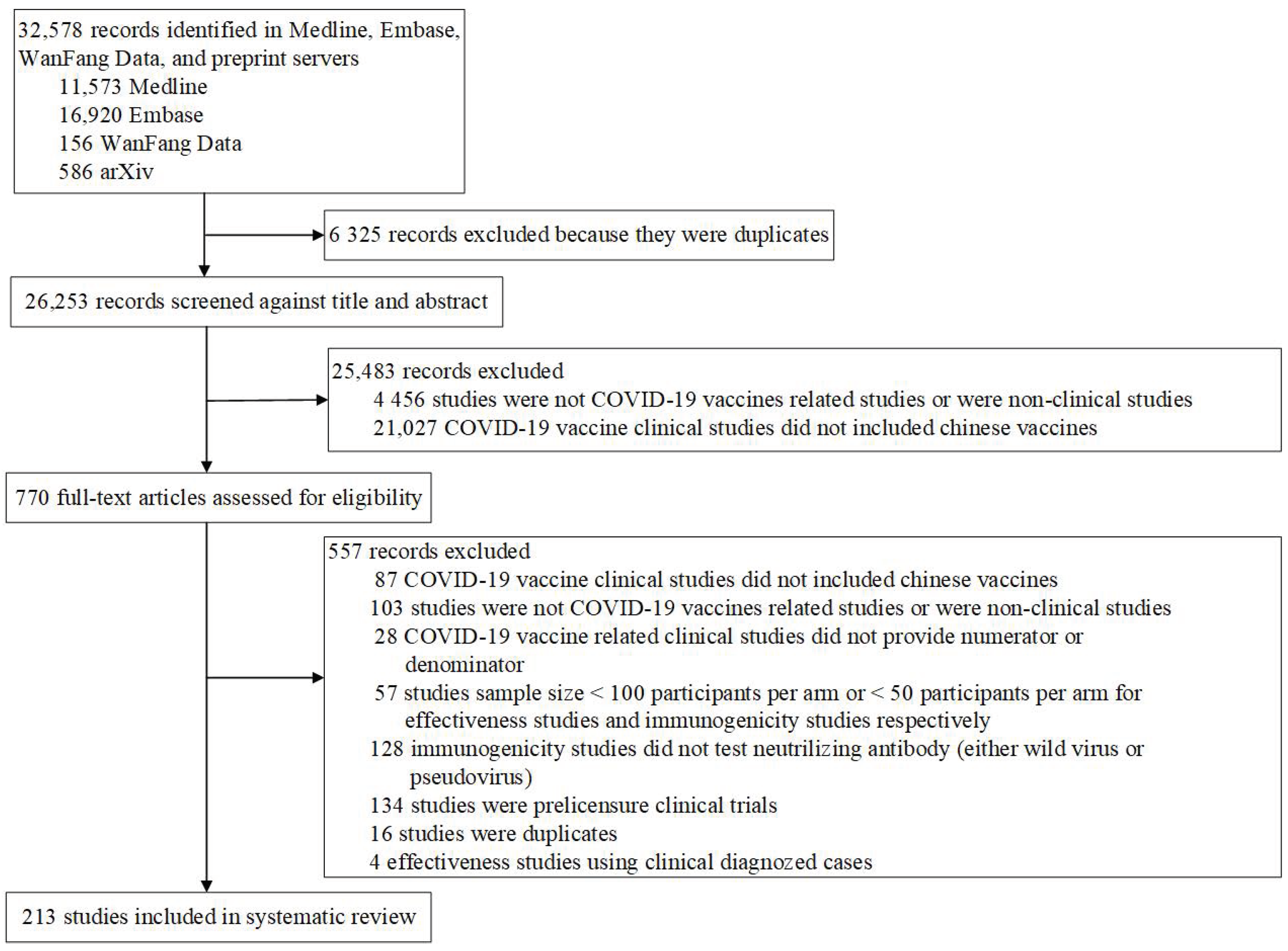
4.1. Selection and Characteristics of the Studies
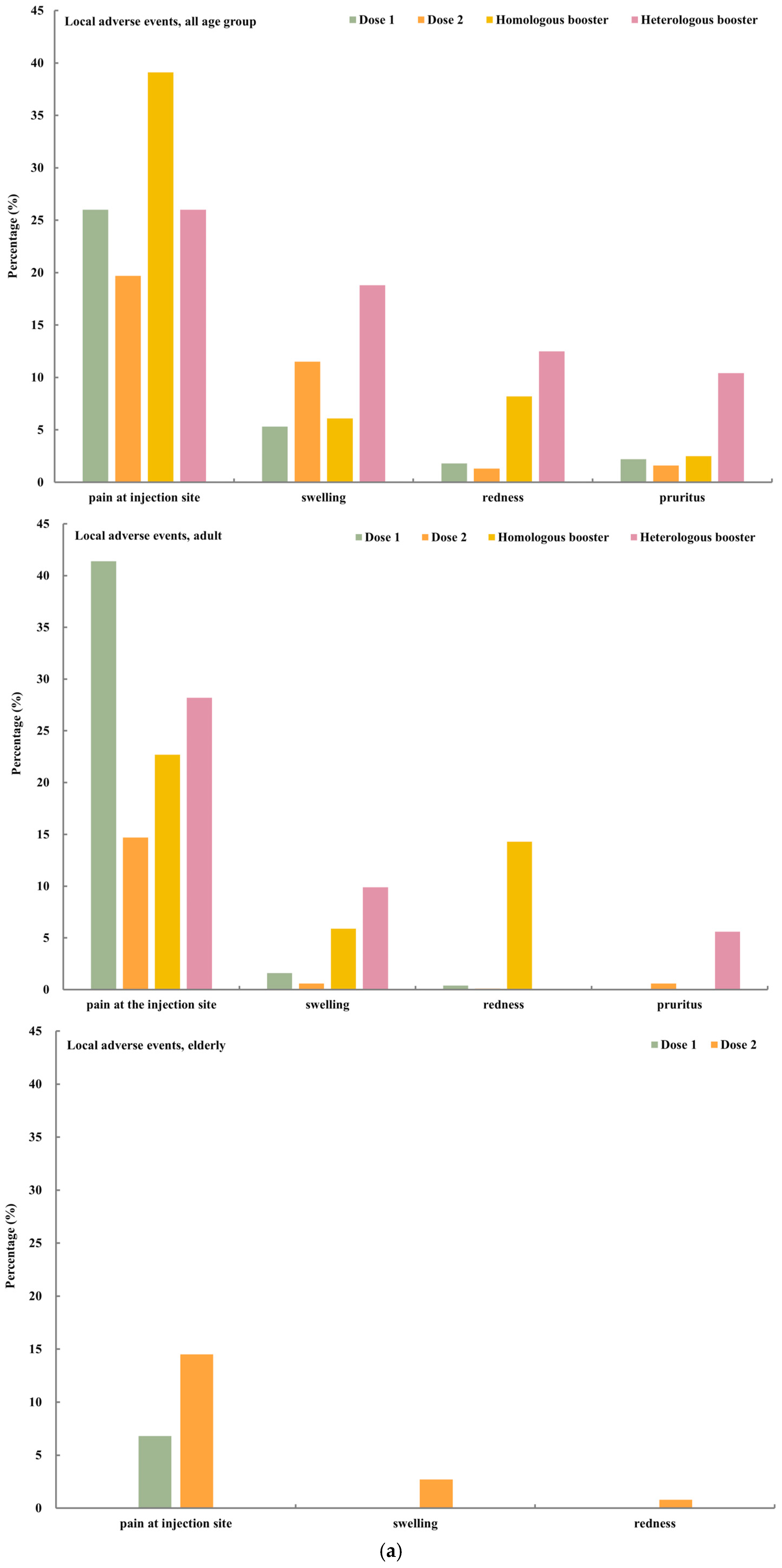
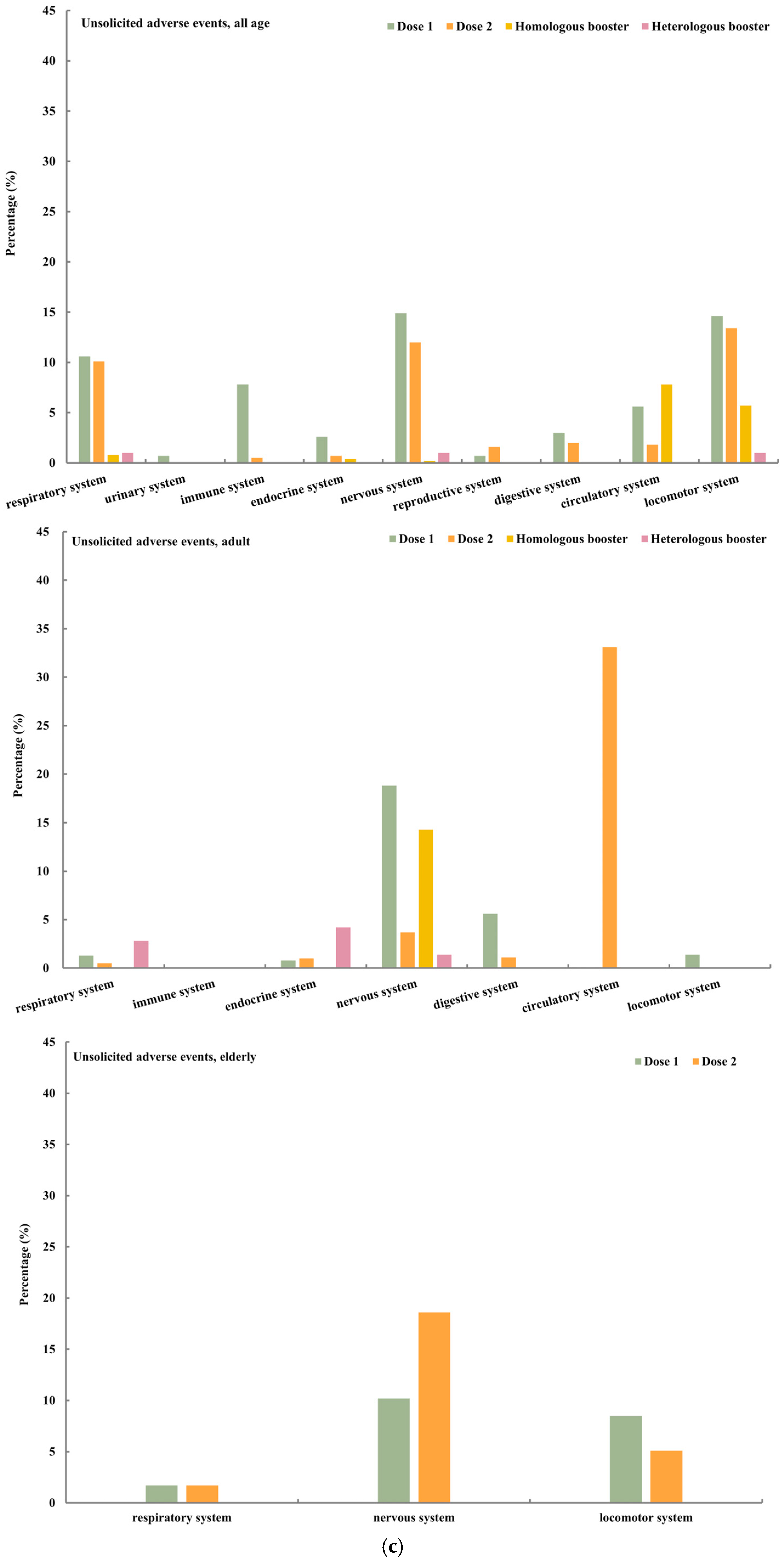
4.2. Safety
4.3. Immunogenicity
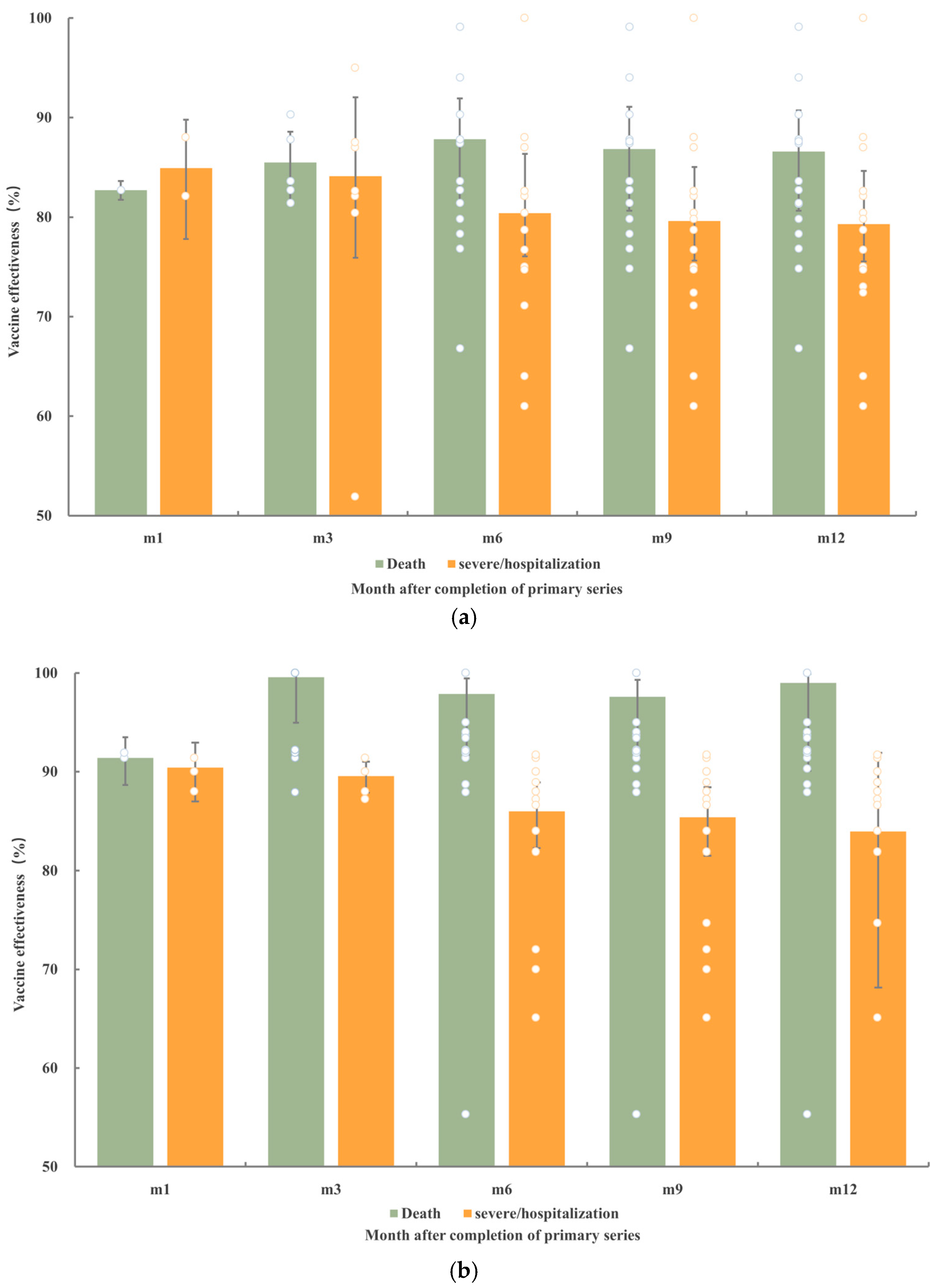
4.4. Effectiveness
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Vaccine Tracker and Landscape; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization. WHO Validates Sinovac COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. 2021. Available online: https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 25 June 2024).
- World Health Organization. WHO Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. 2021. Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 25 June 2024).
- World Health Organization. WHO Validates 11th Vaccine for COVID-19. 2022. Available online: https://www.who.int/news/item/19-05-2022-who-validates-11th-vaccine-for-covid-19 (accessed on 25 June 2024).
- Bolcato, M.; Rodriguez, D.; Feola, A.; Di Mizio, G.; Bonsignore, A.; Ciliberti, R.; Tettamanti, C.; Aurilio, M.T.; Aprile, A. COVID-19 Pandemic and Equal Access to Vaccines. Vaccines 2021, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- China NHCotPsRo. Prevention BoDCa; 2022. Available online: http://www.nhc.gov.cn/xcs/yqjzqk/202211/44ba0f46473c4cbaaf6e8dcbc79818cc.shtml (accessed on 1 December 2022).
- Mallapaty, S. China’s COVID vaccines have been crucial-now immunity is waning. Nature 2021, 598, 398–399. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis 2022, 22, 1435–1443. [Google Scholar] [CrossRef]
- Wu, D.; Ye, Y.; Tang, L.; Wang, A.B.; Zhang, R.; Qian, Z.H.; Wang, F.Z.; Zheng, H.; Huang, C.; Lv, X.Y.; et al. A case-case study on the effect of primary and booster immunization with China-produced COVID-19 vaccines on prevention of pneumonia and viral load among vaccinated persons infected by Delta and Omicron variants. Emerg. Microbes Infect. 2022, 11, 1950–1958. [Google Scholar] [CrossRef]
- Dolgin, E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature 2022, 601, 311. [Google Scholar] [CrossRef]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: An interim analysis in Indonesia. Vaccine 2021, 39, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Concato, J.; Corrigan-Curay, J. Real-World Evidence-Where Are We Now? N. Engl. J. Med. 2022, 386, 1680–1682. [Google Scholar] [CrossRef]
- Elliott, J.H.; Synnot, A.; Turner, T.; Simmonds, M.; Akl, E.A.; McDonald, S.; Salanti, G.; Meerpohl, J.; MacLehose, H.; Hilton, J.; et al. Living systematic review: 1. Introduction-the why, what, when, and how. J. Clin. Epidemiol. 2017, 91, 23–30. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.M.Z. (Ed.) JBI Manual for Evidence Synthesis; The Joanna Briggs Institute: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine, CoronaVac, Developed by Sinovac; WHO/2019-nCoV/vaccines/SAGE_recommendation/Sinovac-CoronaVac/2021.2. Available online: https://iris.who.int/handle/10665/346786?&locale-attribute=es (accessed on 11 November 2022).
- World Health Organization. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-BIBP (accessed on 11 November 2022).
- Kwok, R. Vaccines: The real issues in vaccine safety. Nature 2011, 473, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. ed.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- van Houwelingen, H.C.; Arends, L.R.; Stijnen, T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat. Med. 2002, 21, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.C.; Adams, J.L.; Suttorp, M.J.; Shekelle, P.G. AHRQ Technical Reviews. In Meta-regression Approaches: What, Why, When, and How? Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2004. [Google Scholar]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based. Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Rosa Duque, J.S.; Wang, X.; Leung, D.; Cheng, S.M.S.; Cohen, C.A.; Mu, X.; Hachim, A.; Zhang, Y.; Chan, S.M.; Chaothai, S.; et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat. Commun. 2022, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Lopes de Abreu, A.d.J.; Dias, C.Z.; Meng, X.; Ferreira, R.V.; Gonçalves Pereira, R.; Julian, G.S.; Yin, W. Safety profile of COVID-19 vaccines in pregnant and postpartum women in brazil. medRxiv 2021. [Google Scholar] [CrossRef]
- Fala.BR-Plataforma Integrada de Ouvidoria e Acesso à Informação. Available online: https://falabr.cgu.gov.br/web/home (accessed on 11 November 2022).
- Horne, E.M.F.; Hulme, W.J.; Keogh, R.H.; Palmer, T.M.; Williamson, E.J.; Parker, E.P.K.; Green, A.; Walker, V.; Walker, A.J.; Curtis, H.; et al. Waning effectiveness of BNT162b2 and ChAdOx1 COVID-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records. BMJ 2022, 378, e071249. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: Test negative, case-control study. BMJ 2022, 379, e072141. [Google Scholar] [CrossRef] [PubMed]
- Renia, L.; Goh, Y.S.; Rouers, A.; Le Bert, N.; Chia, W.N.; Chavatte, J.M.; Fong, S.W.; Chang, Z.W.; Zhuo, N.Z.; Tay, M.Z.; et al. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat. Commun. 2022, 13, 4615. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, Y.; Zhao, L.; Wang, L.; Fu, Z.; Tang, L.; Ye, F.; Liu, Q.; Wang, W.; Wang, S.; et al. Safety, immunogenicity, and immune persistence of two inactivated COVID-19 vaccines replacement vaccination in China: An observational cohort study. Vaccine 2022, 40, 5701–5708. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.L.; Cheng, S.M.; Leung, J.N.; Leung, K.; Lee, C.K.; Peiris, J.M.; Wu, J.T. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Euro Surveill. 2022, 27, 2101197. [Google Scholar] [CrossRef] [PubMed]
- Ranzani, O.T.; Hitchings, M.D.T.; de Melo, R.L.; de França, G.V.A.; Fernandes, C.d.F.R.; Lind, M.L.; Torres, M.S.S.; Tsuha, D.H.; David, L.C.S.; Said, R.F.C.; et al. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat. Commun. 2022, 13, 5536. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Ganga, Y.; Bernstein, M.; Jule, Z.; Reedoy, K.; Cele, S.; Lustig, G.; Amoako, D.; Wolter, N.; et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 2022, 13, 4686. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef]
- Lim, J.M.E.; Hang, S.K.; Hariharaputran, S.; Chia, A.; Tan, N.; Lee, E.S.; Chng, E.; Lim, P.L.; Young, B.E.; Lye, D.C.; et al. A comparative characterization of SARS-CoV-2-specific T cells induced by mRNA or inactive virus COVID-19 vaccines. Cell Rep. Med. 2022, 3, 100793. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.K.; Mahase, E. What next for COVID-19 vaccines? BMJ 2022, 379, o2422. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Bonhoeffer, J.; Black, S.; Izurieta, H.; Zuber, P.; Sturkenboom, M. Current status and future directions of post-marketing vaccine safety monitoring with focus on USA and Europe. Biologicals 2012, 40, 393–397. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Number of Studies (%) (n = 213) |
|---|---|
| Publication year | |
| 2021 | 83 (39.0) |
| 2022 | 130 (61.0) |
| Publication status | |
| Peer-reviewed, Chinese | 2 (0.9) |
| Peer-reviewed, English | 189 (88.7) |
| Preprint | 22 (10.4) |
| Study design | |
| Case report | 71 (33.3) |
| Case serials | 14 (6.6) |
| Cross-sectional study | 52 (24.4) |
| Case–control study | 15 (7.0) |
| Cohort study | 52 (24.4) |
| Clinical trials | 9 (4.2) |
| Study objectives a | |
| Safety | 170 (79.8) |
| Neutralization titers | 21 (9.7) |
| Effectiveness | 33 (15.5) |
| WHO regions b | |
| Africa | 0 (0) |
| Americas | 40 (18.8) |
| Eastern Mediterranean | 39 (18.4) |
| Europe | 48 (22.5) |
| Southeast Asia | 26 (12.2) |
| Western Pacific | 60 (28.1) |
| Quality of study | |
| High | 121 (56.8) |
| Medium | 56 (26.3) |
| lLw | 36 (16.9) |
| Potential Impact Factor | OR (%, 95% CI) | p Value |
|---|---|---|
| Death as the endpoint (9 studies, 16 estimates) | ||
| Interval since the last dose | 1.34 (0.08, 22.89) | 0.8402 |
| Interval between the two primary injections | 9.70 (0.44, 212.51) | 0.1491 |
| <3 weeks (Ref.) | ||
| ≥3 weeks | 0.66 (0.11, 4.09) | 0.6586 |
| Prior infection | 1.05 (0.03, 35.98) | 0.9771 |
| Vaccination regimens | ||
| Primary vaccination (Ref.) | - | - |
| Homologous booster vaccination | 0.08 (0.01, 0.48) | 0.0054 |
| Proportion of elderly | 2.32 (0.27, 19.89) | 0.4412 |
| Variant of concern | ||
| Before delta (Ref.) | - | - |
| Delta contained | 1.58 (0.01, 191.25) | 0.8522 |
| Omicron | 1.35 (0.04, 40.85) | 0.8633 |
| Severe disease as the endpoint (12 studies, 21 estimates) | ||
| Interval since the last dose | 1.44 (0.71, 2.91) | 0.3123 |
| Interval between the two primary injections | ||
| <3 weeks (Ref.) | NA | |
| ≥3 weeks | NA | |
| Prior infection | NA | NA |
| Vaccination regimens | ||
| Primary vaccination (Ref.) | - | - |
| Homologous booster vaccination | 0.26 (0.07, 0.95) | 0.0417 |
| Proportion of elderly | 1.62 (0.34, 7.73) | 0.5437 |
| Variant of concern | ||
| Before delta (Ref.) | - | - |
| Delta contained | 0.65 (0.11, 3.62) | 0.6186 |
| Omicron | 0.53 (0.1, 2.96) | 0.4706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zheng, H.; Wang, J.; Chen, Y.; Guo, X.; Li, Z.; Zhang, W.; Zhou, J.; Wang, S.; Lin, B.; et al. Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review. Vaccines 2024, 12, 781. https://doi.org/10.3390/vaccines12070781
Qi Y, Zheng H, Wang J, Chen Y, Guo X, Li Z, Zhang W, Zhou J, Wang S, Lin B, et al. Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review. Vaccines. 2024; 12(7):781. https://doi.org/10.3390/vaccines12070781
Chicago/Turabian StyleQi, Yangyang, Hui Zheng, Jinxia Wang, Yani Chen, Xu Guo, Zheng Li, Wei Zhang, Jiajia Zhou, Songmei Wang, Boyi Lin, and et al. 2024. "Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review" Vaccines 12, no. 7: 781. https://doi.org/10.3390/vaccines12070781
APA StyleQi, Y., Zheng, H., Wang, J., Chen, Y., Guo, X., Li, Z., Zhang, W., Zhou, J., Wang, S., Lin, B., Zhang, L., Yan, T., Clemens, J., Xia, J., An, Z., Yin, Z., Wang, X., & Feng, Z. (2024). Safety, Immunogenicity, and Effectiveness of Chinese-Made COVID-19 Vaccines in the Real World: An Interim Report of a Living Systematic Review. Vaccines, 12(7), 781. https://doi.org/10.3390/vaccines12070781





