Anti-SARS-CoV-2 Antibodies versus Vaccination Status in CAD Patients with COVID-19: A Prospective, Propensity Score-Matched Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Variables
2.3. Data Sources and Measurements
2.4. Ethical Approval
2.5. Statistical Methods
3. Results
3.1. Participants
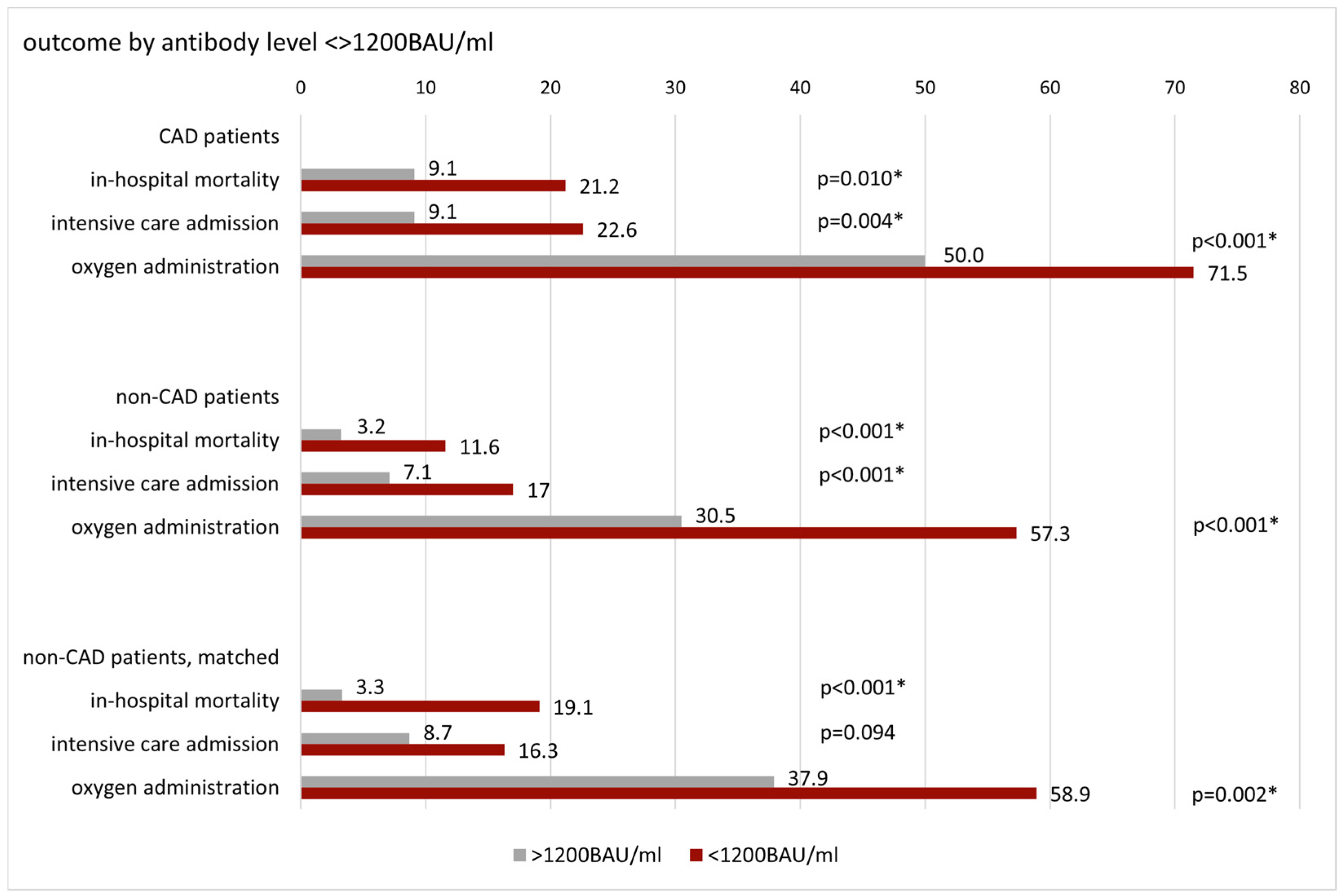
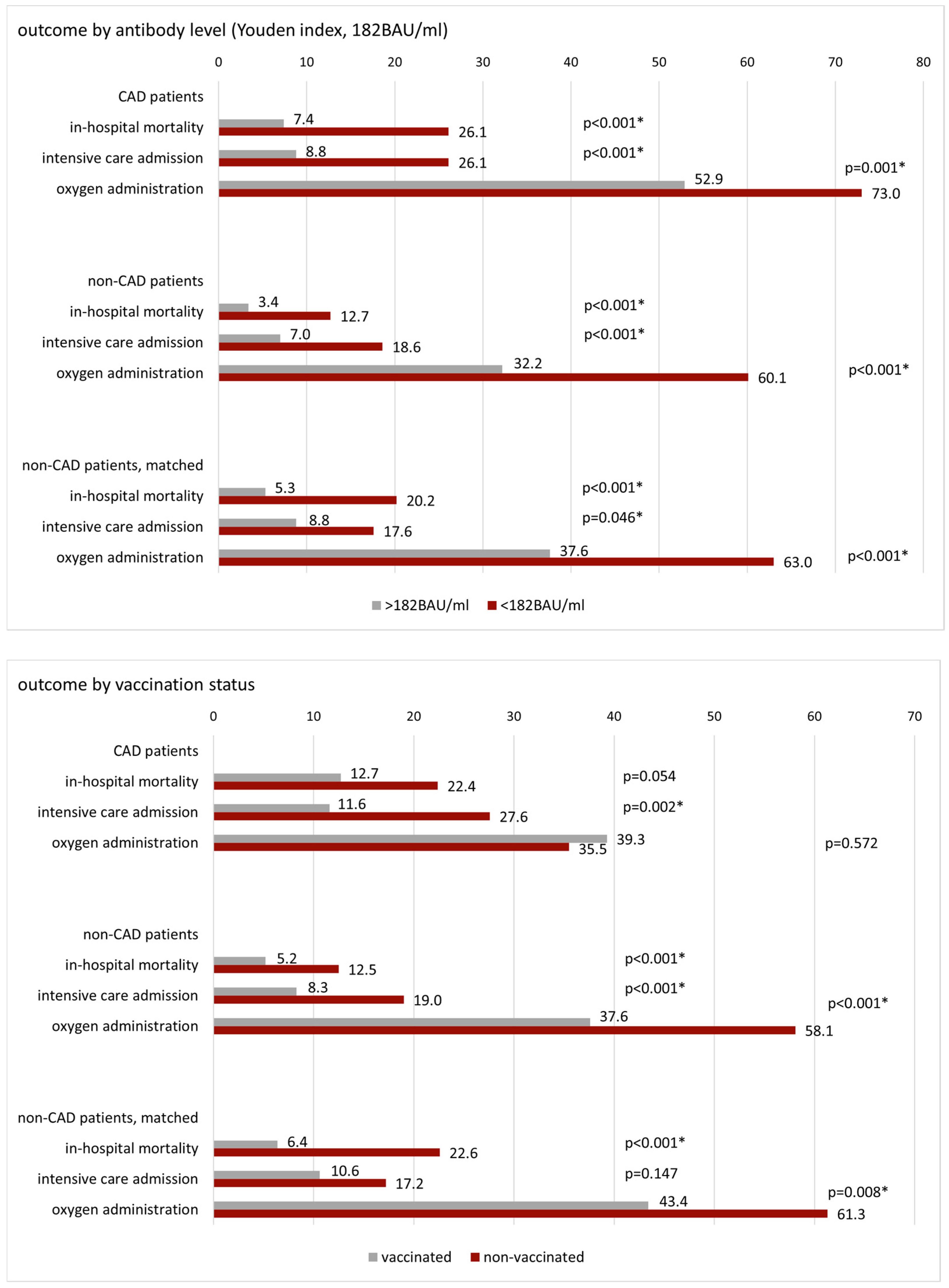
3.2. Patient Outcome by Antibody Level and Vaccination Status
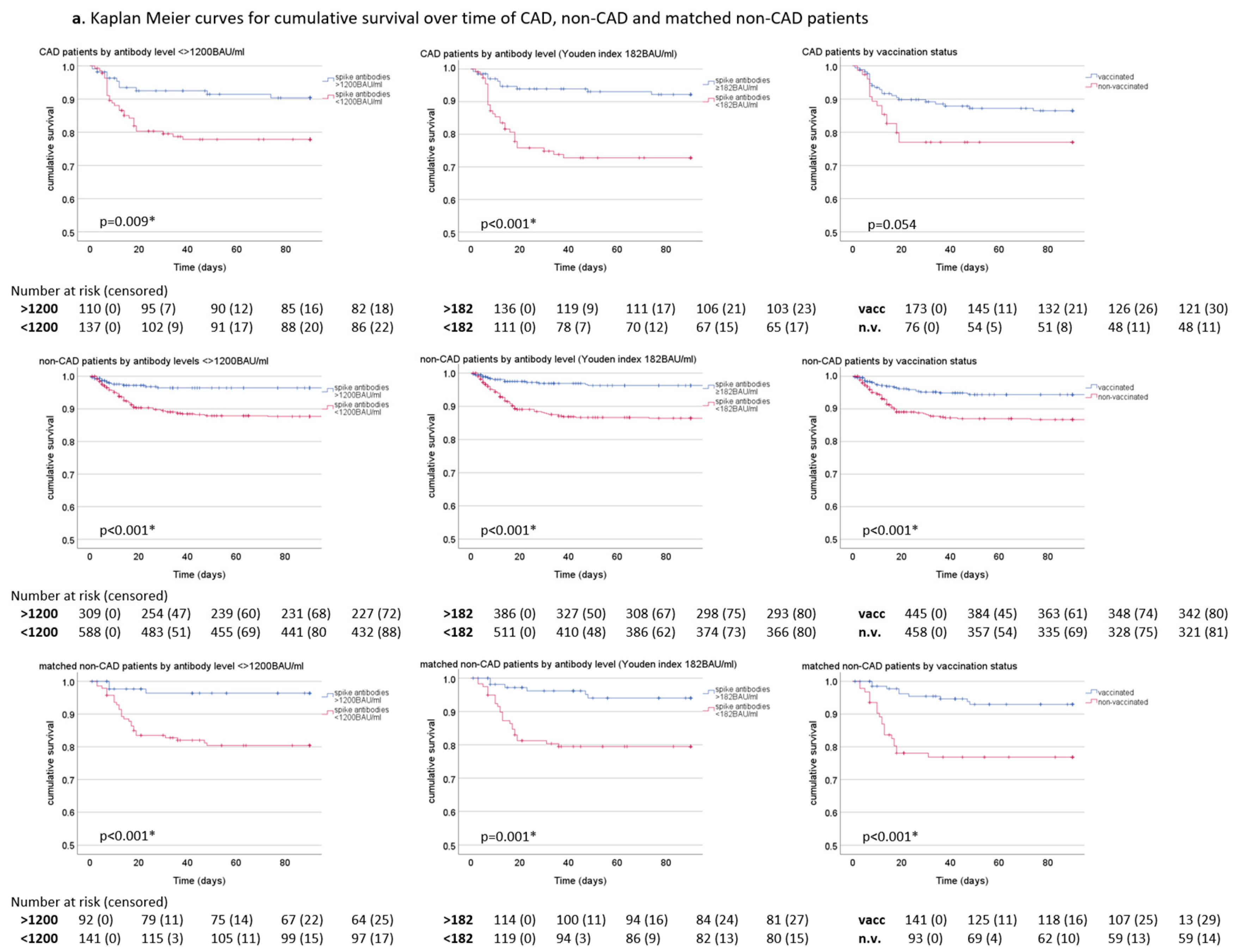
3.3. Survival over Time
3.4. Risk Estimation and Adjustment for Potential Confounders
3.5. Mortality Risk Estimation by Antibody Titer Increment
4. Discussion
4.1. Key Results
4.2. Interpretation
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (a)OR, (a)HR | (adjusted) odds ratio, (adjusted) hazard ratio. |
| BAU/ml | Binding antibody units/ml. |
| BMI | Body mass index. |
| CAD | Coronary artery disease. |
| CI | Confidence interval. |
| COPD | Chronic obstructive pulmonary disease. |
| COVID-19 | Coronavirus disease 2019. |
| CRP | C-reactive protein. |
| CVD | Cerebrovascular disease. |
| ICU | Intensive care unit. |
| IL6 | Interleukin 6. |
| IQR | Interquartile range. |
| PCR | Polymerase chain reaction. |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2. |
| STEMI | ST elevation myocardial infarction. |
| TIA | Transient ischemic attack. |
References
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 April 2024).
- World Health Organization (WHO). Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. 2023. Available online: https://www.who.int/news/item/05–05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 11 December 2023).
- European Centre for Disease Prevention and Control. Data on Testing for COVID-19 by Week and Country. 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-testing (accessed on 19 April 2024).
- Wastewater is a robust proxy for monitoring circulating SARS-CoV-2 variants. Nat. Biotechnol. 2022, 40, 1768–1769. [CrossRef] [PubMed]
- World Health Organization (WHO). Wastewater Environmental Surveillance. 2023. Available online: https://data.who.int/dashboards/covid19/wastewater (accessed on 18 April 2024).
- World Health Organization (WHO). Tracking SARS-CoV-2-Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 18 April 2024).
- Ticona, J.P.A.; Xiao, M.; Li, D.; Nery, N.; Hitchings, M.; Belitardo, E.M.M.A.; Fofana, M.O.; Victoriano, R.; Cruz, J.S.; de Moraes, L.; et al. Extensive transmission of SARS-CoV-2 BQ.1* variant in a population with high levels of hybrid immunity: A prevalence survey. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, 139, 159–167. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Deaths by Select Demographic and Geographic Characteristics. Provisional Death Counts for COVID-19. Available online: https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm (accessed on 19 April 2024).
- Rossi, G.; Salmanton-García, J.; Cattaneo, C.; Marchesi, F.; Dávila-Valls, J.; Martín-Pérez, S.; Itri, F.; López-García, A.; Glenthøj, A.; da Silva, M.G.; et al. Age, successive waves, immunization, and mortality in elderly COVID-19 hematological patients: EPICOVIDEHA findings. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, 137, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Hajikhani, B.; Safavi, M.; Bostanshirin, N.; Sameni, F.; Ghazi, M.; Yazdani, S.; Nasiri, M.J.; Khosravi-Dehaghi, N.; Noorisepehr, N.; Sayyari, S.; et al. COVID-19 and coronary artery disease; A systematic review and meta-analysis. New Microbes New Infect. 2023, 53, 101151. [Google Scholar] [CrossRef] [PubMed]
- Merzah, M.A.; Sulaiman, D.; Karim, A.A.; Khalil, M.E.; Gupta, S.; Almuzaini, Y.; Hashemi, S.; Mathew, S.; Khatoon, S.; Hoque, M.B. A systematic review and meta-analysis on the prevalence and impact of coronary artery disease in hospitalized COVID-19 patients. Heliyon 2023, 9, e19493. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Mierzejewska, M.; Jurek, J.; Kochanowska, A.; Gasecka, A.; Truszewski, Z.; Pruc, M.; Blek, N.; Rafique, Z.; Filipiak, K.J.; et al. Effect of Coronary Artery Disease on COVID-19-Prognosis and Risk Assessment: A Systematic Review and Meta-Analysis. Biology 2022, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Kotlo, S.; Thorgerson, A.; Kulinski, J. Coronary artery calcification as a predictor of adverse outcomes in patients hospitalized with COVID-19. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 28, 100288. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Kermani-Alghoraishi, M. A Review of Coronary Artery Thrombosis: A New Challenging Finding in COVID-19 Patients and ST-elevation Myocardial Infarction. Curr. Probl. Cardiol. 2021, 46, 100744. [Google Scholar] [CrossRef] [PubMed]
- Seeherman, S.; Suzuki, Y.J. Viral Infection and Cardiovascular Disease: Implications for the Molecular Basis of COVID-19 Pathogenesis. Int. J. Mol. Sci. 2021, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; Reimann, P.; Fraunberger, P. Prognostic value of anti-SARS-CoV-2 antibodies: A systematic review. Clin. Chem. Lab. Med. 2024, 62, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Pedrero, M.; Jansen, J.M.; Blume, C.; Stanislawski, N.; Jonczyk, R.; Molle, A.; Hernandez, M.G.; Kaiser, F.K.; Jung, K.; Osterhaus, A.D.M.E.; et al. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat. Aging 2022, 2, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Plonné, D.; Voss, M.; Ivanova, R.; Frodl, R.; Deininger, S. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 130, 104542. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet. Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Vidyasagar, K.; Pathan, S.; Sharma, S.; Chavan, M.R.; Bhagavathula, A.S.; Padmavathi, R.; Manjula, M.; Chhabra, M.; Gupta, R.; et al. Global prevalence and effect of comorbidities and smoking status on severity and mortality of COVID-19 in association with age and gender: A systematic review, meta-analysis and meta-regression. Sci. Rep. 2023, 13, 6415. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Alonzi, T.; Alter, G.; Noonan, D.M.; Landay, A.L.; Albini, A.; Goletti, D. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front. Immunol. 2023, 14, 1146704. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef] [PubMed]
- Petráš, M.; Máčalík, R.; Janovská, D.; Čelko, A.M.; Dáňová, J.; Selinger, E.; DolečEk, J.; Neradová, S.; Franklová, M.; Dlouhý, P.; et al. Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: A meta-analysis and meta-regression. BMC Med. 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Zheng, N.-Y.; Huang, M.; Cabanov, A.; Rojas, K.T.; Kaur, K.; Andrews, S.F.; Palm, A.-K.E.; Chen, Y.-Q.; Li, Y.; et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe 2019, 25, 357–366.e6. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021, 27, 1147–1148. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Tytun, A.; Ury, H.K. A simple approximation for calculating sample sizes for comparing independent proportions. Biometrics 1980, 36, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, X. Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest 2020, 158, S12–S20. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.H.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; de Angelis, M.H.; et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet. Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.; Anderson, E.; Shadid, H.; Berlin, H.; Pan, M.; Azam, T.U.; Khaleel, I.; Padalia, K.; Meloche, C.; O’hayer, P.; et al. Inflammation, Hyperglycemia, and Adverse Outcomes in Individuals with Diabetes Mellitus Hospitalized for COVID-19. Diabetes Care 2022, 45, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Pivina, L.; Srinath, S.; Benahmed, A.G.; Semenova, Y.; Menzel, A.; Dadar, M.; Bjørklund, G. Interrelations between COVID-19 and other disorders. Clin. Immunol. 2021, 224, 108651. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lim, M.A.; Wahjoepramono, E.J.; July, J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020, 29, 104949. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, M.; Yang, Q.; Fairley, C.K.; Chai, Z.-L.; McIntyre, R.; Ong, J.J.; Liu, H.; Lu, P.; Hu, W.; et al. Global Diabetes Prevalence in COVID-19 Patients and Contribution to COVID-19- Related Severity and Mortality: A Systematic Review and Meta-analysis. Diabetes Care 2023, 46, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.H.; Nyberg, T.; Sinnathamby, M.A.; Aziz, N.A.; Ferguson, N.; Seghezzo, G.; Blomquist, P.B.; Bridgen, J.; Chand, M.; Groves, N.; et al. Hospitalisation and mortality risk of SARS-COV-2 variant omicron sub-lineage BA.2 compared to BA.1 in England. Nat. Commun. 2022, 13, 6053. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; List, W.; Hoefle, G.; Frick, M.; Suessenbacher, A.; Winder, T.; Fetz, C.; Boesl, A.; Saely, C.H.; Drexel, H.; et al. Evaluation of SARS-CoV-2 antibody levels on hospital admission as a correlate of protection against mortality. J. Intern. Med. 2023, 293, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; Fraunberger, P. Anti-SARS-CoV-2 Antibody Testing: Role and Indications. J. Clin. Med. 2023, 12, 7575. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; Saely, C.H.; Frick, M.; Leiherer, A.; Drexel, H.; Fraunberger, P. Association between Lipid Levels, Anti-SARS-CoV-2 Spike Antibodies and COVID-19 Mortality: A Prospective Cohort Study. J. Clin. Med. 2023, 12, 5068. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; Saely, C.H.; Leiherer, A.; Frick, M.; Plattner, T.; Drexel, H.; Fraunberger, P. Anti-SARS-CoV-2 antibody levels predict outcome in COVID-19 patients with type 2 diabetes: A prospective cohort study. Sci. Rep. 2023, 13, 18326. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Liao, J.K. The Pleiotropic Effects of Statins—From Coronary Artery Disease and Stroke to Atrial Fibrillation and Ventricular Tachyarrhythmia. Curr. Vasc. Pharmacol. 2019, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Rahhal, A.; Mahfouz, A.; Khir, F.; Okleh, N.; Aljundi, A.H.; AlKhalaila, O.; Hamid, Y.; Al-Amri, M.; Al-Yafei, S.A.; Al Suwaidi, J.; et al. Medications adherence post-primary percutaneous coronary intervention in acute myocardial infarction: A population-based cohort study. J. Clin. Pharm. Ther. 2021, 46, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Siostrzonek, P.; Brath, H.; Zweiker, R.; Drexel, H.; Hoelzl, R.; Hemetsberger, M.; Ray, K.K. Lipid lowering therapy in primary and secondary prevention in Austria: Are LDL-C goals achieved? Results from the DA VINCI study. Wien. Klin. Wochenschr. 2022, 134, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Drexel, H.; Rosano, G.M.C.; Lewis, B.S.; Huber, K.; Vonbank, A.; Dopheide, J.F.; Mader, A.; Niessner, A.; Savarese, G.; Wassmann, S.; et al. The age of randomized clinical trials: Three important aspects of randomized clinical trials in cardiovascular pharmacotherapy with examples from lipid and diabetes trials. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Wise, H.; Templeton, K.; Batchelor, B.; Squires, M.; McCance, K.; Jarvis, L.; Malloy, K.; Furrie, E.; Richardson, C.; et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: A serological analysis. Lancet Microbe 2022, 3, e493–e502. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Wang, P.; Tian, J.; Gao, G.F.; Liu, K.; Liu, W.J. Seeing the T cell Immunity of SARS-CoV-2 and SARS-CoV: Believing the Epitope-Oriented Vaccines. Int. J. Biol. Sci. 2023, 19, 4052–4060. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, E.; Varaklis, C.; Pillet, S.; Shafiani, S.; Lancelotta, M.P.; Steinhubl, S.; Sugden, S.; Clutter, M.; Montamat-Sicotte, D.; Chermak, T.; et al. Integrated antibody and cellular immunity monitoring are required for assessment of the long term protection that will be essential for effective next generation vaccine development. Front. Immunol. 2023, 14, 1166059. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Dopico, X.C.; Dyrdak, R.; Martin, D.P.; Reddy, S.T.; Dillner, J.; Hedestam, G.B.K.; et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: A cross-sectional study. Lancet Infect. Dis. 2022, 22, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Larkin, H.D. Evolving Omicron Subvariants Are More Resistant to Antibody Therapy. JAMA 2022, 328, 518. [Google Scholar] [CrossRef] [PubMed]
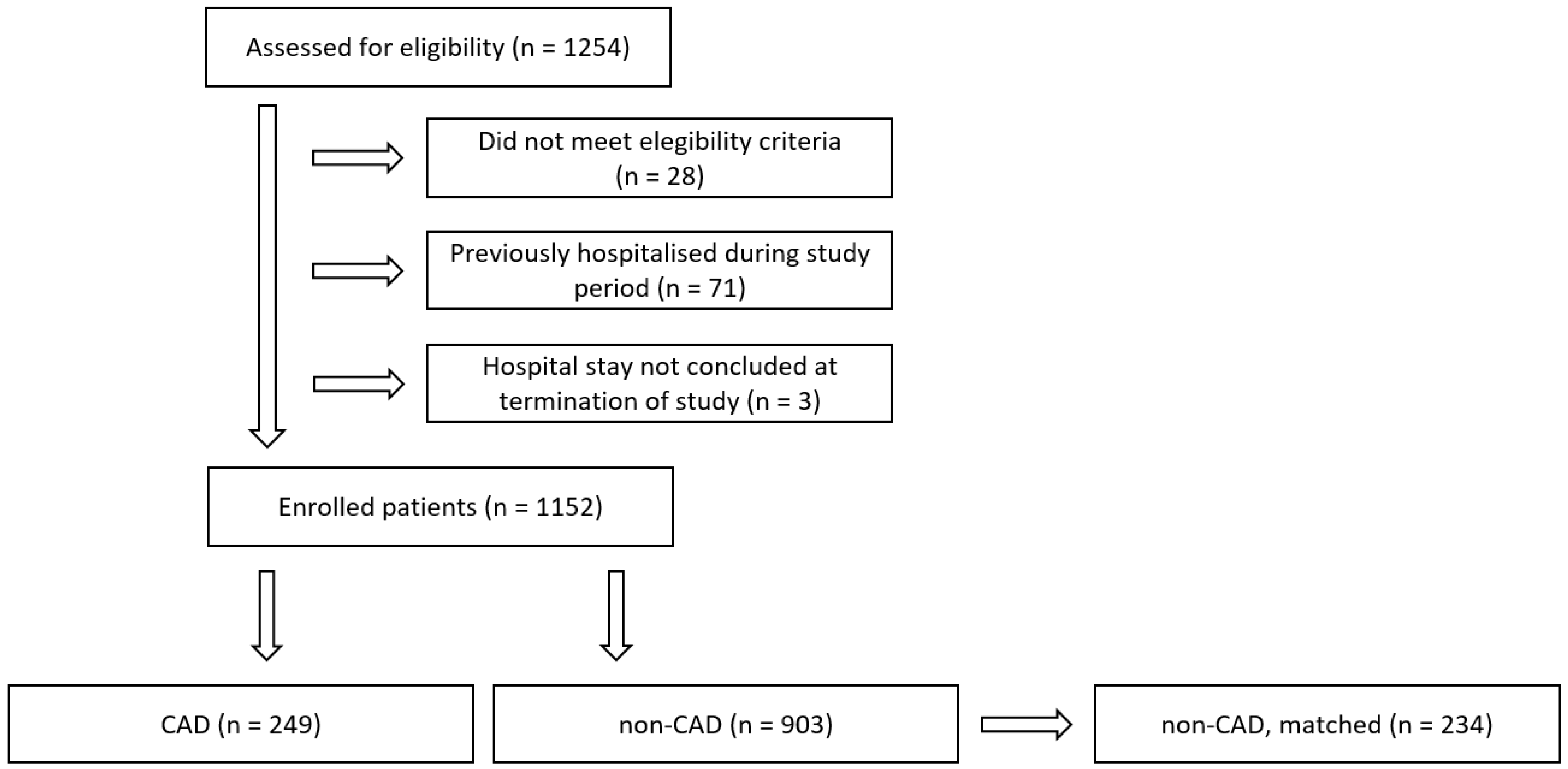


| Study Cohort | Whole Cohort | CAD | Non-CAD | p-Value | Non-CAD, Matched | p-Value | CAD, Omicron | Non-CAD, Omicron | p-Value | Non-CAD, Omicron, Matched | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1152 | 249 | 903 | 234 | 128 | 433 | 123 | ||||
| age (years) | 66.8 ± 20.3 | 78.9 ± 11.1 | 63.5 ± 21.0 | <0.001 | 77.2 ± 14.1 | 0.647 | 79.8 ± 11.0 | 63.0 ± 23.2 | <0.001 | 78.1 ± 13.4 | 0.592 |
| male gender (%) | 53.2 | 57.4 | 52.0 | 0.132 | 54.7 | 0.546 | 57.0 | 57.7 | 0.887 | 62.6 | 0.368 |
| BMI (kg/m2) | 27.1 ± 6.5 | 27.0 ± 5.8 | 27.1 ± 6.7 | 0.688 | 26.8 ± 5.0 | 0.596 | 26.9 ± 6.2 | 25.8 ± 6.0 | 0.091 | 25.7 ± 5.1 | 0.107 |
| obesity (%) | 25.2 | 25.7 | 25.0 | 0.849 | 20.9 | 0.251 | 23.7 | 19.7 | 0.362 | 14.4 | 0.091 |
| statin treatment (%) | 25.7 | 48.2 | 19.5 | <0.001 | 28.2 | <0.001 | 59.4 | 24.0 | <0.001 | 32.5 | <0.001 |
| Comorbidities | |||||||||||
| DM (%) | 24.8 | 42.4 | 20.0 | <0.001 | 46.6 | 0.359 | 40.5 | 20.6 | <0.001 | 51.2 | 0.093 |
| hypertension (%) | 50.5 | 77.9 | 43.0 | <0.001 | 76.5 | 0.711 | 79.7 | 44.8 | <0.001 | 78.9 | 0.872 |
| heart failure (%) | 7.2 | 20.5 | 3.5 | <0.001 | 5.6 | <0.001 | 19.5 | 3.0 | <0.001 | 4.9 | <0.001 |
| COPD (%) | 9.6 | 14.5 | 8.3 | 0.004 | 9.4 | 0.088 | 14.8 | 8.5 | 0.037 | 8.1 | 0.096 |
| asthma (%) | 2.4 | 2.0 | 2.5 | 0.625 | 1.3 | 0.532 | 2.3 | 1.2 | 0.319 | 0.8 | 0.333 |
| renal disease (%) | 23.1 | 46.3 | 16.7 | <0.001 | 37.6 | 0.053 | 53.2 | 18.4 | <0.001 | 39.0 | 0.025 |
| stroke/TIA/CVD (%) | 11.7 | 24.5 | 8.2 | <0.001 | 14.5 | 0.006 | 29.7 | 11.3 | <0.001 | 18.7 | 0.042 |
| comorbidity count | 1.5 ± 1.5 | 3.3 ± 1.2 | 1.0 ± 1.1 | <0.001 | 1.9 ± 1.2 | <0.001 | 3.4 ± 1.3 | 1.1 ± 1.2 | <0.001 | 2.0 ± 1.1 | <0.001 |
| Outcome | |||||||||||
| mortality (%) | 10.2 | 15.7 | 8.7 | 0.001 | 12.8 | 0.372 | 9.4 | 4.4 | 0.030 | 7.3 | 0.556 |
| ICU (%) | 14.3 | 16.5 | 13.7 | 0.276 | 13.2 | 0.321 | 13.3 | 6.4 | 0.013 | 6.5 | 0.073 |
| oxygen req (%) | 51.0 | 61.8 | 48.0 | <0.001 | 50.7 | 0.014 | 48.4 | 27.5 | <0.001 | 31.4 | 0.006 |
| Laboratory analysis | |||||||||||
| CT value | 21.3 ± 6.6 | 20.8 ± 6.5 | 21.4 ± 6.6 | 0.097 | 20.4 ± 6.6 | 0.578 | 20.8 ± 6.5 | 21.0 ± 6.6 | 0.818 | 19.7 ± 6.5 | 0.149 |
| creatinine (mg/dL) | 1.0 (0.8–1.3) | 1.2 (1.0–1.7) | 1.0 (0.8–1.2) | <0.001 | 1.1 (0.9–1.5) | 0.014 | 1.2 (1.0–1.7) | 1.0 (0.8–1.3) | <0.001 | 1.2 (1.0–1.6) | 0.770 |
| NT-proBNP (pg/mL) | 327 (100–1243) | 1387 (477–4333) | 227 (69–767) | <0.001 | 661 (176–1649) | <0.001 | 1588 (646–4504) | 242 (70–831) | <0.001 | 696 (225–6902) | <0.001 |
| IL6 (pg/mL) | 28.7 (11–67) | 39.0 (17–81) | 27.2 (1–64) | <0.001 | 24.9 (11–58) | 0.002 | 27 (13–213) | 18.4 (8–110) | 0.001 | 20.2 (9–45) | 0.071 |
| CRP (mg/dL) | 6.0 ± 6.4 | 6.6 ± 6.5 | 5.8 ± 6.4 | 0.026 | 5.6 ± 6.4 | 0.036 | 6.5 ± 6.8 | 4.4 ± 5.9 | <0.001 | 4.3 ± 6.3 | <0.001 |
| CAD Patients | Omicron | Non-Omicron | p-Value | Non-Vaccinated | Vaccinated | p-Value | Statin Treatment | No Statin Treatment | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| N | 128 | 121 | 76 | 173 | 120 | 129 | |||
| age (years) | 79.8 ± 11.0 | 77.8 ± 11.1 | 0.140 | 77.9 ± 13.1 | 79.3 ± 10.2 | 0.613 | 77.7 ± 10.2 | 79.9 ± 11.9 | 0.037 |
| male gender (%) | 57.0 | 57.9 | 0.896 | 52.6 | 59.5 | 0.310 | 65.8 | 49.6 | 0.010 |
| BMI (kg/m2) | 26.9 ± 6.2 | 27.1 ± 5.3 | 0.594 | 26.3 ± 5.4 | 27.3 ± 5.9 | 0.505 | 27.7 ± 5.4 | 26.3 ± 6.1 | 0.088 |
| obesity (%) | 23.7 | 27.6 | 0.498 | 20.6 | 27.8 | 0.255 | 28.6 | 22.9 | 0.323 |
| statin treatment (%) | 59.4 | 36.4 | <0.001 | 38.2 | 52.6 | 0.036 | |||
| Comorbidities | |||||||||
| DM (%) | 40.5 | 44.3 | 0.549 | 43.1 | 42.1 | 0.888 | 49.5 | 36.0 | 0.036 |
| hypertension (%) | 79.7 | 76.0 | 0.487 | 80.3 | 76.9 | 0.553 | 85.8 | 70.5 | 0.004 |
| heart failure (%) | 19.5 | 21.5 | 0.702 | 17.1 | 22.0 | 0.382 | 17.5 | 23.3 | 0.261 |
| COPD (%) | 14.8 | 14.0 | 0.859 | 9.2 | 16.8 | 0.119 | 15.0 | 14.0 | 0.814 |
| asthma (%) | 2.3 | 1.7 | 0.698 | 1.3 | 2.3 | 0.606 | 3.3 | 0.8 | 0.150 |
| renal disease (%) | 53.2 | 39.2 | 0.028 | 30.3 | 53.5 | <0.001 | 47.5 | 45.2 | 0.722 |
| stroke/TIA/CVD (%) | 29.7 | 19.0 | 0.050 | 26.3 | 23.7 | 0.658 | 33.3 | 16.3 | 0.002 |
| comorbidity count | 3.4 ± 1.3 | 3.1 ± 1.2 | 0.067 | 3.1 ± 1.2 | 3.4 ± 1.2 | 0.051 | 3.5 ± 1.2 | 3.0 ± 1.2 | 0.005 |
| Outcome | |||||||||
| mortality (%) | 9.4 | 22.3 | 0.005 | 22.4 | 12.7 | 0.054 | 12.5 | 18.6 | 0.185 |
| ICU (%) | 13.3 | 19.8 | 0.163 | 27.6 | 11.6 | 0.002 | 14.2 | 18.6 | 0.345 |
| oxygen req (%) | 48.0 | 76.0 | <0.001 | 64.5 | 60.7 | 0.572 | 55.0 | 68.2 | 0.032 |
| Laboratory analysis | |||||||||
| CT value | 20.8 ± 6.5 | 20.7 ± 6.6 | 0.817 | 20.7 ± 6.0 | 20.8 ± 6.8 | 0.768 | 20.2 ± 5.7 | 21.2 ± 7.2 | 0.436 |
| creatinine (mg/dL) | 1.2 (1.0–1.7) | 1.2 (0.9–1.7) | 0.811 | 1.1 (0.9–1.5) | 1.3 (1.0–1.8) | 0.020 | 1.2 (1.0–1.7) | 1.2 (0.9–1.7) | 0.363 |
| NT-proBNP (pg/mL) | 1588 (646–4304) | 975 (388–4333) | 0.070 | 1264 (494–2527) | 1532 (470–5422) | 0.137 | 1324 (426–3427) | 1566 (506–4622) | 0.435 |
| IL6 (pg/mL) | 27.4 (13–78) | 50.8 (22–84) | 0.029 | 38.2 (15–79) | 39.1 (17–83) | 0.773 | 31.6 (12–68) | 46.6 (20–97) | 0.020 |
| CRP (mg/dL) | 6.5 ± 6.8 | 6.7 ± 6.2 | 0.593 | 6.6 ± 6.6 | 6.6 ± 6.5 | 0.746 | 5.7 ± 5.8 | 7.5 ± 7.0 | 0.024 |
| CAD vs. Non-CAD Patients | CAD, Vaccinated | Non-CAD, Vaccinated | p-Value | Non-CAD, Matched Vaccinated | p-Value | CAD, Statin Treatment | Non-CAD, Statin Treatment | p-Value | Non-CAD, Statins, Matched | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 173 | 445 | 141 | 120 | 176 | 66 | ||||
| age (years) | 79.3 ± 10.2 | 68.3 ± 18.9 | <0.001 | 78.3 ± 11.7 | 0.810 | 77.7 ± 10.2 | 75.4 ± 11.8 | 0.175 | 79.6 ± 9.5 | 0.138 |
| male gender (%) | 59.5 | 55.5 | 0.364 | 60.3 | 0.893 | 65.8 | 55.7 | 0.080 | 59.1 | 0.361 |
| BMI (kg/m2) | 27.3 ± 5.9 | 26.7 ± 5.8 | 0.150 | 26.3 ± 4.9 | 0.143 | 27.7 ± 5.4 | 28.5 ± 6.5 | 0.522 | 28.1 ± 5.0 | 0.474 |
| obesity (%) | 27.8 | 21.6 | 0.120 | 16.8 | 0.028 | 28.6 | 32.9 | 0.450 | 29.3 | 0.920 |
| statin treatment (%) | 52.6 | 27.2 | <0.001 | 34.8 | 0.002 | |||||
| Comorbidities | ||||||||||
| DM (%) | 42.1 | 23.8 | <0.001 | 51.1 | 0.116 | 49.5 | 43.9 | 0.354 | 65.2 | 0.043 |
| hypertension (%) | 76.9 | 51.0 | <0.001 | 79.4 | 0.587 | 85.8 | 68.2 | <0.001 | 86.4 | 0.921 |
| heart failure (%) | 22.0 | 4.5 | <0.001 | 6.4 | <0.001 | 17.5 | 6.3 | 0.002 | 6.1 | 0.029 |
| COPD (%) | 16.8 | 12.4 | 0.152 | 14.2 | 0.531 | 15.0 | 17.0 | 0.639 | 10.6 | 0.401 |
| asthma (%) | 2.3 | 2.0 | 0.822 | 2.1 | 0.912 | 3.3 | 2.3 | 0.581 | 3.0 | 0.911 |
| renal disease (%) | 53.5 | 22.7 | <0.001 | 43.3 | 0.071 | 47.5 | 25.0 | <0.001 | 43.9 | 0.641 |
| stroke/TIA/CVD (%) | 23.7 | 11.5 | <0.001 | 15.6 | 0.075 | 33.3 | 21.0 | 0.018 | 22.7 | 0.129 |
| comorbidity count | 3.4 ± 1.2 | 1.3 ± 1.2 | <0.001 | 2.1 ± 1.1 | <0.001 | 3.5 ± 1.2 | 1.8 ± 1.3 | <0.001 | 2.4 ± 1.2 | <0.001 |
| Outcome | ||||||||||
| mortality (%) | 12.7 | 5.2 | 0.001 | 6.4 | 0.061 | 12.5 | 8.5 | 0.266 | 9.1 | 0.482 |
| ICU (%) | 11.6 | 8.3 | 0.211 | 10.6 | 0.796 | 14.2 | 11.4 | 0.474 | 4.5 | 0.043 |
| oxygen req (%) | 60.7 | 37.6 | <0.001 | 43.4 | 0.002 | 55.0 | 51.7 | 0.577 | 54.5 | 0.952 |
| Laboratory analysis | ||||||||||
| CT value | 20.8 ± 6.8 | 20.9 ± 6.6 | 0.736 | 20.8 ± 7.0 | 0.917 | 20.2 ± 5.7 | 20.2 ± 6.1 | 0.903 | 21.3 ± 6.3 | 0.368 |
| creatinine (mg/dL) | 1.3 (1.0–1.8) | 1.0 (0.8–1.3) | <0.001 | 1.2 (0.9–1.6) | 0.077 | 1.2 (1.0–1.7) | 1.1 (0.9–1.4) | <0.001 | 1.2 (0.9–1.5) | 0.103 |
| NT-proBNP (pg/mL) | 1532 (470–5422) | 302 (96–1140) | <0.001 | 734 (211–2039) | <0.001 | 1324 (426–3427) | 384 (165–1388) | <0.001 | 534 (211–1049) | <0.001 |
| IL6 (pg/mL) | 39.1 (17–83) | 22.1 (8–58) | <0.001 | 22.4 (9–44) | <0.001 | 31.6 (12–68) | 26.6 (9–62) | 0.229 | 27.0 (9–57) | 0.430 |
| CRP (mg/dL) | 6.6 ± 6.5 | 5.2 ± 6.4 | <0.001 | 5.2 ± 6.5 | 0.005 | 5.7 ± 5.8 | 5.5 ± 6.5 | 0.412 | 5.9 ± 7.3 | 0.890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mink, S.; Drexel, H.; Leiherer, A.; Cadamuro, J.; Hitzl, W.; Frick, M.; Reimann, P.; Saely, C.H.; Fraunberger, P. Anti-SARS-CoV-2 Antibodies versus Vaccination Status in CAD Patients with COVID-19: A Prospective, Propensity Score-Matched Cohort Study. Vaccines 2024, 12, 855. https://doi.org/10.3390/vaccines12080855
Mink S, Drexel H, Leiherer A, Cadamuro J, Hitzl W, Frick M, Reimann P, Saely CH, Fraunberger P. Anti-SARS-CoV-2 Antibodies versus Vaccination Status in CAD Patients with COVID-19: A Prospective, Propensity Score-Matched Cohort Study. Vaccines. 2024; 12(8):855. https://doi.org/10.3390/vaccines12080855
Chicago/Turabian StyleMink, Sylvia, Heinz Drexel, Andreas Leiherer, Janne Cadamuro, Wolfgang Hitzl, Matthias Frick, Patrick Reimann, Christoph H. Saely, and Peter Fraunberger. 2024. "Anti-SARS-CoV-2 Antibodies versus Vaccination Status in CAD Patients with COVID-19: A Prospective, Propensity Score-Matched Cohort Study" Vaccines 12, no. 8: 855. https://doi.org/10.3390/vaccines12080855






