Plasma EV-miRNAs as Potential Biomarkers of COVID-19 Vaccine Immune Response in Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Storage
2.2. Quantification of Anti-SARS-CoV-2 IgG Antibody Levels in Immunoessay ELISA
2.3. Plasma Purification and Extracellular Vesicle Isolation
2.4. Extracellular Vesicle NTA Analysis
2.5. Transmission Electron Microscopy Characterization of EVs
2.6. EV-CD63 Quantification
2.7. RNA Extraction and cDNA Synthesis
2.8. Quantification of the Selected EV-hsa-miRNAs Profile by Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Data Analysis
3. Results
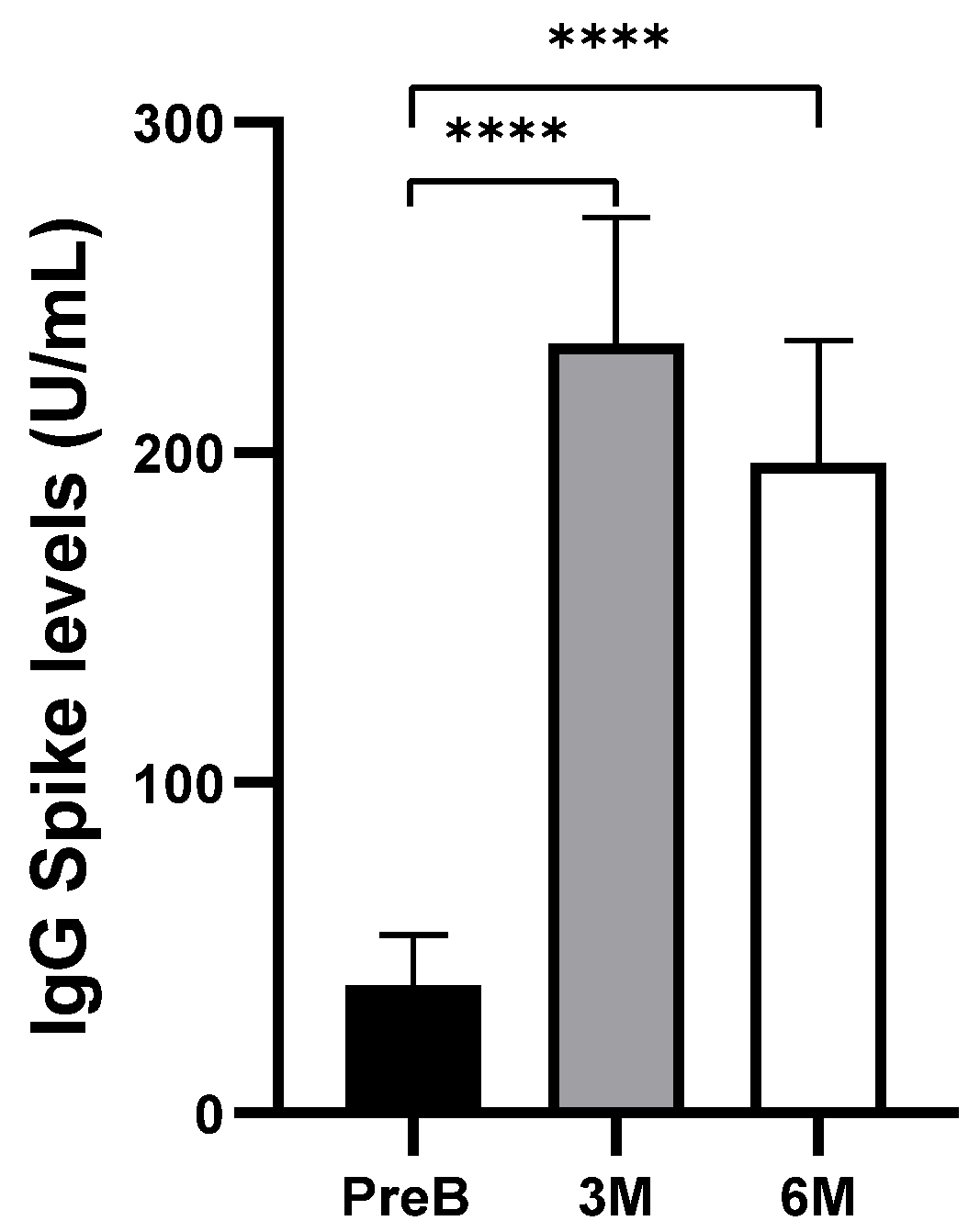
3.1. Humoral Response to COVID-19 Vaccination
3.2. Extracellular Vesicle Characterization
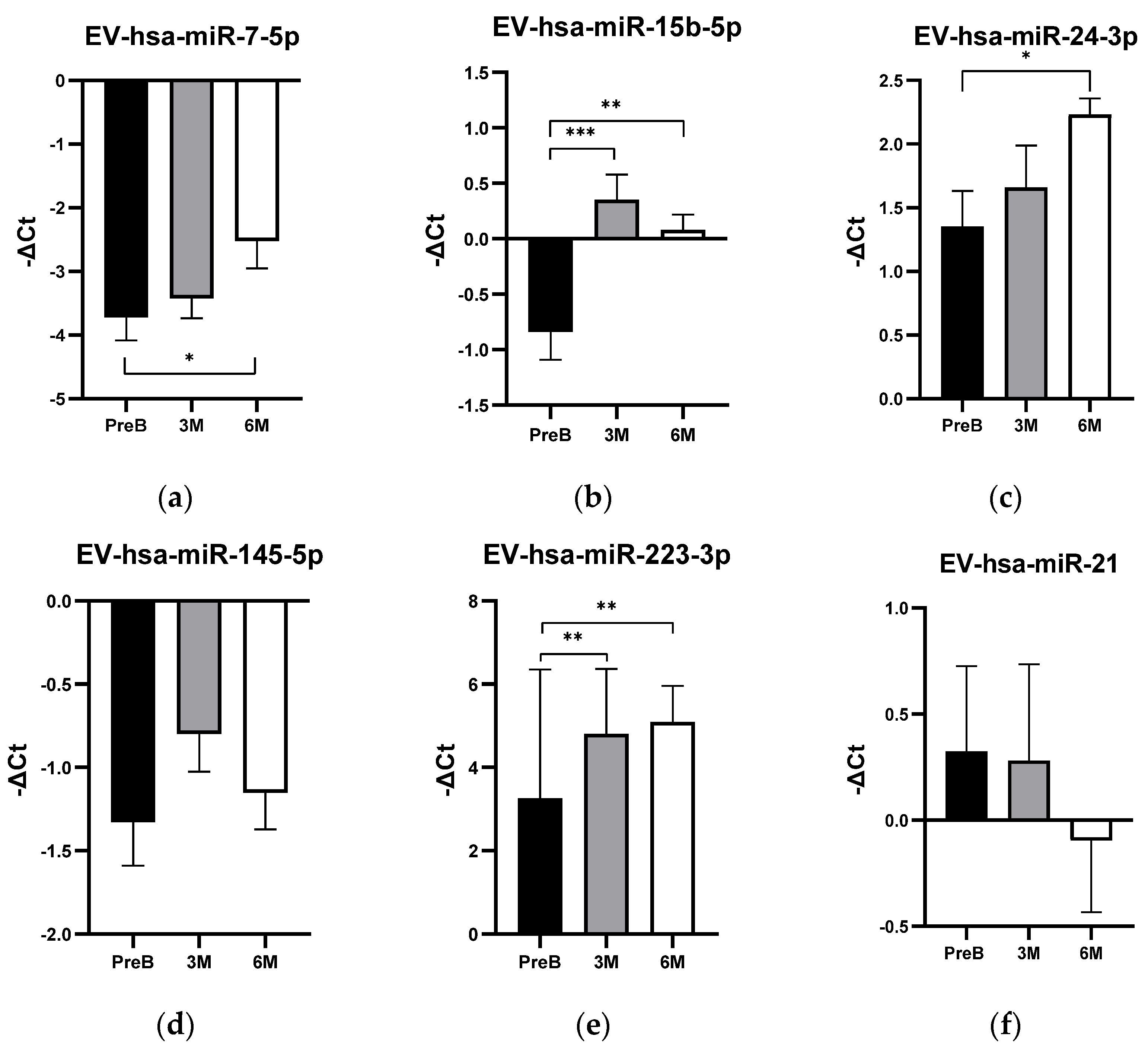
3.3. EV-hsa-miRNA Profile Levels in Cancer Patients’ Plasma Samples and Their Relationship with Humoral Response
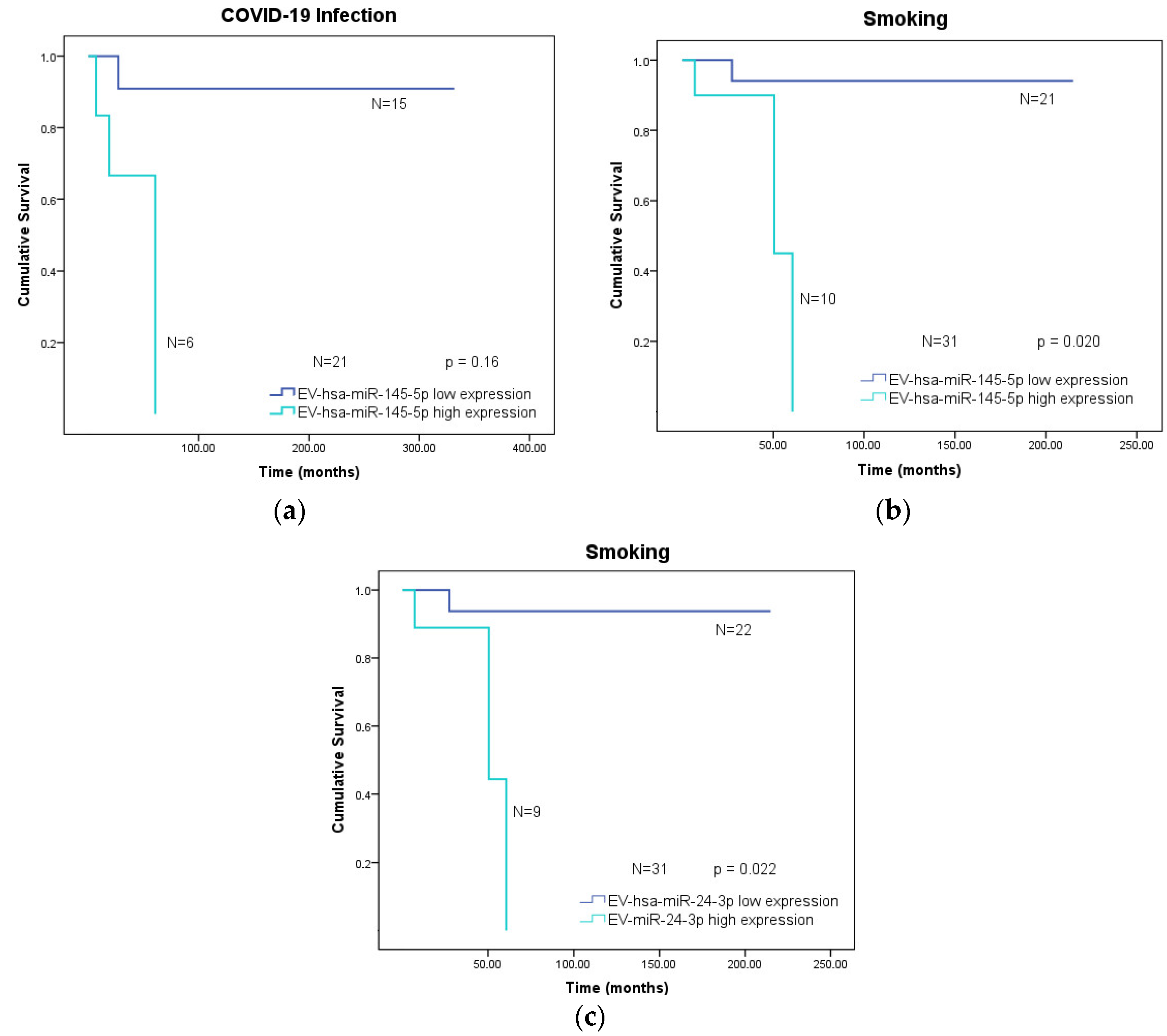
3.4. Overall Survival of Cancer Patients According to Clinical Variables and Low or High Expression of the EV-hsa-miRNA Profile
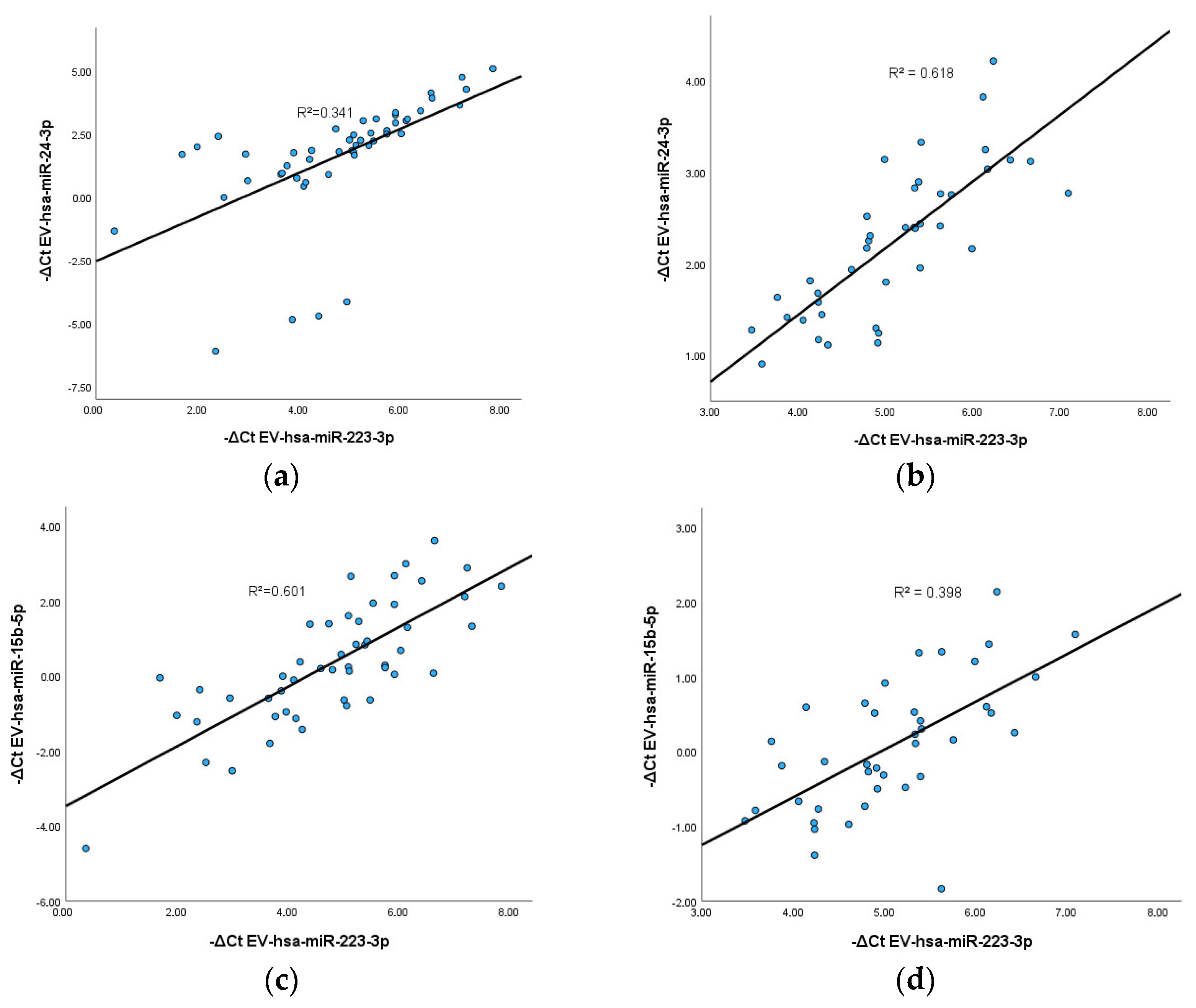
3.5. Correlation between EV-hsa-miRNAs
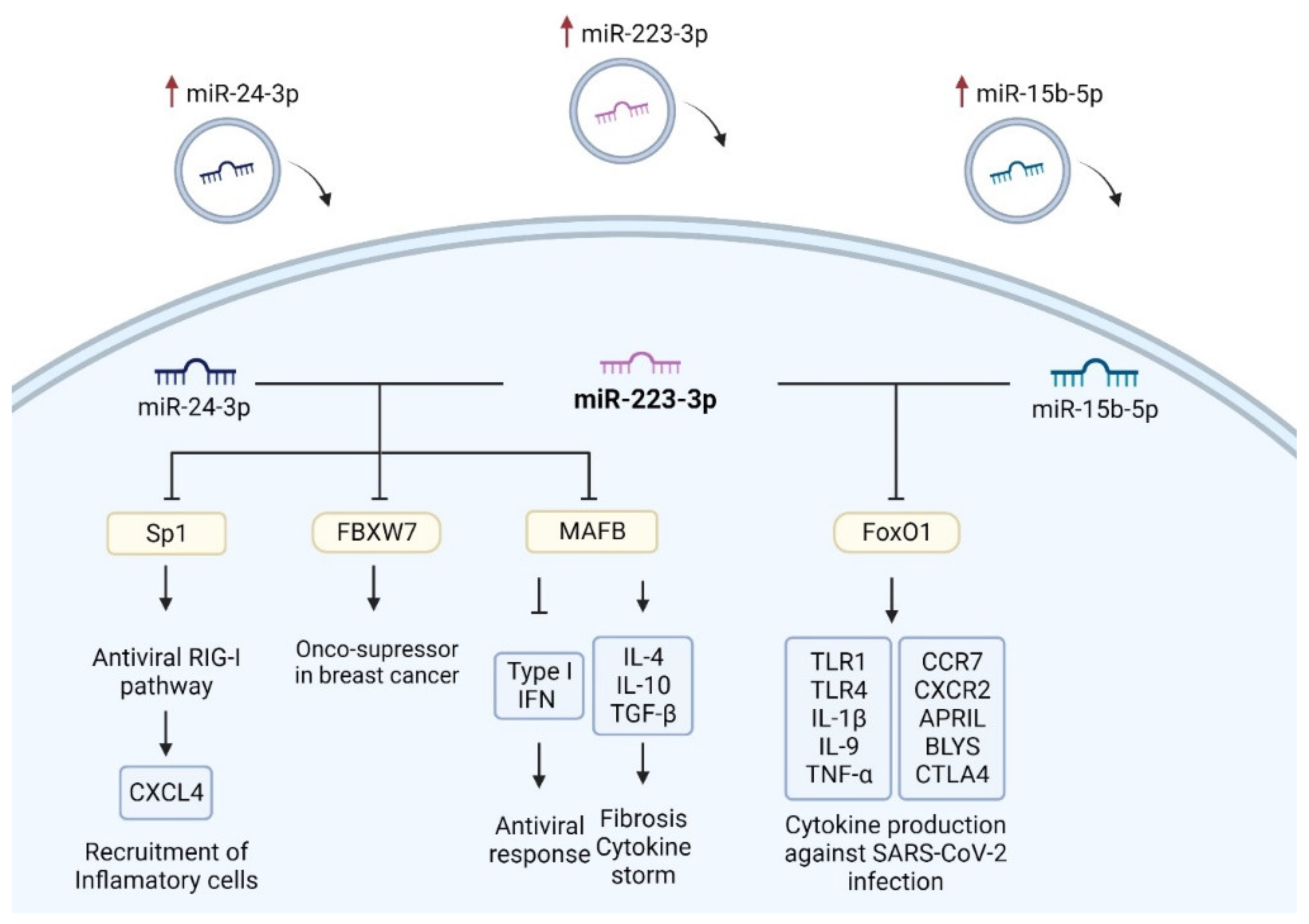
3.6. Common Targets of EV-hsa-miR-223-3p with EV-hsa-miR-24-3p and with EV-hsa-miR-15b-5p
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, R.; Gautam, R.K.; Chopra, H.; Dubey, A.K.; Singla, R.K.; Rayan, R.A.; Kamal, M.A. Comparative highlights on MERS-CoV, SARS-CoV-1, SARS-CoV-2, and NEO-CoV. Excli J. 2022, 21, 1245–1272. [Google Scholar] [CrossRef]
- Rahimi, A.; Mirzazadeh, A.; Tavakolpour, S. Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics 2021, 113, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, J.; Chen, S.; Wang, L.; Yau, T.O.; Zhao, Q.; Hong, Z.; Ruan, J.; Duan, G.; Gao, S. Genomic Feature Analysis of Betacoronavirus Provides Insights Into SARS and COVID-19 Pandemics. Front. Microbiol. 2021, 12, 614494. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Yang, H.; Zhang, M.; Liu, Q.; Alotaibi, G.; Irfan, M.; He, H.; Chang, J.; Liang, X.J.; Weng, Y.; et al. mRNA vaccines for COVID-19 and diverse diseases. J. Control. Release 2022, 345, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Szabó, G.T.; Mahiny, A.J.; Vlatkovic, I. COVID-19 mRNA vaccines: Platforms and current developments. Mol. Ther. 2022, 30, 1850–1868. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 71–110. [Google Scholar] [CrossRef] [PubMed]
- Hajiaghapour Asr, M.; Dayani, F.; Saedi Segherloo, F.; Kamedi, A.; Neill, A.O.; MacLoughlin, R.; Doroudian, M. Lipid Nanoparticles as Promising Carriers for mRNA Vaccines for Viral Lung Infections. Pharmaceutics 2023, 15, 1127. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12, 2202688. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Lee, Y.; Park, J.; Jung, H.; Lee, H. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv. Drug Deliv. Rev. 2023, 200, 114990. [Google Scholar] [CrossRef] [PubMed]
- Echaide, M.; Chocarro de Erauso, L.; Bocanegra, A.; Blanco, E.; Kochan, G.; Escors, D. mRNA Vaccines against SARS-CoV-2: Advantages and Caveats. Int. J. Mol. Sci. 2023, 24, 5944. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castaño, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e1015. [Google Scholar] [CrossRef]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e1285. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Song, N.J.; Chakravarthy, K.B.; Jeon, H.; Bolyard, C.; Reynolds, K.; Weller, K.P.; Reisinger, S.; Wang, Y.; Li, A.; Jiang, S.; et al. mRNA vaccines against SARS-CoV-2 induce divergent antigen-specific T-cell responses in patients with lung cancer. J. Immunother. Cancer 2024, 12, e007922. [Google Scholar] [CrossRef] [PubMed]
- Rouf, N.Z.; Biswas, S.; Tarannum, N.; Oishee, L.M.; Muna, M.M. Demystifying mRNA vaccines: An emerging platform at the forefront of cryptic diseases. RNA Biol. 2022, 19, 386–410. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; de Vries, E.G.E.; GeurtsvanKessel, C.H.; Haanen, J.B.; Wörmann, B.; Turajlic, S.; von Lilienfeld-Toal, M. COVID-19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat. Rev. Clin. Oncol. 2022, 19, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, Y.; Yoshida, T.; Takashima, A.; Yonemori, K.; Ohba, A.; Yazaki, S.; Yagishita, S.; Nakahama, H.; Kobayashi, O.; Yanagida, M.; et al. Immunogenicity after vaccination of COVID-19 vaccines in patients with cancer: A prospective, single center, observational study. Int. J. Clin. Oncol. 2024, 29, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, L.; Citterio, C.; Toscani, I. COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis. Vaccines 2021, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Singh, P.; Samaddar, A.; Singh, D.; Verma, M.; Rakesh, A.; Ranjan, R. Vaccination of cancer patients against COVID-19: Towards the end of a dilemma. Med. Oncol. 2021, 38, 92. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Bednash, J.S.; Horowitz, J.C.; Rubinstein, M.P.; Vlasova, A.N. Brief research report: Impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune Responses to COVID-19 mRNA Vaccines in Patients with Solid Tumors on Active, Immunosuppressive Cancer Therapy. medRxiv 2021. [Google Scholar] [CrossRef]
- Oshiumi, H. Circulating Extracellular Vesicles Carry Immune Regulatory miRNAs and Regulate Vaccine Efficacy and Local Inflammatory Response After Vaccination. Front. Immunol. 2021, 12, 685344. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-De Prada, L.; García-Concejo, A.; Tamayo-Velasco, Á.; Martín-Fernández, M.; Gonzalo-Benito, H.; Gorgojo-Galindo, Ó.; Montero-Jodra, A.; Peláez, M.T.; Martínez Almeida, I.; Bardají-Carrillo, M.; et al. miRNome profiling of extracellular vesicles in severe COVID-19 patients and identification of predictors of mortality. J. Infect. Dis. 2024, jiae310. [Google Scholar] [CrossRef] [PubMed]
- Takaaki, T.; Yusuke, Y.; Shinichi, S.; Tomohiko, I.; Takahiro, O. Extracellular vesicles as a promising biomarker resource in liquid biopsy for cancer. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 148–174. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, X.; Jiang, X.-M.; Guo, J.; Fu, Z.; Zhou, Z.; Yang, P.; Guo, H.; Guo, X.; Liang, G.; et al. Decreased inhibition of exosomal miRNAs on SARS-CoV-2 replication underlies poor outcomes in elderly people and diabetic patients. Signal Transduct. Target. Ther. 2021, 6, 300. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.R.; Dias, F.; Teixeira, A.L.; Sousa, H.; Oliveira, J.; Medeiros, R. MicroRNAs as Potential Tools for Predicting Cancer Patients’ Susceptibility to SARS-CoV-2 Infection and Vaccination Response. Cells 2022, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Blixt, L.; Wullimann, D.; Aleman, S.; Lundin, J.; Chen, P.; Gao, Y.; Cuapio, A.; Akber, M.; Lange, J.; Rivera-Ballesteros, O.; et al. T-cell immune responses following vaccination with mRNA BNT162b2 against SARS-CoV-2 in patients with chronic lymphocytic leukemia: Results from a prospective open-label clinical trial. Haematologica 2022, 107, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Pradhan, K.; Jindal, S.; Cui, Z.; Rockwell, B.; Shah, A.P.; Packer, S.; Sica, R.A.; Sparano, J.; Goldstein, D.Y.; et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat. Cancer 2021, 2, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090.e1082. [Google Scholar] [CrossRef]
- Janzic, U.; Bidovec-Stojkovic, U.; Mohorcic, K.; Mrak, L.; Dovnik, N.F.; Ivanovic, M.; Ravnik, M.; Caks, M.; Skof, E.; Debeljak, J.; et al. Solid cancer patients achieve adequate immunogenicity and low rate of severe adverse events after SARS-CoV-2 vaccination. Future Oncol. 2022, 18, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, Y.; Lim, C.-W.; Park, J.-M.; Yu, S.-H.; Kim, Y.; Han, H.J.; Kim, C.-H.; Song, Y.-S.; Kim, C.; et al. Antiviral effects of miRNAs in extracellular vesicles against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and mutations in SARS-CoV-2 RNA virus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mishra, R.; Banerjea, A.C. SARS-CoV-2 Spike Targets USP33-IRF9 Axis via Exosomal miR-148a to Activate Human Microglia. Front. Immunol. 2021, 12, 656700. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Guiot, J.; Henket, M.; Remacle, C.; Cambier, M.; Struman, I.; Winandy, M.; Moermans, C.; Louis, E.; Malaise, M.; Ribbens, C.; et al. Systematic review of overlapping microRNA patterns in COVID-19 and idiopathic pulmonary fibrosis. Respir. Res. 2023, 24, 112. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Yang, L.; Guo, J.; Hu, H.; Li, F.; Liu, Y.; Pang, Y. miR-145-5p is associated with smoke-related chronic obstructive pulmonary disease via targeting KLF5. Chem. Biol. Interact. 2019, 300, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nouws, J.; Wan, F.; Finnemore, E.; Roque, W.; Kim, S.J.; Bazan, I.; Li, C.X.; Skold, C.M.; Dai, Q.; Yan, X.; et al. MicroRNA miR-24-3p reduces DNA damage responses, apoptosis, and susceptibility to chronic obstructive pulmonary disease. JCI Insight 2021, 6, e134218. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Q.; Zhang, W.; Sun, H.; Kuang, Z.; Zhang, G.; Huang, Z. Changes and Clinical Value of Serum miR-24 and miR-223 Levels in Patients with Severe Pneumonia. Int. J. Gen. Med. 2023, 16, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M.P.; Boudewijn, I.M.; van Nijnatten, J.L.L.; Faiz, A.; Vermeulen, C.J.; van Oosterhout, A.J.; Affleck, K.; Timens, W.; Bracke, K.R.; Maes, T.; et al. Identification of asthma-associated microRNAs in bronchial biopsies. Eur. Respir. J. 2022, 59, 2101294. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Shirai, T.; Shimoshikiryo, T.; Ueda, M.; Gon, Y.; Maruoka, S.; Itoh, K. Circulating microRNA-15b-5p as a biomarker for asthma-COPD overlap. Allergy 2021, 76, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Roffel, M.; Boudewijn, I.; Guryev, V.; Brandsma, C.-A.; Berge, M.V.D.; Bracke, K.; Maes, T.; Heijink, I. Unraveling the role of miR-223-3p in the regulation of airway inflammation in asthma. Eur. Respir. J. 2018, 52, PA4998. [Google Scholar] [CrossRef]
- Roffel, M.P.; Bracke, K.R.; Heijink, I.H.; Maes, T. miR-223: A Key Regulator in the Innate Immune Response in Asthma and COPD. Front. Med. 2020, 7, 196. [Google Scholar] [CrossRef]
- Yuan, S.; Wu, Q.; Wang, Z.; Che, Y.; Zheng, S.; Chen, Y.; Zhong, X.; Shi, F. miR-223: An Immune Regulator in Infectious Disorders. Front. Immunol. 2021, 12, 781815. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Wang, X.P.; Luoreng, Z.M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.W. miR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int. J. Biol. Sci. 2021, 17, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Oliveros, J.C.; Enjuanes, L.; Sola, I. Contribution of Host miRNA-223-3p to SARS-CoV-Induced Lung Inflammatory Pathology. mBio 2022, 13, e0313521. [Google Scholar] [CrossRef] [PubMed]
- Houshmandfar, S.; Saeedi-Boroujeni, A.; Rashno, M.; Khodadadi, A.; Mahmoudian-Sani, M.R. miRNA-223 as a regulator of inflammation and NLRP3 inflammasome, the main fragments in the puzzle of immunopathogenesis of different inflammatory diseases and COVID-19. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.A.; Simón-Fuentes, M.; González de la Aleja, A.; Nieto, C.; Colmenares, M.; Herrero, C.; Domínguez-Soto, Á.; Corbí, Á.L. MAFB and MAF Transcription Factors as Macrophage Checkpoints for COVID-19 Severity. Front. Immunol. 2020, 11, 603507. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. The transcription factor MafB promotes anti-inflammatory M2 polarization and cholesterol efflux in macrophages. Sci. Rep. 2017, 7, 7591. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Maurin, V.; Brinza, L.; Baguet, J.; Plantamura, E.; Schicklin, S.; Chambion, S.; Macari, C.; Tomkowiak, M.; Deniaud, E.; Leverrier, Y.; et al. Overexpression of the transcription factor Sp1 activates the OAS-RNAse L-RIG-I pathway. PLoS ONE 2015, 10, e0118551. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, H. FOXO1: A potential target for human diseases. Curr. Drug Targets 2011, 12, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Ortega, A.A.; Feinberg, D.; Liang, Y.; Rossa, C., Jr.; Graves, D.T. The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells. Crit. Rev. Immunol. 2017, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheema, P.S.; Nandi, D.; Nag, A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021, 11, 210069. [Google Scholar] [CrossRef] [PubMed]


| Cohort Population | ||
|---|---|---|
| Number of patients | n | % |
| Timepoint 1 (pre-boost) | 54 | 100 |
| Timepoint 2 (3 months post-boost) | 50 | 92.59 |
| Timepoint 3 (6 months post-boost) | 40 | 74.07 |
| Mortality | ||
| Yes | 9 | 16.67 |
| No | 45 | 83.33 |
| Tumor types | ||
| Breast | 16 | 29.64 |
| Lymphoma | 8 | 14.82 |
| Lung | 7 | 12.96 |
| Head and Neck | 6 | 11.11 |
| Rectal | 6 | 11.11 |
| Colon | 4 | 7.41 |
| Myeloma | 2 | 3.70 |
| Prostate | 2 | 3.70 |
| Bladder | 1 | 1.85 |
| Melanoma | 1 | 1.85 |
| Gastric | 1 | 1.85 |
| Type of therapy | ||
| Chemotherapy | 35 | 47.30 |
| Targeted | 12 | 16.22 |
| Others | 27 | 36.48 |
| Sex | ||
| Female | 25 | 46.30 |
| Male | 29 | 53.70 |
| Age (Mean ± SD) | 58.85 ± 10.82 | |
| Boost vaccination brand | ||
| Pfizer-BioNTech | 33 | 61.1 |
| Moderna | 16 | 29.63 |
| Unknown | 5 | 9.26 |
| Positive Anti-SARS-CoV-2 Spike IgG Levels | Negative Anti-SARS-CoV-2 Spike IgG Levels | |||
|---|---|---|---|---|
| Solid tumors | Hematologic tumors | Solid tumors | Hematologic tumors | |
| PreB (N = 54) | 41 | 2 | 3 | 8 |
| 3 M (N = 50) | 41 | 2 | 0 | 7 |
| 6 M (N = 40) | 36 | 1 | 0 | 4 |
| EV-hsa-miR-7-5p | EV-hsa-miR-15b-5p | EV-hsa-miR-24-3p | EV-hsa-miR-145-5p | EV-hsa-miR-223-3p | |
|---|---|---|---|---|---|
| Spearman rho | 0.085 | −0.006 | 0.123 | −0.075 | 0.088 |
| p-value | 0.624 | 0.970 | 0.394 | 0.607 | 0.545 |
| N | 36 | 50 | 50 | 50 | 50 |
| EV-hsa-miR-7-5p | EV-hsa-miR-15b-5p | EV-hsa-miR-24-3p | EV-hsa-miR-145-5p | EV-hsa-miR-223-3p | |
|---|---|---|---|---|---|
| Spearman rho | 0.009 | 0.059 | 0.024 | −0.144 | 0.151 |
| p-value | 0.965 | 0.717 | 0.881 | 0.376 | 0.351 |
| N | 25 | 40 | 40 | 40 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, B.; Dias, T.R.; Cruz, P.; Sousa-Pimenta, M.; Teixeira, A.L.; Pereira, C.E.; Costa-Silva, B.; Oliveira, J.; Medeiros, R.; Dias, F. Plasma EV-miRNAs as Potential Biomarkers of COVID-19 Vaccine Immune Response in Cancer Patients. Vaccines 2024, 12, 848. https://doi.org/10.3390/vaccines12080848
Almeida B, Dias TR, Cruz P, Sousa-Pimenta M, Teixeira AL, Pereira CE, Costa-Silva B, Oliveira J, Medeiros R, Dias F. Plasma EV-miRNAs as Potential Biomarkers of COVID-19 Vaccine Immune Response in Cancer Patients. Vaccines. 2024; 12(8):848. https://doi.org/10.3390/vaccines12080848
Chicago/Turabian StyleAlmeida, Beatriz, Tânia R. Dias, Pedro Cruz, Mário Sousa-Pimenta, Ana Luísa Teixeira, Catarina Esteves Pereira, Bruno Costa-Silva, Júlio Oliveira, Rui Medeiros, and Francisca Dias. 2024. "Plasma EV-miRNAs as Potential Biomarkers of COVID-19 Vaccine Immune Response in Cancer Patients" Vaccines 12, no. 8: 848. https://doi.org/10.3390/vaccines12080848
APA StyleAlmeida, B., Dias, T. R., Cruz, P., Sousa-Pimenta, M., Teixeira, A. L., Pereira, C. E., Costa-Silva, B., Oliveira, J., Medeiros, R., & Dias, F. (2024). Plasma EV-miRNAs as Potential Biomarkers of COVID-19 Vaccine Immune Response in Cancer Patients. Vaccines, 12(8), 848. https://doi.org/10.3390/vaccines12080848










