Humoral and Cellular Response Induced by Primary Series and Booster Doses of mRNA Coronavirus Disease 2019 Vaccine in Patients with Cardiovascular Disease: A Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
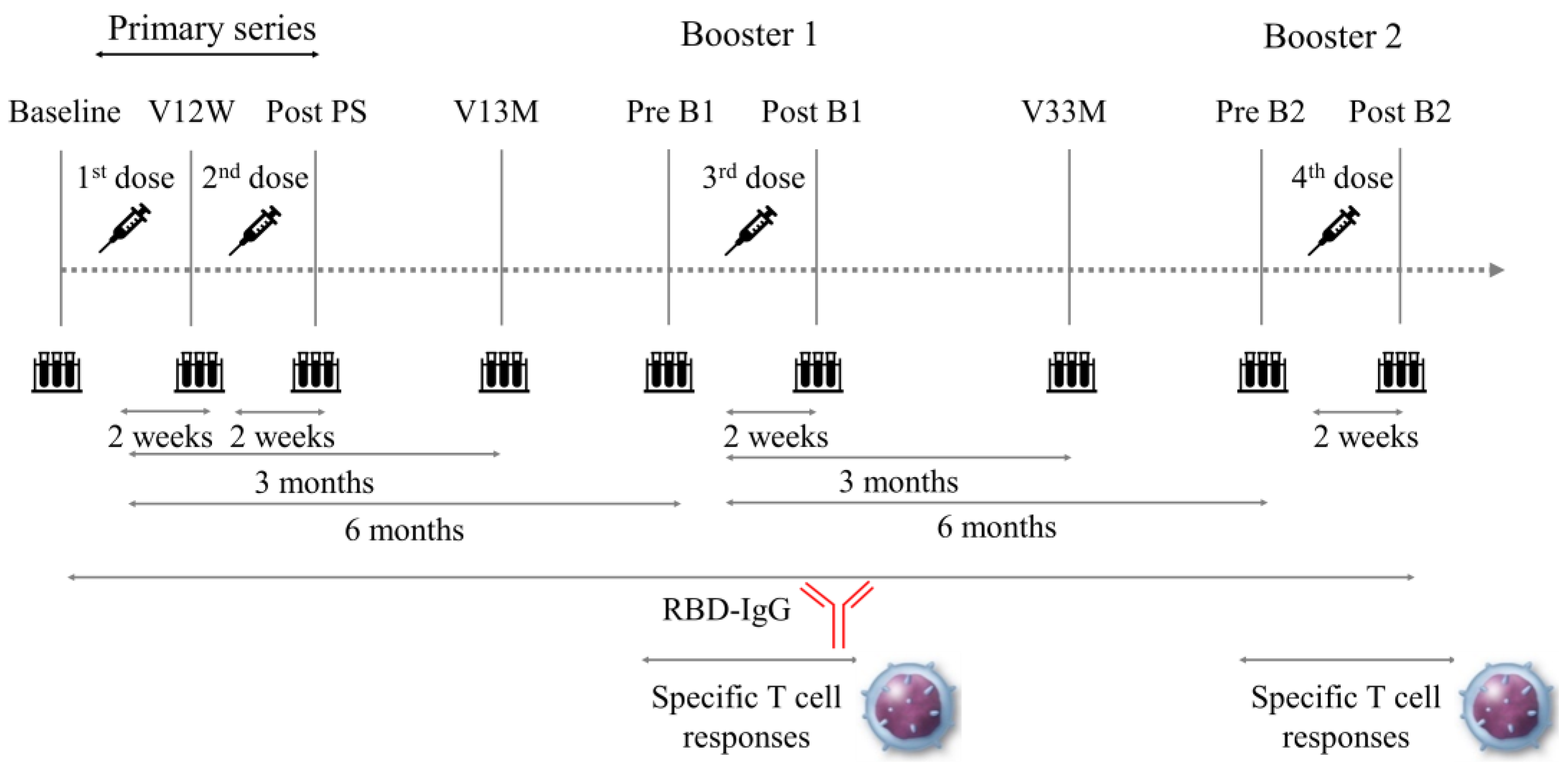
2.2. Participants and Blood Samples
2.3. Data Collection and Analysis
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Dynamics of RBD-IgG Titers
3.3. Vaccine Immunogenicity before and after Booster Vaccinations
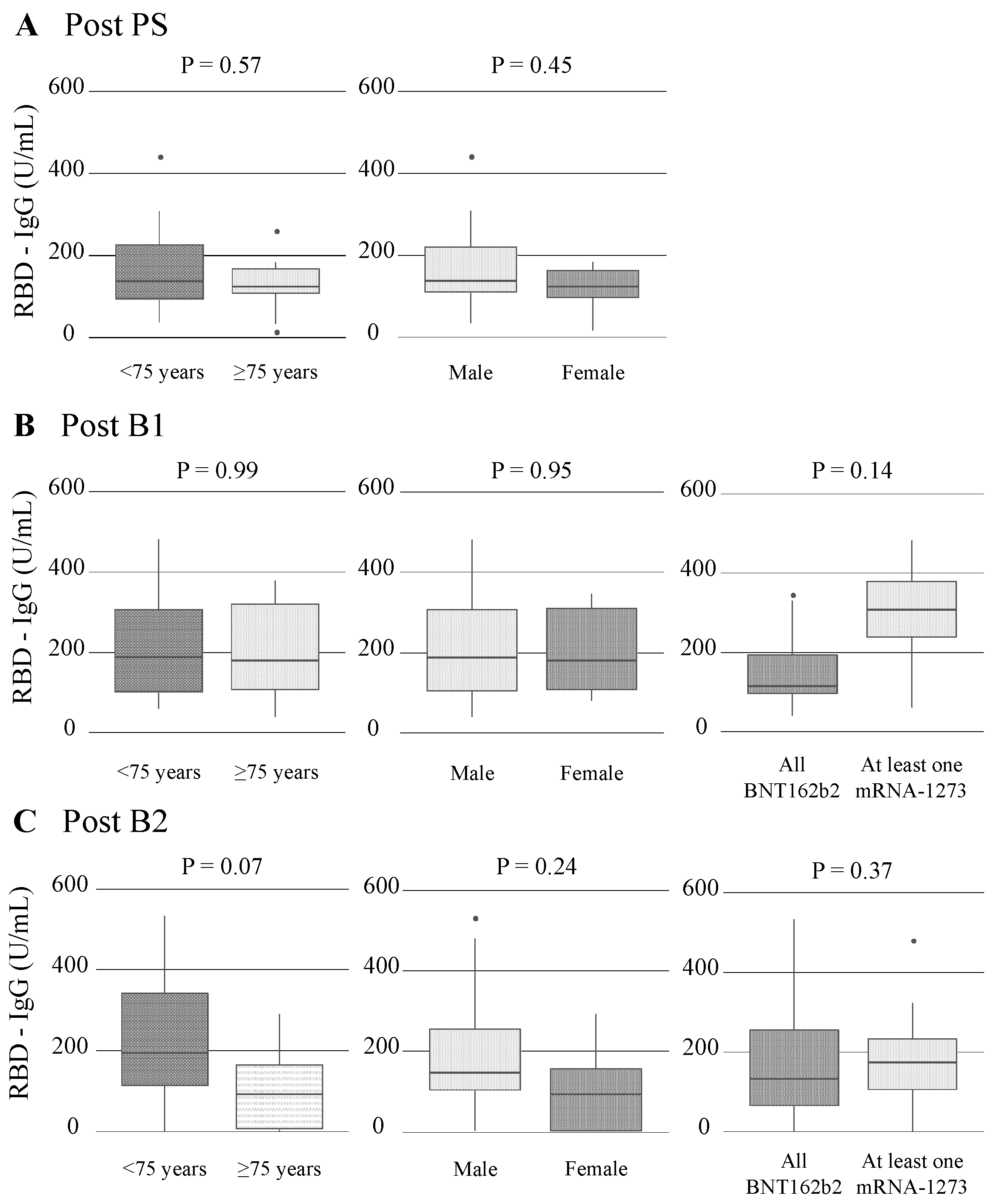
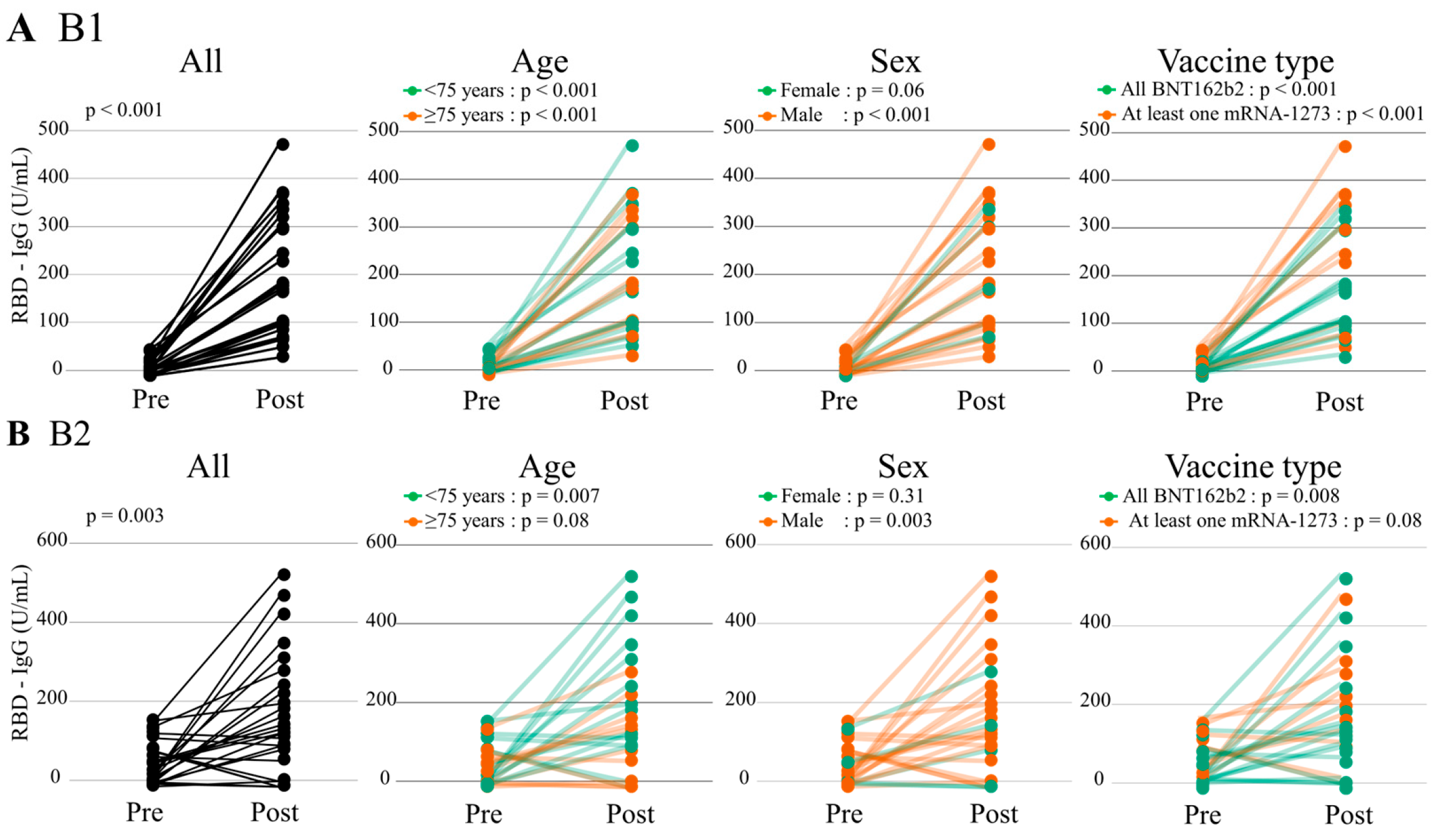
3.3.1. Humoral Immunogenicity
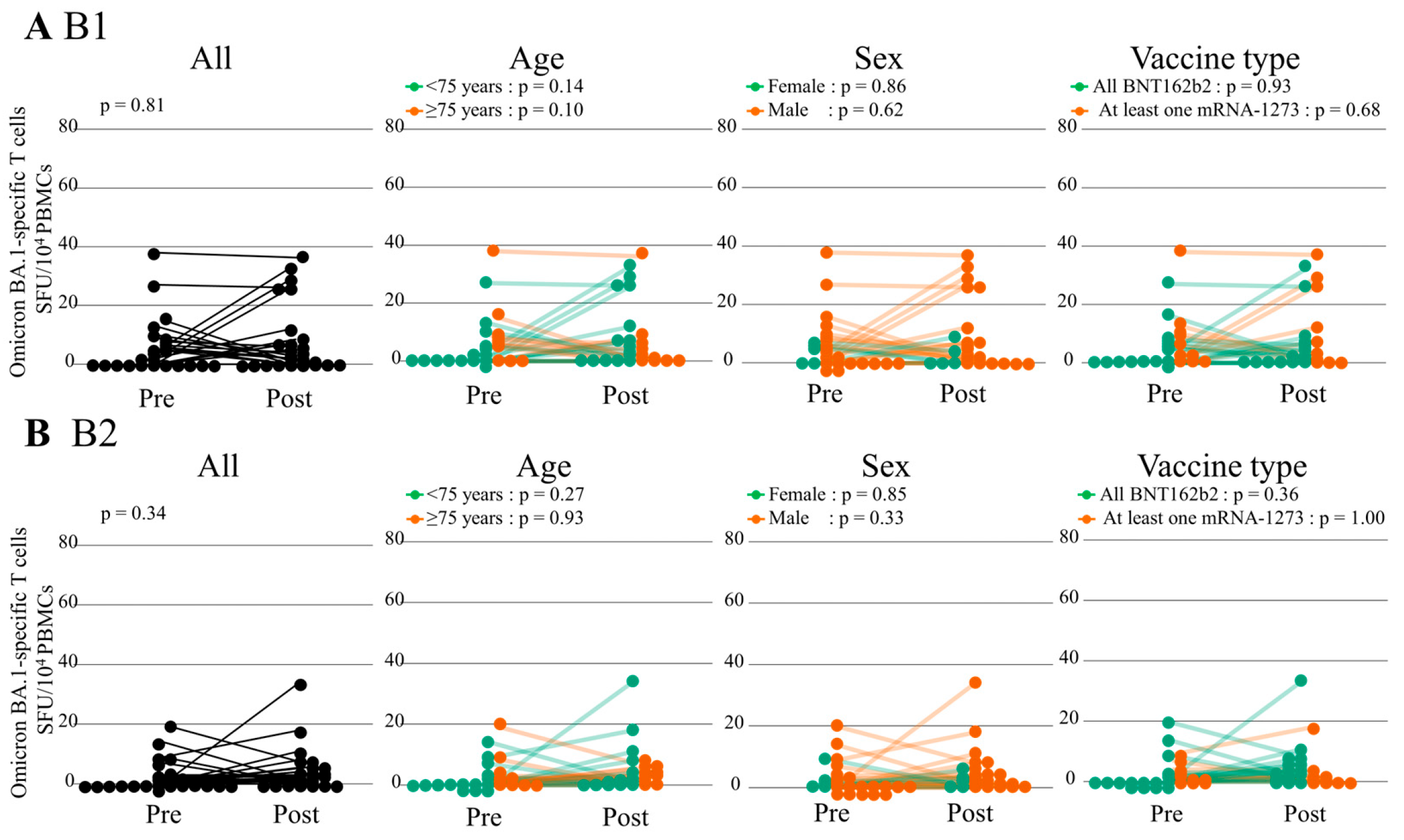
3.3.2. Cellular Immunity
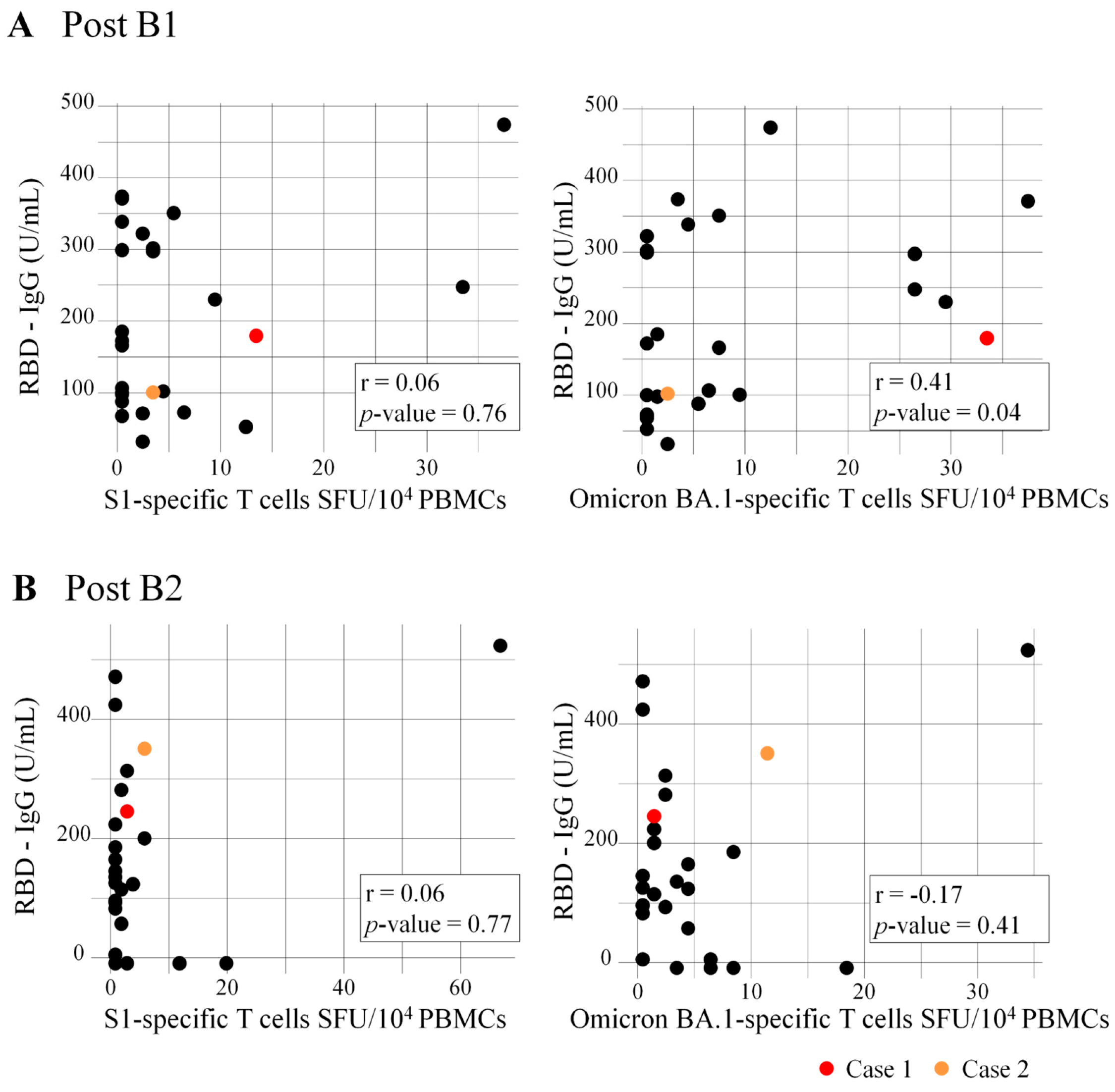
3.4. Correlation of RBD-IgG Titers and Numbers of Specific T Cells
3.5. Safety and Breakthrough Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidal-Perez, R.; Brandão, M.; Pazdernik, M.; Kresoja, K.-P.; Carpenito, M.; Maeda, S.; Casado-Arroyo, R.; Muscoli, S.; Pöss, J.; Fontes-Carvalho, R.; et al. Cardiovascular disease and COVID-19, a deadly combination: A review about direct and indirect impact of a pandemic. World J. Clin. Cases 2022, 10, 9556–9572. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA-J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Tam, C.-C.F.; Cheung, K.-S.; Lam, S.; Wong, A.; Yung, A.; Sze, M.; Lam, Y.-M.; Chan, C.; Tsang, T.-C.; Tsui, M.; et al. Impact of Coronavirus Disease 2019 (COVID-19) Outbreak on ST-Segment-Elevation Myocardial Infarction Care in Hong Kong, China. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006631. [Google Scholar] [CrossRef]
- Oba, S.; Hosoya, T.; Amamiya, M.; Mitsumura, T.; Kawata, D.; Sasaki, H.; Kamiya, M.; Yamamoto, A.; Ando, T.; Shimada, S.; et al. Arterial and Venous Thrombosis Complicated in COVID-19: A Retrospective Single Center Analysis in Japan. Front. Cardiovasc. Med. 2021, 8, 767074. [Google Scholar] [CrossRef]
- Burger, A.L.; Kaufmann, C.C.; Jäger, B.; Pogran, E.; Ahmed, A.; Wojta, J.; Farhan, S.; Huber, K. Direct cardiovascular complications and indirect collateral damage during the COVID-19 pandemic: A review. Wien. Klin. Wochenschr. 2021, 133, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.; Turlacu, M.; Micheu, M.M. Acute myocardial injury in patients with COVID-19: Possible mechanisms and clinical implications. World J. Clin. Cases 2022, 10, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Khan, M.S.; Rashid, S.; Niyaz, A.; Farooq, R.; Bhat, S.A.; Wani, H.A.; Qureshi, W. COVID-19: Diagnostics, Therapeutic Advances, and Vaccine Development. Curr. Clin. Microbiol. Rep. 2021, 8, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Stuart, A.S.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; I Stuart, D.; et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022, 399, 234–236. [Google Scholar] [CrossRef]
- Chu, L.; Vrbicky, K.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: An open-label phase 2 trial. Nat. Med. 2022, 28, 1042–1049. [Google Scholar] [CrossRef]
- Fujigaki, H.; Inaba, M.; Osawa, M.; Moriyama, S.; Takahashi, Y.; Suzuki, T.; Yamase, K.; Yoshida, Y.; Yagura, Y.; Oyamada, T.; et al. Comparative Analysis of Antigen-Specific Anti–SARS-CoV-2 Antibody Isotypes in COVID-19 Patients. J. Immunol. 2021, 206, 2393–2401. [Google Scholar] [CrossRef]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thummler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2-Specific IgG. Emerg. Infect. Dis. 2021, 27, 122–129. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Naruse, H.; Ito, H.; Izawa, H.; Sarai, M.; Ishii, J.; Sakaguchi, E.; Murakami, R.; Ando, T.; Fujigaki, H.; Saito, K. Immunogenicity of bnt162b2 mrna COVID-19 vaccine in patients with cardiovascular disease. J. Clin. Med. 2021, 10, 5498. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Cosme, J.; Dutheil, F.; Lambert, C.; Junda, M.; Mirand, A.; Ollier, A.; et al. Decline of Humoral and Cellular Immune Responses Against SARS-CoV-2 6 Months After Full BNT162b2 Vaccination in Hospital Healthcare Workers. Front. Immunol. 2022, 13, 842912. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; Sugiyama, K. Attenuation of Antibody Titers from 3 to 6 Months after the Second Dose of the BNT162b2 Vaccine Depends on Sex, with Age and Smoking Risk Factors for Lower Antibody Titers at 6 Months. Vaccines 2021, 9, 1500. [Google Scholar] [CrossRef]
- Weigert, A.; Bergman, M.-L.; Gonçalves, L.A.; Godinho, I.; Duarte, N.; Abrantes, R.; Borges, P.; Brennand, A.; Malheiro, V.; Matoso, P.; et al. Longitudinal Analysis of Antibody Responses to the mRNA BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis: A 6-Month Follow-Up. Front. Med. 2021, 8, 796676. [Google Scholar] [CrossRef] [PubMed]
- Kanai, D.; Wakui, H.; Haze, T.; Azushima, K.; Kinguchi, S.; Tsukamoto, S.; Kanaoka, T.; Urate, S.; Toya, Y.; Hirawa, N.; et al. SARS-CoV-2 spike protein antibody titers 6 months after SARS-CoV-2 mRNA vaccination among patients undergoing hemodialysis in Japan. Clin. Exp. Nephrol. 2022, 26, 988–996. [Google Scholar] [CrossRef]
- Akhtar, M.; Islam, R.; Khaton, F.; Soltana, U.H.; Jafrin, S.A.; Rahman, S.I.A.; Tauheed, I.; Ahmed, T.; Khan, I.I.; Akter, A.; et al. Appearance of tolerance-induction and non-inflammatory SARS-CoV-2 spike-specific IgG4 antibodies after COVID-19 booster vaccinations. Front Immunol. 2023, 14, 1309997. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Moderbacher, C.R.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. SARS-CoV-2 Variants of Concern Partially Escape Humoral but Not T Cell Responses in COVID-19 Convalescent Donors and Vaccine Recipients. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Durkee-Shock, J.; Jensen-Wachspress, M.; Kankate, V.V.; Lang, H.; Lazarski, C.A.; Keswani, A.; Webber, K.C.; Montgomery-Recht, K.; Walkiewicz, M.; et al. Robust Antibody and T Cell Responses to SARS-CoV-2 in Patients with Antibody Deficiency. J. Clin. Immunol. 2021, 41, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Picchianti-Diamanti, A.; Sebastiani, G.D.; Aiello, A.; Laganà, B.; Cuzzi, G.; Vanini, V.; Gualano, G.; Grifoni, A.; Ferraioli, M.; et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int. J. Infect. Dis. 2022, 121, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Elmer, A.; Kingston, N.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Dudley, H.M.; O’Mara, M.; Auma, A.; Gong, J.; Ross, Y.; Gurevich, N.; Carbone, S.; Reihs, A.; Nguyen, Y.; McComsey, G.A.; et al. Rheumatoid arthritis and older age are associated with lower humoral and cellular immune response to primary series COVID-19 mRNA vaccine. Vaccine 2023, 41, 6112–6119. [Google Scholar] [CrossRef] [PubMed]
- Jo, N.; Hidaka, Y.; Kikuchi, O.; Fukahori, M.; Sawada, T.; Aoki, M.; Yamamoto, M.; Nagao, M.; Morita, S.; Nakajima, T.E.; et al. Impaired CD4+ T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nat. Aging 2023, 3, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Dietz, L.L.; Juhl, A.K.; Sogaard, O.S.; Reekie, J.; Nielsen, H.; Johansen, I.S.; Benfield, T.; Wiese, L.; Staerke, N.B.; Jensen, T.O.; et al. Impact of age and comorbidities on SARS-CoV-2 vaccine-induced T cell immunity. Commun. Med. 2023, 3, 58. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Bozkurt, B. Shedding Light on Mechanisms of Myocarditis with COVID-19 mRNA Vaccines. Circulation 2023, 147, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]



| Subjects (N = 26) | |
|---|---|
| Age, y | 72 ± 7 |
| Male | 21 (81%) |
| Hypertension | 20 (77%) |
| Dyslipidemia | 18 (69%) |
| Diabetes | 8 (31%) |
| Allergic disease | 4 (15%) |
| Systolic blood pressure, mmHg | 131 ± 14 |
| Heart rate, beats per minutes | 71 ± 12 |
| Diagnosis | |
| Coronary artery disease | 20 (77%) |
| Hypertensive heart disease | 4 (15%) |
| Dilated cardiomyopathy | 1 (4%) |
| Aortic dissection | 1 (4%) |
| Previous myocardial infarction | 5 (19%) |
| Previous coronary revascularization | 12 (46%) |
| Paroxysmal or persistent atrial fibrillation | 2 (8%) |
| Laboratory data | |
| White blood cell count, ×103/μL | 5.9 ± 1.7 |
| Hemoglobin, g/dL | 14.0 ± 1.4 |
| Platelet count, ×104/μL | 19.6 ± 4.1 |
| Creatinine-based estimated glomerular filtration rate, mL/min/1.73 m2 | 58.0 ± 14.4 |
| Chronic kidney disease | 15 (58%) |
| Low-density lipoprotein-cholesterol, mg/dL | 91.5 ± 21.8 |
| Hemoglobin A1c, % | 6.3 ± 0.9 |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 116.5 (48.0–356.0) |
| High-sensitivity Troponin I, pg/mL | 3.70 (2.10–12.0) |
| Left ventricular ejection fraction, % | 54 ± 10 |
| Medications | |
| Renin–angiotensin–aldosterone system inhibitors | 11 (42%) |
| Beta-blockers | 10 (38%) |
| Diuretics | 3 (12%) |
| Statins | 14 (54%) |
| Antiplatelet drugs | 12 (46%) |
| Anticoagulant drugs | 4 (15%) |
| Intervals between, day | |
| V1 and sampling (V12W) | 14.7 ± 2.4 |
| V2 and sampling (post PS) | 15.2 ± 2.1 |
| B1 and sampling (post B1) | 15.3 ± 2.6 |
| B2 and sampling (post B2) | 14.9 ± 2.9 |
| V1 and V2 | 21.6 ± 1.9 |
| V2 and B1 | 222.7 ± 16.8 |
| B1 and B2 | 188.0 ± 9.9 |
| Vaccine type | |
| V1 | |
| BNT162b2 | 26 (100%) |
| V2 | |
| BNT162b2 | 26 (100%) |
| V3 | |
| BNT162b2 | 19 (73%) |
| mRNA-1273 | 7 (27%) |
| V4 | |
| BNT162b2 | 18 (69%) |
| mRNA-1273 | 8 (31%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, Y.; Naruse, H.; Fujigaki, H.; Murakami, R.; Ando, T.; Sakurai, K.; Uehara, K.; Shimomae, K.; Sakaguchi, E.; Hattori, H.; et al. Humoral and Cellular Response Induced by Primary Series and Booster Doses of mRNA Coronavirus Disease 2019 Vaccine in Patients with Cardiovascular Disease: A Longitudinal Study. Vaccines 2024, 12, 786. https://doi.org/10.3390/vaccines12070786
Ishihara Y, Naruse H, Fujigaki H, Murakami R, Ando T, Sakurai K, Uehara K, Shimomae K, Sakaguchi E, Hattori H, et al. Humoral and Cellular Response Induced by Primary Series and Booster Doses of mRNA Coronavirus Disease 2019 Vaccine in Patients with Cardiovascular Disease: A Longitudinal Study. Vaccines. 2024; 12(7):786. https://doi.org/10.3390/vaccines12070786
Chicago/Turabian StyleIshihara, Yuya, Hiroyuki Naruse, Hidetsugu Fujigaki, Reiko Murakami, Tatsuya Ando, Kouhei Sakurai, Komei Uehara, Koki Shimomae, Eirin Sakaguchi, Hidekazu Hattori, and et al. 2024. "Humoral and Cellular Response Induced by Primary Series and Booster Doses of mRNA Coronavirus Disease 2019 Vaccine in Patients with Cardiovascular Disease: A Longitudinal Study" Vaccines 12, no. 7: 786. https://doi.org/10.3390/vaccines12070786
APA StyleIshihara, Y., Naruse, H., Fujigaki, H., Murakami, R., Ando, T., Sakurai, K., Uehara, K., Shimomae, K., Sakaguchi, E., Hattori, H., Sarai, M., Ishii, J., Fujii, R., Ito, H., Saito, K., & Izawa, H. (2024). Humoral and Cellular Response Induced by Primary Series and Booster Doses of mRNA Coronavirus Disease 2019 Vaccine in Patients with Cardiovascular Disease: A Longitudinal Study. Vaccines, 12(7), 786. https://doi.org/10.3390/vaccines12070786






