Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency
Abstract
1. Introduction
1.1. Actuality
- assessment of the cell-mediated component of the immune response (for example, by quantification of T lymphocytes specific for vaccine antigen(s) and/or antigens isolated from wild-type microorganisms in vitro by direct marker incorporation or based on cytokine release);
- assessment of cellular immunity: number and proportion of participants before and after vaccination with sensitized (i.e., antigen specific) T lymphocytes (including sensitized CD4+ and CD8+ T lymphocytes) depending on the antigenic substance(s) used for stimulation and cytokines detected during the assay(s).
1.2. Cellular Immunity in Patients with CVID
1.3. Close Interaction of Innate and Adaptive Immunity
1.4. Adjuvanted Influenza Virus Vaccines
2. Materials and Methods
2.1. Aim
2.2. Participants
2.3. Interventions
- Diagnosis of CVID confirmed in accordance with the diagnostic guidelines established by the European Society for Immunodeficiency Diseases and the American Academy of Allergy, Asthma, and Immunology for the diagnosis and management of IEI.
- IVIg therapy administered no more than 28 days before vaccination and no less than 21 days after, ensuring a minimum gap of 7 weeks between consecutive immunoglobulin doses.
- Secondary hypogammaglobulinemia causes ruled out.
- No use of glucocorticosteroids or other immunosuppressive therapies at the time of the study or within 3 months before initiation.
- No evidence of protein-losing enteropathy or suspicion of oncological or lymphoproliferative diseases in the CVID patients at the time of the study.
- Absence of specific antiviral antibodies at protective levels (above 1:40) in pre-vaccination blood samples.
- No vaccination against other infections within 1.5–2 months prior to study enrollment.
- All contraindications stated in the instructions for the vaccines.
2.4. Outcomes
2.5. Statistical Methods
3. Results
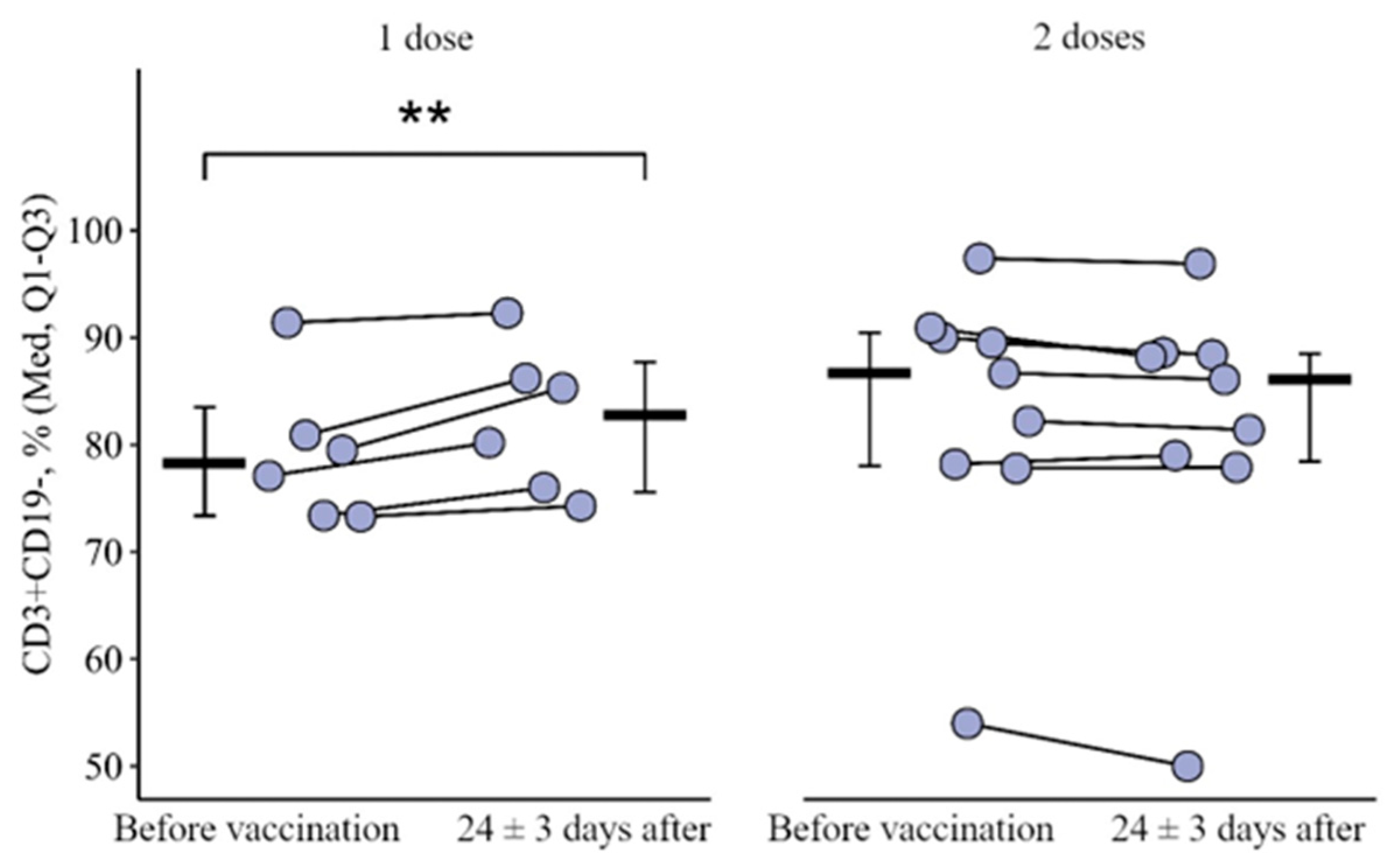
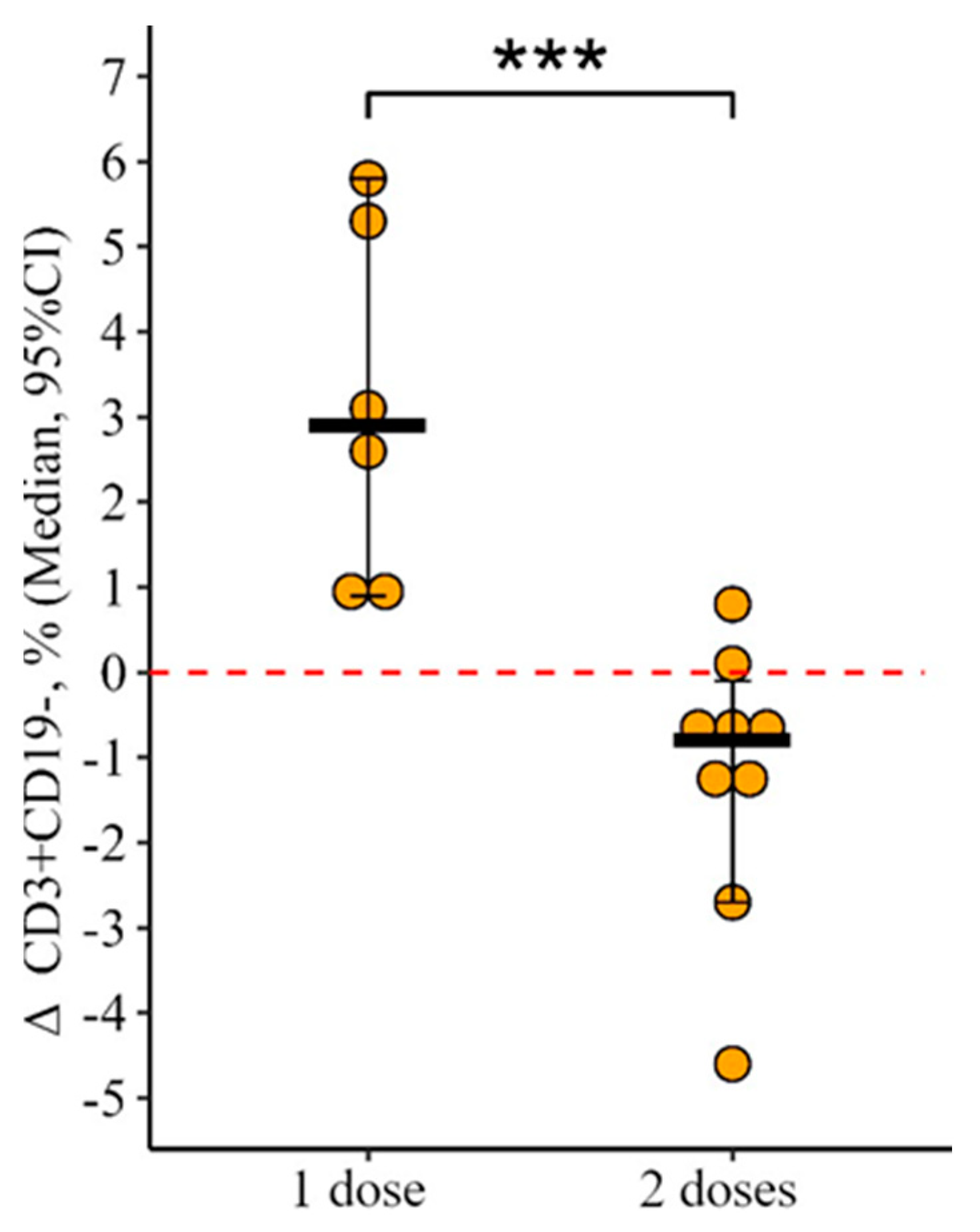
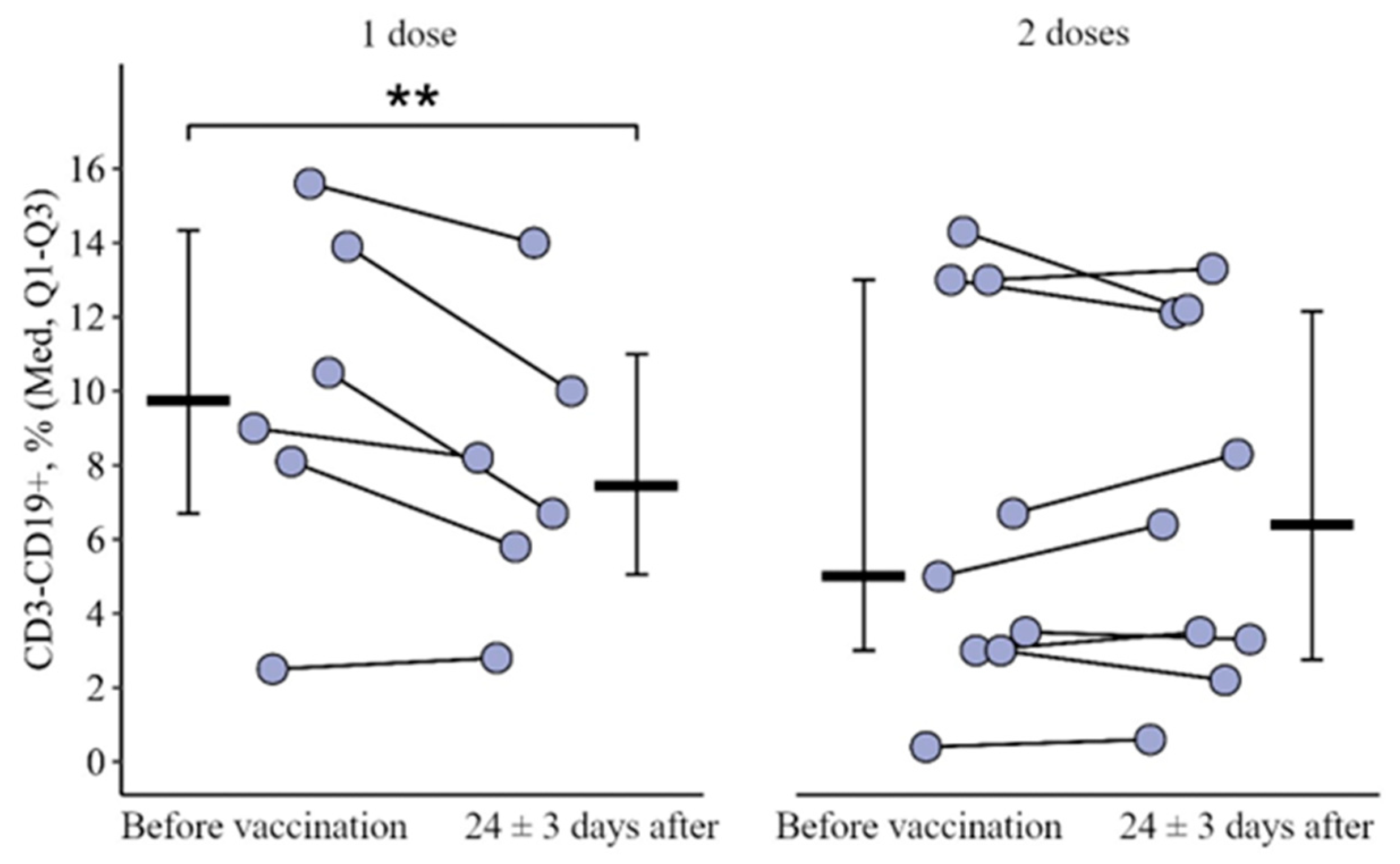
3.1. Changes in the Main Peripheral Blood Lymphocyte Subpopulation in Patients with CVID Vaccinated with One and Two (Simultaneous) Doses
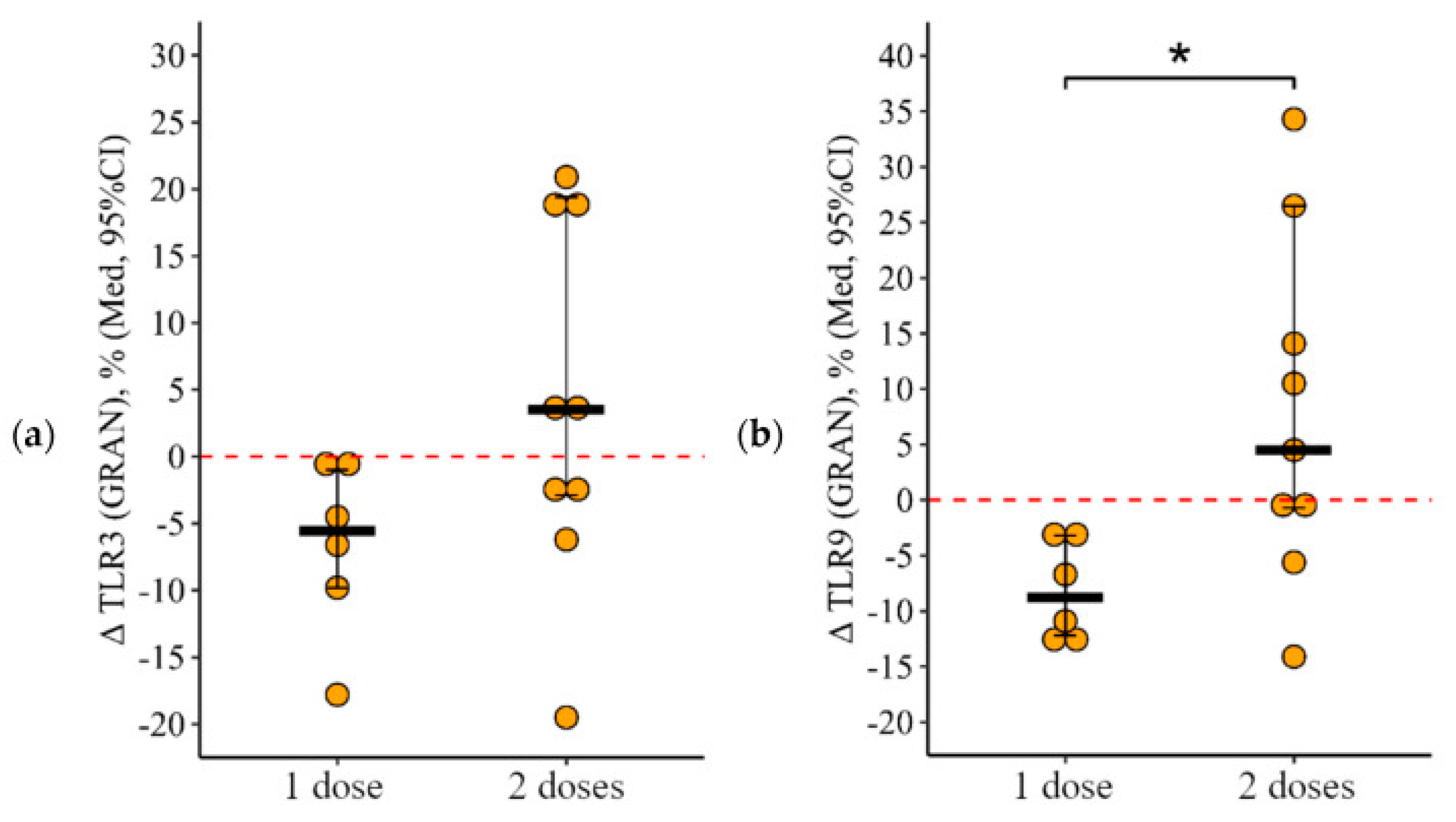
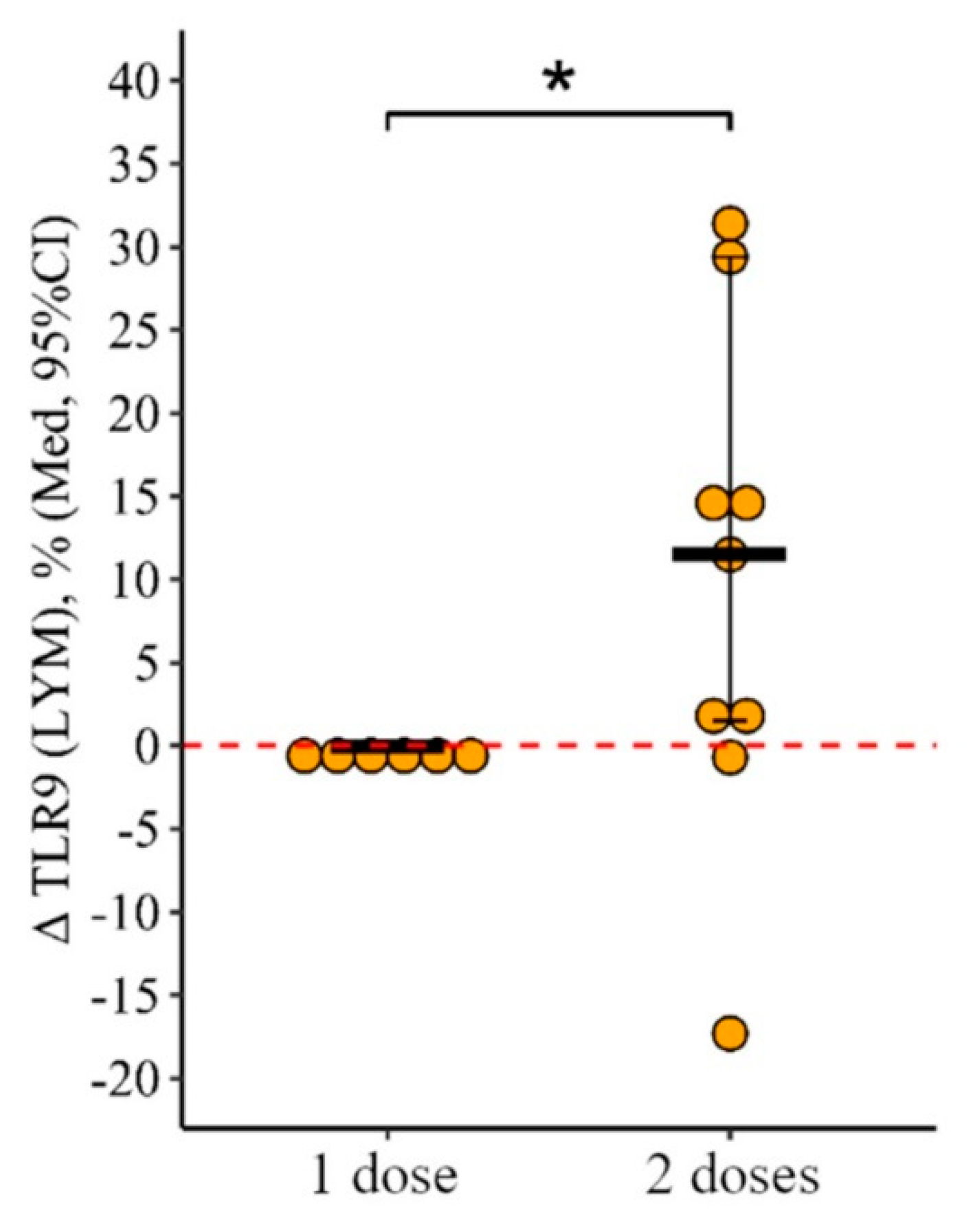
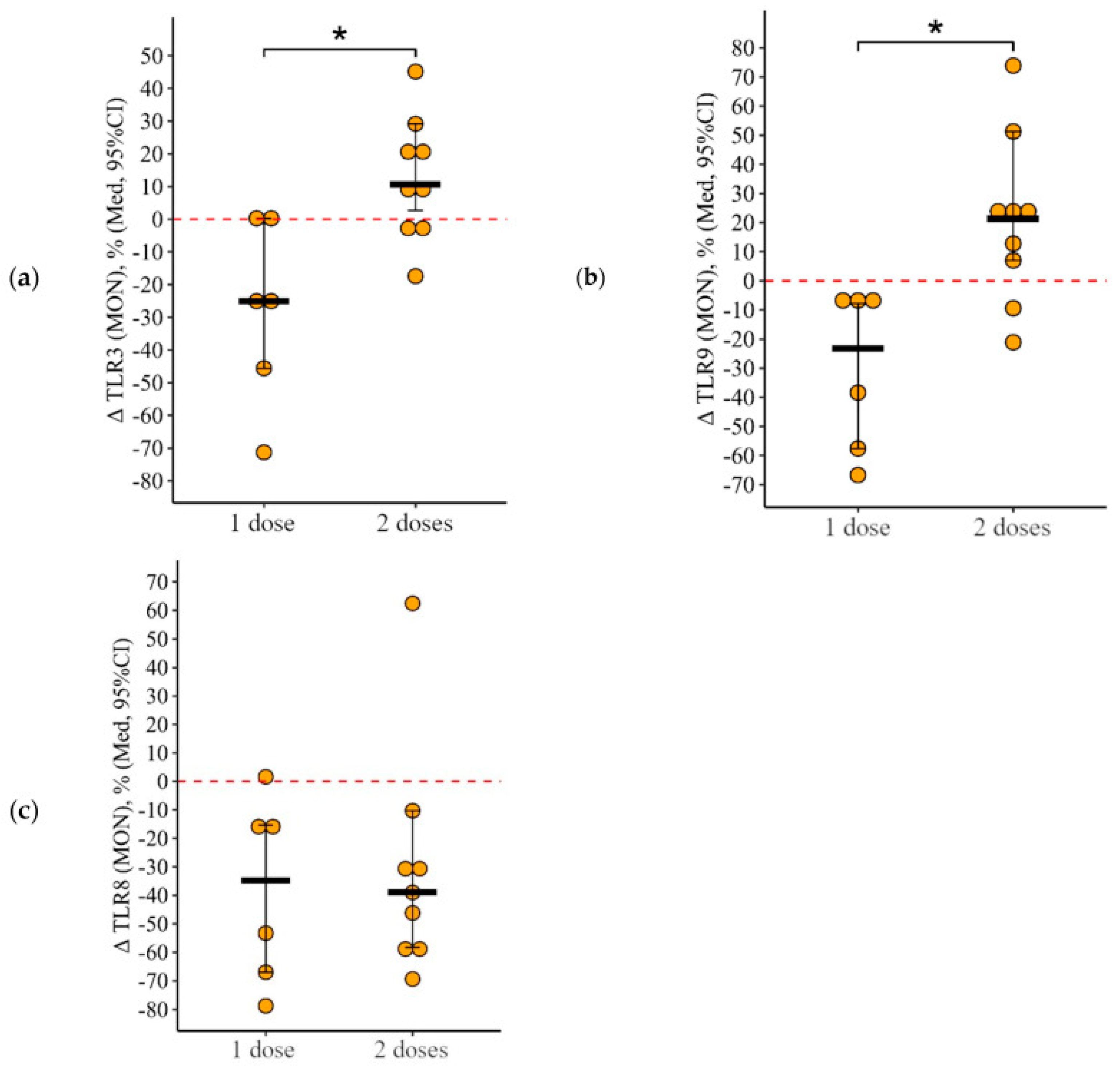
3.2. Changes in the Expression of Toll-like Receptors in Patients with CVID Vaccinated with One and Two (Simultaneous) Doses
3.2.1. Granulocytes
3.2.2. Lymphocytes
3.2.3. Monocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVID | common variable immune deficiency |
| CD | clusters of differentiation |
| IEI | inborn errors of immunity |
| IVIG | intravenous immunoglobulin |
| IVV | influenza virus vaccination |
| PAMPs | pathogen-associated molecular patterns |
| PID | primary immune deficiency |
| QIV | quadrivalent inactivated vaccine |
| aQIV | adjuvant quadrivalent inactivated vaccine |
| TIV | trivalent inactivated vaccine |
| aTIV | adjuvant trivalent inactivated vaccine |
| TLRs | Toll-like receptors |
References
- Tangye, S.G.; Al Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020, 40, 24–64. [Google Scholar] [CrossRef]
- Wasserman, R.L. Recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin infusion in primary immunodeficiency diseases. Immunotherapy 2017, 9, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, L.D.; Bacchetta, R.; Casanova, J.L.; Su, H.C. Human inborn errors of immunity: An expanding universe. Sci. Immunol. 2020, 5, eabb1662. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.E. Fundamental Immunology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; 1603p. [Google Scholar]
- Eibl, M.M.; Wolf, H.M. Vaccination in patients with primary immunedeficiency, secondary immunedeficiency and autoimmunity with immune regulatory abnormalities. Immunotherapy 2015, 7, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bousfiha, A.; Jeddane, L.; Picard, C.; Al-Herz, W.; Ailal, F.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; et al. Human Inborn Errors of Immunity: 2019 Update of the IUIS Phenotypical Classification. J. Clin. Immunol. 2020, 40, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Mukhina; Kuzmenko, N.B.; Rodina, Y.A.; Kondratenko, I.V.; Bologov, A.A. Characteristics of patients with primary immunodeficiency states in the Russian Federation: From birth to old age. Pediatr. N.A. G.N. Speransky 2019, 98, 24–31. (In Russian) [Google Scholar] [CrossRef]
- World Health Organization. Global Influenza Strategy 2019–2030; World Health Organization: Geneva, Switzerland, 2019; 34p. [Google Scholar]
- Hanitsch, L.G.; Löbel, M.; Mieves, J.F.; Bauer, S.; Babel, N.; Schweiger, B.; Wittke, K.; Grabowski, P.; Volk, H.-D.; Scheibenbogen, C. Cellular and humoral influenza-specific immune response upon vaccination in patients with common variable immunodeficiency and unclassified antibody deficiency. Vaccine 2016, 34, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- van Assen, S.; Holvast, A.; Telgt, D.S.C.; Benne, C.A.; de Haan, A.; Westra, J.; Kallenberg, C.G.; Bijl, M. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine. Clin. Immunol. 2010, 136, 228–235. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; Bosco, J.J.; Ojaimi, S.; Stirling, R.G.; Cameron, P.U.; Flanagan, K.; Plebanski, M.; Hogarth, P.M.; E O’hehir, R.; et al. Influenza-specific IgG1 + memory B-cell numbers increase upon booster vaccination in healthy adults but not in patients with predominantly antibody deficiency. Clin. Transl. Immunol. 2020, 9, e1199. [Google Scholar] [CrossRef]
- Gardulf, A.; Abolhassani, H.; Gustafson, R.; Eriksson, L.E.; Hammarström, L. Predictive markers for humoral influenza vaccine response in patients with common variable immunodeficiency. J. Allergy Clin. Immunol. 2018, 142, 1922–1931.e2. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, G.; Halstensen, A.; Sjursen, H.; Naess, A.; Kristoffersen, E.K.; Cox, R.J. Pandemic Influenza Vaccination Elicits Influenza-Specific CD4+ Th1-cell Responses in Hypogammaglobulinaemic Patients: Four case reports. Scand. J. Immunol. 2011, 74, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Alexia, C.; Cren, M.; Louis-Plence, P.; Vo, D.-N.; El Ahmadi, Y.; Dufourcq-Lopez, E.; Lu, Z.-Y.; Hernandez, J.; Shamilov, F.; Chernysheva, O.; et al. Polyoxidonium® Activates Cytotoxic Lymphocyte Responses Through Dendritic Cell Maturation: Clinical Effects in Breast Cancer. Front. Immunol. 2019, 10, 2693. [Google Scholar] [CrossRef] [PubMed]
- Kostinov, M.P.; Cherdantsev, A.P.; Kuselman, A.I.; Akhmatova, N.K.; Kostinova, A.M.; Deryabina, E.V.; Demina, E.O. Prospective randomized open-label comparative study of immunogenicity after subunit and polymeric subunit influenza vaccines administration among mothers and infants. Human. Vaccines Immunother. 2018, 14, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Barlan, I.; Chapel, H.; Costa-Carvalho, B.T.; Cunningham-Rundles, C.; de la Morena, M.T.; Espinosa-Rosales, F.J.; Hammarström, L.; Nonoyama, S.; Quinti, I.; et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2016, 4, 38–59. [Google Scholar] [CrossRef] [PubMed]
- Kostinov, M.P.; Akhmatova, N.K.; Khromova, E.A.; Skhodova, S.A.; Stolpnikova, V.N.; Cherdantsev, A.P.; Vlasenko, A.E. The impact of adjuvanted and non-adjuvanted influenza vaccines on the innate and adaptive immunity effectors. In Influenza-Therapeutics and Challenges; Saxena, S.K., Ed.; Intech Open Book Series. Infectious Diseases; InTech: London, UK, 2018; Volume 1, Chapter 5; pp. 83–109. [Google Scholar]
- Sobh, A.; Bonilla, F.A. Vaccination in Primary Immunodeficiency Disorders. J. Allergy Clin. Immunol. Pract. 2016, 4, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- de Waure, C.; Boccalini, S.; Bonanni, P.; Amicizia, D.; Poscia, A.; Bechini, A.; Barbieri, M.; Capri, S.; Specchia, M.L.; Di Pietro, M.L.; et al. Adjuvanted influenza vaccine for the Italian elderly in the 2018/19 season: An updated health technology assessment. Eur. J. Public Health 2019, 29, 900–905. [Google Scholar] [CrossRef]
- Azizi, G.; Mirshafiey, A.; Abolhassani, H.; Yazdani, R.; Jafarnezhad-Ansariha, F.; Shaghaghi, M.; Mortazavi-Jahromi, S.S.; Noorbakhsh, F.; Rezaei, N.; Aghamohammadi, A. Circulating Helper T-Cell Subsets and Regulatory T Cells in Patients With Common Varia-ble Immunodeficiency Without Known Monogenic Disease. J. Investig. Allergol. Clin. Immunol. 2018, 28, 172–181. [Google Scholar] [CrossRef]
- Chapel, H.; Lucas, M.; Lee, M.; Bjorkander, J.; Webster, D.; Grimbacher, B.; Fieschi, C.; Thon, V.; Abedi, M.R.; Hammarstrom, L. Com-mon variable immunodeficiency disorders: Division into distinct clinical phenotypes. Blood 2008, 112, 277–286. [Google Scholar] [CrossRef]
- Azizi, G.; Ahmadi, M.; Abolhassani, H.; Yazdani, R.; Mohammadi, H.; Mirshafiey, A.; Rezaei, N.; Aghamohammadi, A. Autoimmunity in Primary Antibody Deficiencies. Int. Arch. Allergy Immunol. 2016, 171, 180–193. [Google Scholar] [CrossRef]
- Yazdani, R.; Heydari, A.; Azizi, G.; Abolhassani, H.; Aghamohammadi, A. Asthma and Allergic Diseases in a Selected Group of Patients With Common Variable Immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2016, 26, 209–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yazdani, R.; Abolhassani, H.; Rezaei, N.; Azizi, G.; Hammarström, L.; Aghamohammadi, A. Evaluation of Known Defective Signal-ing-Associated Molecules in Patients Who Primarily Diagnosed as Common Variable Immunodeficiency. Int. Rev. Immunol. 2016, 35, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, R.; Ganjalikhani-Hakemi, M.; Esmaeili, M.; Abolhassani, H.; Vaeli, S.; Rezaei, A.; Sharifi, Z.; Azizi, G.; Rezaei, N.; Aghamo-hammadi, A. Impaired Akt phosphorylation in B-cells of patients with common variable immunodeficiency. Clin. Immunol. 2017, 175, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Abolhassani, H.; Mahdaviani, S.A.; Chavoshzadeh, Z.; Eshghi, P.; Yazdani, R.; Kiaee, F.; Shaghaghi, M.; Mohammadi, J.; Re-zaei, N.; et al. Clinical, immunologic, molecular analyses and outcomes of iranian patients with LRBA deficiency: A longitudinal study. Pediatr. Allergy Immunol. 2017, 28, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Shearer, W.T.; Fleisher, T.A.; Buckley, R.H.; Ballas, Z.; Ballow, M.; Blaese, R.M.; Bonilla, F.A.; Conley, M.E.; Cunningham-Rundles, C.; Fil-ipovich, A.H.; et al. Medical Adviso-ry Committee of the Immune Deficiency Foundation. Recommendations for live viral and bacterial vaccines in immunodefi-cient patients and their close contacts. J. Allergy Clin. Immunol. 2014, 133, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A. Update: Vaccines in primary immunodeficiency. J. Allergy Clin. Immunol. 2018, 141, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.K.; Tremoulet, A.H.; Burns, J.C.; Lewis, D.B. Cross-reactive neutralizing antibody against pandemic 2009 H1N1 influenza a virus in intravenous immunoglobulin preparations. Pediatr. Infect. Dis. J. 2011, 30, 67–69. [Google Scholar] [CrossRef] [PubMed]
- ESID Registry–Working Definitions for Clinical Diagnosis of PID. Latest Version, Now Including OMIM and Orpha Codes and HPO Terms: November. 2019. Available online: https://esid.org/Working-Parties/Registry-Working-Party/Diagnosis-criteria (accessed on 21 November 2019).
- Unger, S.; Seidl, M.; van Schouwenburg, P.; Rakhmanov, M.; Bulashevska, A.; Frede, N.; Grimbacher, B.; Pfeiffer, J.; Schrenk, K.; Munoz, L.; et al. The TH1 phenotype of follicular helper T cells indicates an IFN-γ-associated immune dysregulation in patients with CD21low common variable immunodeficiency. J. Allergy Clin. Immunol. 2018, 141, 730–740. [Google Scholar] [CrossRef]
- Giovannetti, A.; Pierdominici, M.; Mazzetta, F.; Marziali, M.; Renzi, C.; Mileo, A.M.; De Felice, M.; Mora, B.; Esposito, A.; Carello, R.; et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J. Immunol. 2007, 178, 3932–3943. [Google Scholar] [CrossRef]
- Horn, J.; Manguiat, A.; Berglund, L.J.; Knerr, V.; Tahami, F.; Grimbacher, B.; Fulcher, D.A. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin. Exp. Immunol. 2009, 156, 446–454. [Google Scholar] [CrossRef]
- Bayry, J.; Hermine, O.; Webster, D.A.; Lévy, Y.; Kaveri, S.V. Common variable immunodeficiency: The immune system in chaos. Trends Mol. Med. 2005, 11, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Rezaei, N.; Kiaee, F.; Tavakolinia, N.; Yazdani, R.; Mirshafiey, A.; Aghamohammadi, A. T-Cell Abnormalities in Common Variable Immunodeficiency. J. Investig. Allergol. Clin. Immunol. 2016, 26, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Hafezi, N.; Mohammadi, H.; Yazdani, R.; Alinia, T.; Tavakol, M.; Aghamohammadi, A.; Mirshafiey, A. Abnormality of regu-latory T cells in common variable immunodeficiency. Cell Immunol. 2017, 315, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Abolhassani, H.; Kiaee, F.; Tavakolinia, N.; Rafiemanesh, H.; Yazdani, R.; Mahdaviani, S.A.; Mohammadikhajehdehi, S.; Tavakol, M.; Ziaee, V.; et al. Autoimmunity and its association with regulatory T cells and B cell subsets in patients with common variable immunodeficiency. Allergol. Immunopathol. 2018, 46, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Arandi, N.; Mirshafiey, A.; Abolhassani, H.; Jeddi-Tehrani, M.; Edalat, R.; Sadeghi, B.; Shaghaghi, M.; Aghamohammadi, A. Frequency and expression of inhibitory markers of CD4(+) CD25(+) FOXP3(+) regulatory T cells in patients with common variable im-munodeficiency. Scand. J. Immunol. 2013, 77, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.A.; Ayers, L.; Sadler, R.; Lucas, M.; Roberts, C.; Woods, A.; Packwood, K.; Burden, J.; Harrison, D.; Kaenzig, N.; et al. T cell phenotypes in patients with common variable immunodeficiency disorders: Associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin. Exp. Immunol. 2012, 170, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Viallard, J.F.; Blanco, P.; André, M.; Etienne, G.; Liferman, F.; Neau, D.; Vidal, E.; Moreau, J.F.; Pellegrin, J.L. CD8+HLA-DR+ T lympho-cytes are increased in common variable immunodeficiency patients with impaired memory B-cell differentiation. Clin. Immunol. 2006, 119, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.M.; Iglesias, J.; Hernández, M.; Matamoros, N. Alterations in interleukin secretion (IL-2 and IL-4) by CD4 and CD4 CD45RO cells from common variable immunodeficiency (CVI) patients. Clin. Exp. Immunol. 1995, 102, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Vigano, S.; Bellanger, F.; Pellaton, C.; Buss, G.; Comte, D.; Roger, T.; Lacabaratz, C.; Bart, P.A.; Levy, Y.; et al. Exhaus-tion of bacteria-specific CD4 T cells and microbial translocation in common variable immunodeficiency disorders. J. Exp. Med. 2014, 211, 2033–2045. [Google Scholar] [CrossRef]
- Le Coz, C.; Bengsch, B.; Khanna, C.; Trofa, M.; Ohtani, T.; Nolan, B.E.; Henrickson, S.E.; Lambert, M.P.; Kim, T.O.; Despotovic, J.M.; et al. Common variable immunodeficiency-associated endotoxemia promotes early commitment to the T follicular lineage. J. Allergy Clin. Immunol. 2019, 144, 1660–1673. [Google Scholar] [CrossRef]
- Hultberg, J.; Ernerudh, J.; Larsson, M.; Nilsdotter-Augustinsson, Å.; Nyström, S. Plasma protein profiling reflects TH1-driven immune dysregulation in common variable immunodeficiency. J. Allergy Clin. Immunol. 2020, 146, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Stuchlý, J.; Kanderová, V.; Vlková, M.; Heřmanová, I.; Slámová, L.; Pelák, O.; Taraldsrud, E.; Jílek, D.; Králíc Ková, P.; Fevang, B.; et al. Common Variable Immunodeficiency patients with a phenotypic profile of immunosenescence present with thrombocytopenia. Sci. Rep. 2017, 7, 39710. [Google Scholar] [CrossRef] [PubMed]
- Berbers, R.M.; van der Wal, M.M.; van Montfrans, J.M.; Ellerbroek, P.M.; Dalm, V.A.S.H.; van Hagen, P.M.; Leavis, H.L.; van Wijk, F. Chronically Activated T-cells Retain Their Inflammatory Properties in Common Variable Immunodeficiency. J. Clin. Immunol. 2021, 41, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.; Goldacker, S.; Peter, H.H.; Warnatz, K. Preserved Cellular Immunity Upon Influenza Vaccination in Most Patients with Common Variable Immunodeficiency. J. Allergy Clin. Immunol. Pract. 2020, 8, 2332–2340.e5. [Google Scholar] [CrossRef] [PubMed]
- Berbers, R.M.; Drylewicz, J.; Ellerbroek, P.M.; van Montfrans, J.M.; Dalm, V.A.S.H.; van Hagen, P.M.; Keller, B.; Warnatz, K.; van de Ven, A.; van Laar, J.M.; et al. Targeted Proteomics Reveals Inflammatory Pathways that Classify Immune Dysreg-ulation in Common Variable Immunodeficiency. J. Clin. Immunol. 2021, 41, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Scott-Taylor, T.H.; Green, M.R.; Raeiszadeh, M.; Workman, S.; Webster, A.D. Defective maturation of dendritic cells in common variable immunodeficiency. Clin. Exp. Immunol. 2006, 145, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pomar, N.; Raga, S.; Ferrer, J.; Pons, J.; Munoz-Saa, I.; Julia, M.R.; de Gracia, J.; Matamoros, N. Elevated serum interleukin (IL)-12p40 levels in common variable immunodeficiency disease and decreased peripheral blood dendritic cells: Analysis of IL-12p40 and interferon-gamma gene. Clin. Exp. Immunol. 2006, 144, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Cambronero, R.; Sewell, W.A.; North, M.E.; Webster, A.D.; Farrant, J. Up-regulation of IL-12 in monocytes: A fundamental defect in common variable immunodeficiency. J. Immunol. 2000, 164, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Aspalter, R.M.; Sewell, W.A.; Dolman, K.; Farrant, J.; Webster, A.D. Deficiency in circulating natural killer (NK) cell subsets in common variable immunodeficiency and X-linked agammaglobulinaemia. Clin. Exp. Immunol. 2000, 121, 506–514. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C.; Radigan, L.; Knight, A.K.; Zhang, L.; Bauer, L.; Nakazawa, A. TLR9 activation is defective in common variable immune deficiency. J. Immunol. 2006, 176, 1978–1987. [Google Scholar] [CrossRef]
- Pasare, C.; Medzhitov, R. Control of B-cell responses by Toll-like receptors. Nature 2005, 438, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Heer, A.K.; Shamshiev, A.; Donda, A.; Uematsu, S.; Akira, S.; Kopf, M.; Marsland, B.J. TLR Signaling Fine-Tunes Anti-Influenza B Cell Responses without Regulating Effector T Cell Responses. J. Immunol. 2007, 178, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Lanzavecchia, A.; Bernasconi, N.; Traggiai, E.; Ruprecht, C.R.; Corti, D.; Sallusto, F. Understanding and making use of human memory B cells. Immun. Rev. 2006, 211, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ruprecht, C.R.; Lanzavecchia, A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur. J. Immunol. 2006, 36, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.; Wilson, H.; Jimbo, S.; Mutwiri, G. Modulation of B cell responses by Toll-like receptors. Cell Tissue Res. 2011, 343, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, N.L.; Onai, N.; Lanzavecchia, A. A role for Toll-like receptors in acquired immunity: Up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 2003, 101, 4500–4504. [Google Scholar] [CrossRef] [PubMed]
- Bourke, E.; Bosisio, D.; Golay, J.; Polentarutti, N.; Mantovani, A. The toll-like receptor repertoire of human B lymphocytes: Inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 2003, 102, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Knight, A.K.; Radigan, L.; Marron, T.U.; Zhang, L.; Sanchez-Ramón, S.; Cunningham-Rundles, C. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. J. Allergy Clin. Immunol. 2009, 124, 349–356. [Google Scholar] [CrossRef]
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert. Rev. Vaccines 2013, 12, 1085–1094. [Google Scholar] [CrossRef]
- Mondiale de la Santé Organisation; World Health Organization. Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season. Wkly. Epidemiol. Rec. = Relev. Épidémiologique Hebd. 2018, 93, 133–141. [Google Scholar]
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccin. Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef]
- Caini, S.; Huang, Q.S.; Ciblak, M.A.; Kusznierz, G.; Owen, R.; Wangchuk, S.; Henriques, C.M.P.; Njouom, R.; Fasce, R.A.; Yu, H.; et al. Epidemiological and virological characteristics of influenza B: Results of the Global Influenza B Study. Influenza Other Respir. Viruses 2015, 9, 3–12. [Google Scholar] [CrossRef]
- Kharit, S.M.; Rudakova, A.M.; Uskov, A.N.; Konovalova, L.N.; Lobzin, Y.V. The averted costs due to influenza vaccination with trivalent and quadrivalent vaccines. J. Infectology 2017, 9, 17–22. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science 2002, 298, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Eichner, M.; Schwehm, M.; Hain, J.; Uphoff, H.; Salzberger, B.; Knuf, M.; Schmidt-Ott, R. 4Flu—An individual based simulation tool to study the effects of quadrivalent vaccination on seasonal influenza in Germany. BMC Infect. Dis. 2014, 14, 365–385. [Google Scholar] [CrossRef]
- Crépey, P.; Boer, P.T.; Postma, M.J.; Pitman, R. Retrospective public health impact of a quadrivalent influenza vaccine in the United States. Influenza Other Respir. Viruses 2015, 9, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Moa, A.M.; Chughtai, A.A.; Muscatello, D.J.; Turner, R.M.; MacIntyre, C.R. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials. Vaccine 2016, 34, 4092–4102. [Google Scholar] [CrossRef]
- Guidance. Summary of Data to Support the Choice of Influenza Vaccination for Adults in Primary Care. Public Health England. Published 29 January 2018. Available online: https://www.gov.uk/government/publications/flu-vaccination-supporting-data-for-adult-vaccines/summary-of-data-to-support-the-choice-of-influenza-vaccination-for-adults-in-primary-care (accessed on 1 June 2024).
- Crampton, S.P.; Voynova, E.; Bolland, S. Innate pathways to B-cell activation and tolerance. Ann. N. Y. Acad. Sci. 2010, 1183, 58–68. [Google Scholar] [CrossRef]
- Khaitov, R.M. Results and prospects of research on the development of synthetic vaccines. Immunology 1982, 6, 35–40. (In Russian) [Google Scholar]
- Vaccination of Pregnant Women against Influenza. Federal Clinical Recommendations. Moscow. 2015. 41p. Available online: https://minzdrav.gov.ru/ru (accessed on 24 February 2015). (In Russian)
- Kostinov, M.P.; Cherdantsev, A.P.; Semenova, S.S.; Tarbaeva, D.A.; Savisko, A.A.; Serova, O.F.; Iozefson, S.A.; Akhmatova, N.K.; Kosti-nova, T.A.; Praulova, D.A. Obstetric and perinatal outcomes among pregnant women after influenza vaccination and after transferred respiratory infection. Gynecology 2015, 17, 43–46. (In Russian) [Google Scholar] [CrossRef]
- Cherdantsev, A.P.; Kuselman, A.I.; Sinitsyna, M.N.; Shalyagina, M.E.; Kostinov, M.P.; Tarbaeva, D.A. Study of the clinical safety of influenza vaccination in pregnant women. Med. Alman 2011, 4, 120–122. (In Russian) [Google Scholar]
- Kostinov, M.P.; Cherdantsev, A.P.; Savisko, A.A.; Tarbaeva, D.A.; Solov’yova, I.L. True and false reactions to influenza vaccine administration in pregnant women. Probl. Gynecol. Obstet. Perinatol. 2011, 10, 44–48. (In Russian) [Google Scholar]
- Kostinov, M.P.; Cherdantsev, A.P. Health state of children born from pregnant women vaccinated against influenza. Pediatr.-Zhurnal Im G.N. Speranskogo. 2016, 95, 67–71. (In Russian) [Google Scholar]
- Koller, M. Robustlmm: An R Package for Robust Estimation of Linear Mixed-Effects Models. J. Stat. Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- Luke, S.G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 2017, 49, 1494–1502. [Google Scholar] [CrossRef]
- Lenth Russell, V. Package ‘Emmeans’: Estimated Marginal Means, Aka Least Squares Means, Version 1.6.1; CRAN: Vienna, Austria, 2021.
- Topham, D.J.; Tripp, R.A.; Hamilton-Easton, A.M.; Sarawar, S.R.; Doherty, P.C. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J. Immunol. 1996, 157, 2947–2952. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Verhoeven, D.; Page, C.A.; Turner, D.; Farber, D.L. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J. Virol. 2010, 84, 9217–9226. [Google Scholar] [CrossRef]
- Zhong, W.; Roberts, A.D.; Woodland, D.L. Antibody-independent antiviral function of memory CD4+ T cells in vivo requires regulatory signals from CD8+ effector T cells. J. Immunol. 2001, 167, 1379–1386. [Google Scholar] [CrossRef]
- Paroli, M.; Accapezzato, D.; Francavilla, V.; Insalaco, A.; Plebani, A.; Balsano, F.; Barnaba, V. Long-lasting memory-resting and memory-effector CD4+ T cells in human X-linked agammaglobulinemia. Blood 2002, 99, 2131–2137. [Google Scholar] [CrossRef]
- van Assen, S.; de Haan, A.; Holvast, A.; Horst, G.; Gorter, L.; Westra, J.; Kallenberg, C.G.; Telgt, D.S.; Palache, A.M.; Giezeman, K.M.; et al. Cell-mediated immune responses to inactivated trivalent influenza-vaccination are decreased in patients with common variable immunodeficiency. Clin. Immunol. 2011, 141, 161–168. [Google Scholar] [CrossRef]
- Bowyer, G.; Rampling, T.; Powlson, J.; Morter, R.; Wright, D.; Hill, A.V.S.; Ewer, K.J. Activation-induced Markers Detect Vaccine-Specific CD4+ T Cell Responses Not Measured by Assays Conventionally Used in Clinical Trials. Vaccines 2018, 6, 50. [Google Scholar] [CrossRef]
- Nowak, E.; Sulicka-Grodzicka, J.; Strach, M.; Bukowska-Strakova, K.; Siedlar, M.; Korkosz, A.; Grodzicki, T. Decreased number of regulatory T lymphocytes is related to inflammation and number of CD8+ T cells expressing programmed cell death protein-1 in common variable immunodeficiency. Folia Med. Cracov. 2020, 60, 5–16. [Google Scholar] [CrossRef]
- Engel, A.L.; Holt, E.G.; Lu, H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert. Rev. Clin. Pharmacol. 2011, 4, 275–289. [Google Scholar] [CrossRef]
- Schulz, O.; Diebold, S.S.; Chen, M.; Näslund, T.I.; Nolte, M.A.; Alexopoulou, L.; Azuma, Y.-T.; Flavell, R.A.; Liljestrom, P.; Reis ESousa, C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Azuma, M.; Ebihara, T.; Oshiumi, H.; Matsumoto, M.; Seya, T. Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c+/CD8α+dendritic cells. OncoImmunology 2012, 1, 581–592. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ohishi, H.M.M.; Aki, D.; Hanada, T. Regulation of TLR signaling and inflammation by SOCS family proteins. J. Leukoc. Biol. 2004, 75, 422–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hyang, L.T.; Paredes, C.J.; Papoutsakis, E.T.; Miller, W.M. Gene expression analysis illuminates the transcriptional programs underlying the functional activity of ex vivo-expanded granulocytes. Physiol. Genom. 2007, 31, 114–125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Kumagai, Y.; Takeuchi, O.; Kato, H.; Kumar, H.; Matsui, K.; Morii, E.; Aozasa, K.; Kawai, T.; Akira, S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity 2007, 27, 240–252. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Khromova, E.A.; Akhmatova, E.A.; Skhodova, S.A.; Semochkin, I.A.; Khomenkov, V.G.; Akhmatova, N.K.; Kostinov, M.P. Effect of influenza vaccines on subpopulations of blood dendritic cells. J. Microbiol. Epidemiol. Immunobiol. 2016, 5, 23–28. [Google Scholar] [CrossRef]
- Kompier, R.; Neels, P.; Beyer, W.; Hardman, T.; Lioznov, D.; Kharit, S.; Kostinov, M. Analysis of the safety and immunogenicity profile of an azoximer bromide polymer-adjuvanted subunit influenza vaccine. F1000Research 2022, 11, 259. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostinova, A.M.; Latysheva, E.A.; Kostinov, M.P.; Akhmatova, N.K.; Skhodova, S.A.; Vlasenko, A.E.; Cherdantsev, A.P.; Soloveva, I.L.; Khrapunova, I.A.; Loktionova, M.N.; et al. Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency. Vaccines 2024, 12, 843. https://doi.org/10.3390/vaccines12080843
Kostinova AM, Latysheva EA, Kostinov MP, Akhmatova NK, Skhodova SA, Vlasenko AE, Cherdantsev AP, Soloveva IL, Khrapunova IA, Loktionova MN, et al. Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency. Vaccines. 2024; 12(8):843. https://doi.org/10.3390/vaccines12080843
Chicago/Turabian StyleKostinova, Aristitsa Mikhailovna, Elena Alexandrovna Latysheva, Mikhail Petrovich Kostinov, Nelly Kimovna Akhmatova, Svetlana Anatolyevna Skhodova, Anna Egorovna Vlasenko, Alexander Petrovich Cherdantsev, Irina Leonidovna Soloveva, Isabella Abramovna Khrapunova, Marina Nikolaevna Loktionova, and et al. 2024. "Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency" Vaccines 12, no. 8: 843. https://doi.org/10.3390/vaccines12080843
APA StyleKostinova, A. M., Latysheva, E. A., Kostinov, M. P., Akhmatova, N. K., Skhodova, S. A., Vlasenko, A. E., Cherdantsev, A. P., Soloveva, I. L., Khrapunova, I. A., Loktionova, M. N., Khromova, E. A., & Poddubikov, A. A. (2024). Comparison of Post-Vaccination Cellular Immune Response in Patients with Common Variable Immune Deficiency. Vaccines, 12(8), 843. https://doi.org/10.3390/vaccines12080843





