A Comprehensive Review on the Intricate Interplay between COVID-19 Immunization and the New Onset of Pemphigus Foliaceus
Abstract
:1. Introduction
2. Existing Data on PF Onset after SARS-CoV-2 Vaccination
3. Discussion
3.1. The Immunological Interplay between COVID-19 Vaccines and AIBDs
3.2. Mechanisms of Vaccine-Induced Skin Findings
3.2.1. T Cell-Mediated Response Resulting from Molecular Mimicry to Viral Epitopes
3.2.2. Bystander Activation of Immune Cells
3.2.3. RNA-Mediated Activation of Innate Immunity
4. Future Perspectives on mRNA Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertl, M.; Jedlickova, H.; Karpati, S.; Marinovic, B.; Uzun, S.; Yayli, S.; Mimouni, D.; Borradori, L.; Feliciani, C.; Ioannides, D.; et al. Pemphigus. S2 Guideline for diagnosis and treatment—Guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, A.; Naumescu, I.P.; Popa, L.G.; Wafi, G.; Balaceanu, B.; Dorobantu, A.M.; Ali, S.; Orzan, O.A. Development of pemphigus foliaceus during pregnancy following COVID vaccination—Case report. Dermatovenerol. J. 2022, 67, 19–22. [Google Scholar]
- Huang, X.; Liang, X.; Zhang, J.; Su, H.; Chen, Y. Pemphigus during the COVID-19 Epidemic: Infection Risk, Vaccine Responses and Management Strategies. J. Clin. Med. 2022, 11, 3968. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Kasperkiewicz, M.; Joly, P. Pemphigus. Lancet 2019, 394, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Melchionda, V.; Harman, K.E. Pemphigus vulgaris and pemphigus foliaceus: An overview of the clinical presentation, investigations and management. Clin. Exp. Dermatol. 2019, 44, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S. Pemphigus trigger factors: Special focus on pemphigus vulgaris and pemphigus foliaceus. Arch. Dermatol. Res. 2018, 310, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Canfora, F.; Mascolo, M.; Varricchio, S.; Mignogna, M.D.; Adamo, D. Autoimmune mucocutaneous blistering diseases after SARS-CoV-2 vaccination: A Case report of Pemphigus Vulgaris and a literature review. Pathol. Res. Pract. 2022, 232, 153834. [Google Scholar] [CrossRef] [PubMed]
- Marcellusi, A.; Fabiano, G.; Sciattella, P.; Andreoni, M.; Mennini, F.S. The impact of COVID-19 vaccination on the italian healthcare system: A scenario analysis. Clin. Drug Investig. 2022, 42, 237–242. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Freeman, E.E.; Sun, Q.; McMahon, D.E.; Singh, R.; Fathy, R.; Tyagi, A.; Blumenthal, K.; Hruza, G.J.; French, L.E.; Fox, L.P. Skin reactions to COVID-19 vaccines: An American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J. Am. Acad. Dermatol. 2021, 86, e165. [Google Scholar] [CrossRef] [PubMed]
- Báez-Negrón, L.; Vilá, L.M. New-onset systemic lupus erythematosus after mRNA SARS-CoV-2 vaccination. Case Rep. Rheumatol. 2022, 11, 6436839. [Google Scholar] [CrossRef] [PubMed]
- Prabhahar, A.; Naidu, G.S.R.S.N.K.; Chauhan, P.; Sekar, A.; Sharma, A.; Sharma, A.; Kumar, A.; Nada, R.; Rathi, M.; Kohli, H.S.; et al. ANCA-associated vasculitis following ChAdOx1 nCoV19 vaccination: Case-based review. Rheumatol. Int. 2022, 42, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2021, 165, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Damsky, W.; Fathy, R.; McMahon, D.E.; Turner, N.; Valentin, M.N.; Rallis, T.; Aivaz, O.; Fox, L.P.; Freeman, E.E. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J. Allergy Clin. Immunol. 2021, 148, 750–751. [Google Scholar] [CrossRef]
- Thongprasom, K.; Pengpis, N.; Phattarataratip, E.; Samaranayake, L. Oral pemphigus after COVID-19 vaccination. Oral Dis. 2021, 28, 2597–2598. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Woodley, D.T. Association between vaccination and autoimmune bullous diseases: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Alami, S.; Benzekri, L.; Senouci, K.; Meziane, M. Pemphigus foliaceus triggered after inactivated SARS-CoV-2 vaccine: Coincidence or causal link? Dermatol. Ther. 2022, 35, e15775. [Google Scholar] [CrossRef] [PubMed]
- Mohaghegh, F.; Hatami, P.; Refaghat, A.; Mehdizadeh, M.; Aryanian, Z.; Mohandesi, N.A.; Afshar, Z.M. New-onset pemphigus foliaceus following SARS-CoV-2 infection and unmasking multiple sclerosis: A case report. Clin. Case Rep. 2022, 10, e05910. [Google Scholar] [CrossRef]
- Guo, M.; Liu, X.; Chen, X.; Li, Q. Insights into new-onset autoimmune diseases after COVID-19 vaccination. Autoimmun. Rev. 2023, 22, 103340. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S. COVID-19 disease and autoimmune disorders: A mutual pathway. World J. Methodol. 2022, 12, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R. Molecular Mimicry and HLA Polymorphisms May Drive Autoimmunity in Recipients of the BNT-162b2 mRNA Vaccine: A Computational Analysis. Microorganisms 2023, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.N.; Nguyen, T.T.P.; Vu, T.T.P.; Nguyen, H.T. Pemphigus Foliaceus after COVID-19 Vaccination: A Report of Two Cases. Case Rep. Dermatol. Med. 2023, 2023, 1218388. [Google Scholar] [CrossRef] [PubMed]
- Pourani, M.; Bidari-Zerehpoosh, F.; Ayatollahi, A.; Robati, R.M. New onset of pemphigus foliaceus following BBIBP COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15816. [Google Scholar] [CrossRef] [PubMed]
- Lua, A.C.Y.; Ong, F.L.L.; Choo, K.J.L.; Yeo, Y.W.; Oh, C.C. An unusual presentation of pemphigus foliaceus following COVID-19 vaccination. Australas. J. Dermatol. 2022, 63, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Corrá, A.; Barei, F.; Genovese, G.; Zussino, M.; Spigariolo, C.B.; Mariotti, E.B.; Quintarelli, L.; Verdelli, A.; Caproni, M.; Marzano, A.V. Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019. J. Dermatol. 2023, 50, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Hali, F.; Araqi, L.; Marnissi, F.; Meftah, A.; Chiheb, S. Autoimmune bullous dermatosis following COVID-19 vaccination: A series of five cases. Cureus 2022, 14, e23127. [Google Scholar] [CrossRef] [PubMed]
- Yıldırıcı, Ş.; Yaylı, S.; Demirkesen, C.; Vural, S. New onset of pemphigus foliaceus following BNT162b2 vaccine. Dermatol. Ther. 2022, 35, e15381. [Google Scholar] [CrossRef]
- Rouatbi, J.; Aounallah, A.; Lahouel, M.; Sriha, B.; Belajouza, C.; Denguezli, M. Two cases with new onset of pemphigus foliaceus after SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15827. [Google Scholar] [CrossRef]
- Gui, H.; Young, P.A.; So, J.Y.; Pol-Rodriguez, M.; Rieger, K.E.; Lewis, M.A.; Winge, M.C.G.; Bae, G.H. New-onset pemphigus vegetans and pemphigus foliaceus after SARS-CoV-2 vaccination: A report of 2 cases. JAAD Case Rep. 2022, 27, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Nogueira, M.; Figueiras, O.; Coelho, A.; Cunha Velho, G.; Raposo, I. Pemphigus foliaceous after mRNA COVID-19 vaccine. Eur. J. Dermatol. 2022, 32, 428–429. [Google Scholar] [CrossRef]
- Kridin, K. Pemphigus group: Overview, epidemiology, mortality, and comorbidities. Immunol. Res. 2018, 66, 255–270. [Google Scholar] [CrossRef]
- Payne, A.S.; Stanley, J.R. Pemphigus. In Fitzpatrick’s Dermatology in General Medicine; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw-Hill: New York, NY, USA, 2008; pp. 459–468. [Google Scholar]
- Del Mar Sáez-de-Ocariz, M.; Vega-Memije, M.E.; Zúñiga, J.; Salgado, N.; Ruíz, J.; Balbuena, A.; Domínguez-Soto, L.; Granados, J. HLA-DRB1*0101 is associated with foliaceous pemphigus in Mexicans. Int. J. Dermatol. 2005, 44, 350. [Google Scholar] [CrossRef]
- De Sena Nogueira Maehara, L.; De-Souza-Santana, F.C.; Porro, A.M.; Marcos, E.V.C.; Ura, S.; Nolte, I.M.; Pas, H.H.; Jonkman, M.F.; Tomimori, J. HLA class II alleles of susceptibility and protection in Brazilian and Dutch pemphigus foliaceus. Br. J. Dermatol. 2018, 178, e212–e214. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.L.; Mercuro, O.; Ruocco, V.; Lo Schiavo, A.; Lombari, V.; Guerrera, V.; Pirozzi, G.; Manzo, C. Common human leukocyte antigen alleles in pemphigus vulgaris and pemphigus foliaceus Italian patients. J. Investig. Dermatol. 1999, 113, 107–110. [Google Scholar] [CrossRef]
- Loiseau, P.; Lecleach, L.; Prost, C.; Lepage, V.; Busson, M.; Bastuji-Garin, S.; Roujeau, J.C.; Charron, D. HLA class II polymorphism contributes to specify desmoglein derived peptides in pemphigus vulgaris and pemphigus foliaceus. J. Autoimmun. 2000, 15, 67–73. [Google Scholar] [CrossRef]
- Hinterseher, J.; Hertl, M.; Didona, D. Autoimmune skin disorders and SARS-CoV-2 vaccination—A meta-analysis. J. Dtsch. Dermatol. Ges. 2023, 21, 853–861. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nousari, H.C.; Kimyai-Asadi, A.; Ketabchi, N.; Diaz, L.A.; Anhalt, G.J. Severe nonendemic pemphigus foliaceus presenting in the postpartum period. J. Am. Acad. Dermatol. 1999, 40 Pt 2, 845–846. [Google Scholar] [CrossRef]
- Moro, F.; Sinagra, J.L.M.; Salemme, A.; Fania, L.; Mariotti, F.; Pira, A.; Didona, B.; Di Zenzo, G. Pemphigus: Trigger and predisposing factors. Front. Med. 2023, 10, 1326359. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Shah, S.; Mahajan, R.; Handa, S. Pemphigus and Pregnancy. Indian Dermatol. Online J. 2024, 1–9. [Google Scholar] [CrossRef]
- Wu, P.C.; Huang, I.H.; Wang, C.Y.; Chi, C.C. New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review. Vaccines 2024, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Poddighe, D.; Laurino, C.; Palmieri, B. Vaccination and autoimmune diseases: Is prevention of adverse health effects on the horizon? EPMA J. 2017, 8, 295–311. [Google Scholar] [CrossRef] [PubMed]
- BNT162b2 vaccine for prevention of COVID-19. Aust. Prescr. 2021, 44, 57–58. [CrossRef] [PubMed]
- Grieco, T.; Maddalena, P.; Sernicola, A.; Muharremi, R.; Basili, S.; Alvaro, D.; Cangemi, R.; Rossi, A.; Pellacani, G. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol Ther. 2021, 34, e15153. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Ram, M.; Shoenfeld, Y. Viral infection can induce the production of autoantibodies. Curr. Opin. Rheumatol. 2007, 19, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin. Immunol. 2021, 224, 108665. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Seida, I.; Seida, R.; Elsalti, A.; Mahroum, N. Vaccines and Autoimmunity-From Side Effects to ASIA Syndrome. Medicina 2023, 59, 364. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Farid, A.S.; Nili, A.; Dayani, D.; Tavakolpour, S.; Soori, T.; Teimourpour, A.; Isazade, A.; Abedini, R.; Balighi, K.; et al. Characteristics and outcomes of COVID-19 in patients with autoimmune bullous diseases: A retrospective cohort study. J. Am. Acad. Dermatol. 2021, 84, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.A.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef] [PubMed]
- Wack, S.; Patton, T.; Ferris, L.K. COVID-19 vaccine safety and efficacy in patients with immune-mediated inflammatory disease: Review of available evidence. J. Am. Acad. Dermatol. 2021, 85, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 vaccine race: Challenges and opportunities in vaccine formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Jaycox, J.R.; Lucas, C.; Yildirim, I.; Dai, Y.; Wang, E.Y.; Monteiro, V.; Lord, S.; Carlin, J.; Kita, M.; Buckner, J.H.; et al. SARS-CoV-2 mRNA vaccines decouple anti-viral immunity from humoral autoimmunity. Nat. Commun. 2023, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, D.; Kalra, A.; Misir, B.R.; Kanga, U.; Mukerji, M. HLA-SPREAD: A natural language processing based resource for curating HLA association from PubMed abstracts. BMC Genom. 2022, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Mansour, Y.; Didona, D.; Dilling, A.; Ghoreschi, K.; Meier, K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e649–e651. [Google Scholar] [CrossRef] [PubMed]
- Koutlas, I.G.; Camara, R.; Argyris, P.P.; Davis, M.D.P.; Miller, D.D. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-CoV-2 vaccine. Oral Dis. 2022, 28 (Suppl. S2), 2612–2613. [Google Scholar] [CrossRef]
- Cozzani, E.; Gasparini, G.; Russo, R.; Parodi, A. May bullous pemphigoid be worsened by COVID-19 vaccine? Front. Med. 2022, 9, 931872. [Google Scholar] [CrossRef]
- Hertl, M.; Eming, R.; Veldman, C. T cell control in autoimmune bullous skin disorders. J. Clin. Investig. 2006, 116, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sernicola, A.; Mazzetto, R.; Tartaglia, J.; Ciolfi, C.; Miceli, P.; Alaibac, M. Role of Human Leukocyte Antigen Class II in Antibody-Mediated Skin Disorders. Medicina 2023, 59, 1950. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Chiappini, E.; Galli, L. Vaccines and autoimmunity. Int. J. Immunopathol. Pharmacol. 2013, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, C.; Kato, M.; Otsuka, A. Cutaneous manifestations of COVID-19 and COVID-19 vaccination. J. Dermatol. 2023, 50, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Muellenhoff, M.; Cukrowski, T.; Morgan, M.; Dorton, D. Oral pemphigus vulgaris after anthrax vaccine administration: Association or coincidence? J. Am. Acad. Dermatol. 2004, 50, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Aashish; Rai, A.; Khatri, G.; Priya; Hasan, M.M. Bullous pemphigoid following COVID-19 vaccine: An autoimmune disorder. Ann. Med. Surg. 2022, 80, 104266. [Google Scholar] [CrossRef]
- Hatano, H.; Ishigaki, K. Functional Genetics to Understand the Etiology of Autoimmunity. Genes 2023, 14, 572. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Bednarek, M.; Tukaj, S. Case Report: Circulating Anti-SARS-CoV-2 Antibodies Do Not Cross-React With Pemphigus or Pemphigoid Autoantigens. Front. Med. 2021, 8, 807711. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K.; Casali, P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008, 4, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Daveluy, S. Pemphigus vulgaris after COVID-19 infection and vaccination. J. Am. Acad. Dermatol. 2022, 87, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.; Solimani, F.; Baum, C.; Meier, K.; Pollmann, R.; Didona, D.; Tekath, T.; Dugas, M.; Casadei, N.; Hudemann, C.; et al. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J. Allergy Clin. Immunol. 2021, 147, 2358–2369. [Google Scholar] [CrossRef]
- Sagy, I.; Zeller, L.; Raviv, Y.; Porges, T.; Bieber, A.; Abu-Shakra, M. New-onset systemic lupus erythematosus following BNT162b2 mRNA COVID-19 vaccine: A case series and literature review. Rheumatol. Int. 2022, 42, 2261–2266. [Google Scholar] [CrossRef]
- Gergen, J.; Petsch, B. RNA-Based Vaccines and Mode of Action. Curr. Top. Microbiol. Immunol. 2022, 440, 1–30. [Google Scholar] [CrossRef]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant effects of a sequence-engineered mRNA vaccine: Translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017, 15, 1. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Thran, M.; Jordan, I. mRNA as novel technology for passive immunotherapy. Cell Mol. Life Sci. 2019, 76, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Bafaloukos, D.; Gazouli, I.; Koutserimpas, C.; Samonis, G. Evolution and Progress of mRNA Vaccines in the Treatment of Melanoma: Future Prospects. Vaccines 2023, 11, 636. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Agmon-Levin, N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J. Autoimmun. 2014, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, B.; Betterle, C.; Zanoni, G. Vaccinations and Autoimmune Diseases. Vaccines 2021, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.T.; Perricone, C.; Kivity, S.; Cipriano, E.; Ceccarelli, F.; Valesini, G.; Shoenfeld, Y. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol. Res. 2017, 65, 82–98. [Google Scholar] [CrossRef]
- Fuchs, S.; Aricha, R.; Reuveni, D.; Souroujon, M.C. Experimental Autoimmune Myasthenia Gravis (EAMG): From immunochemical characterization to therapeutic approaches. J. Autoimmun. 2014, 54, 51–59. [Google Scholar] [CrossRef]
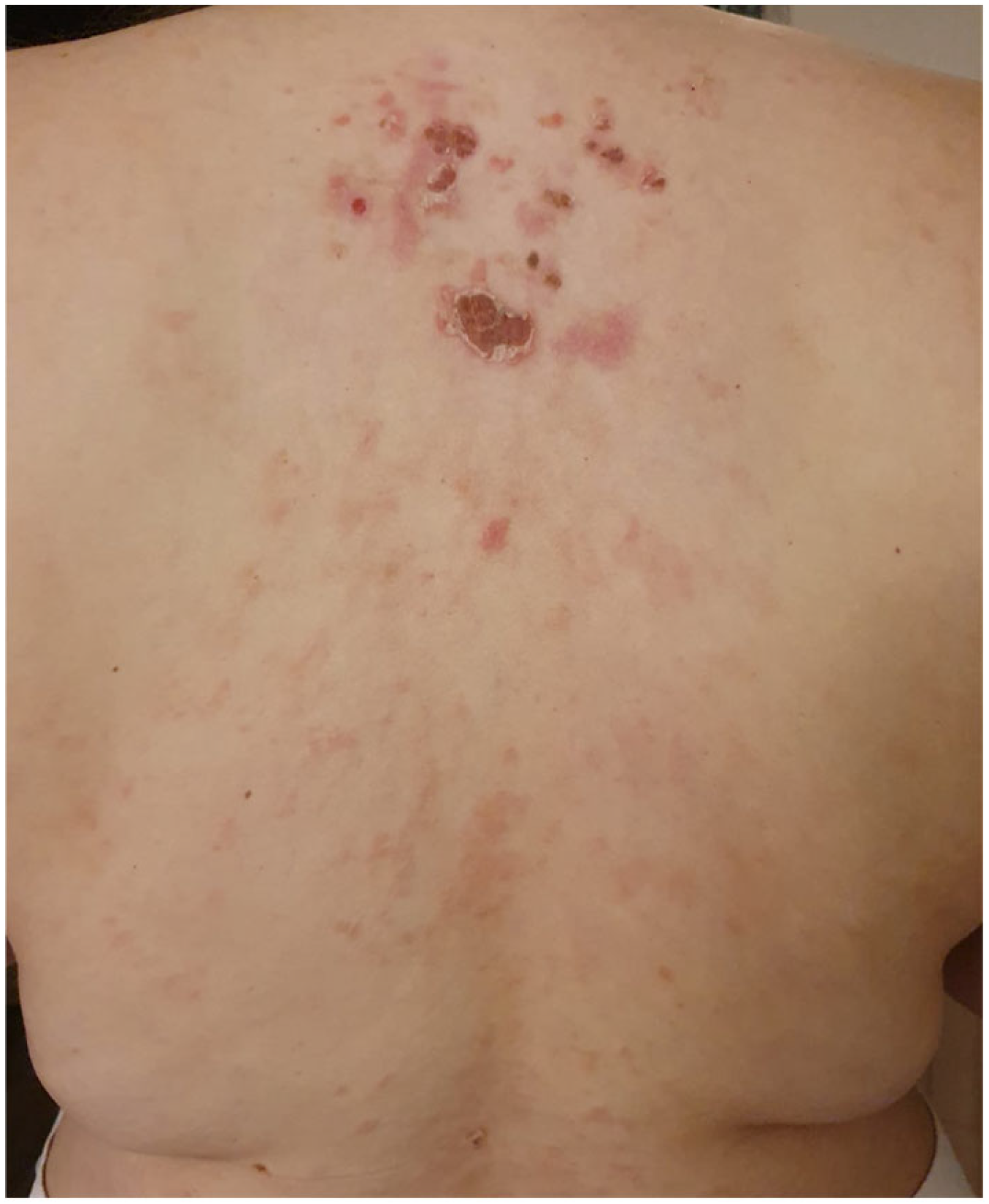
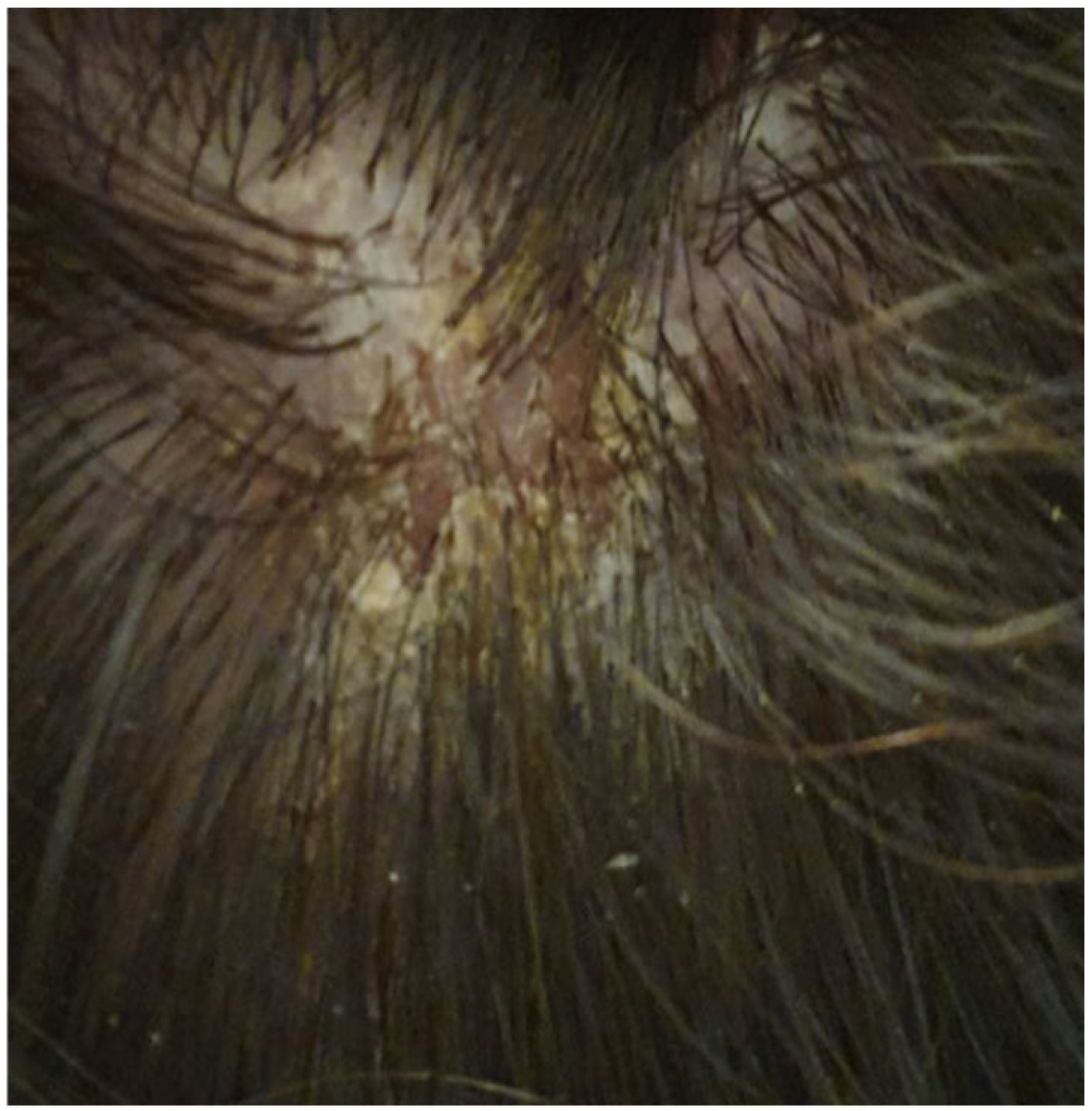
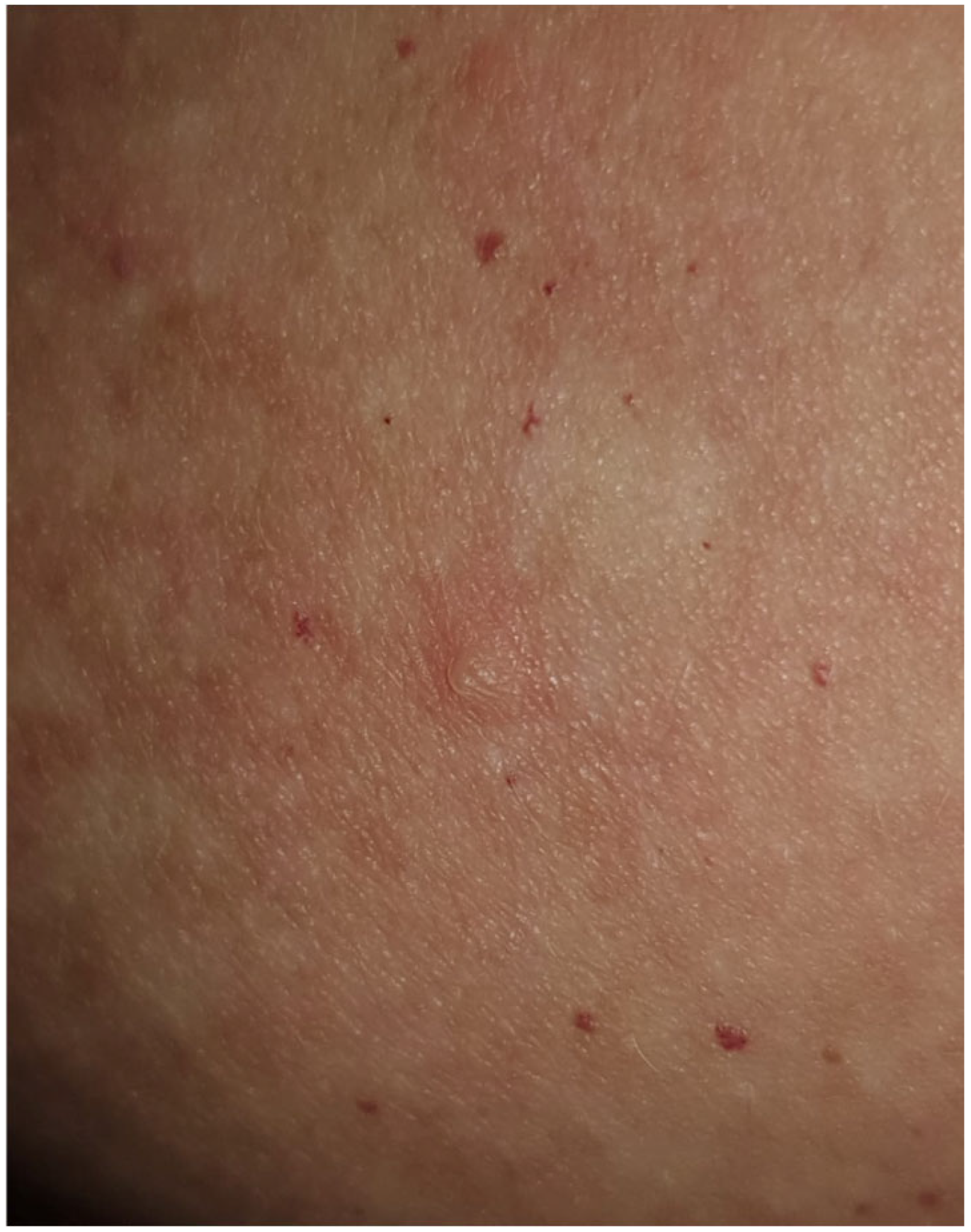
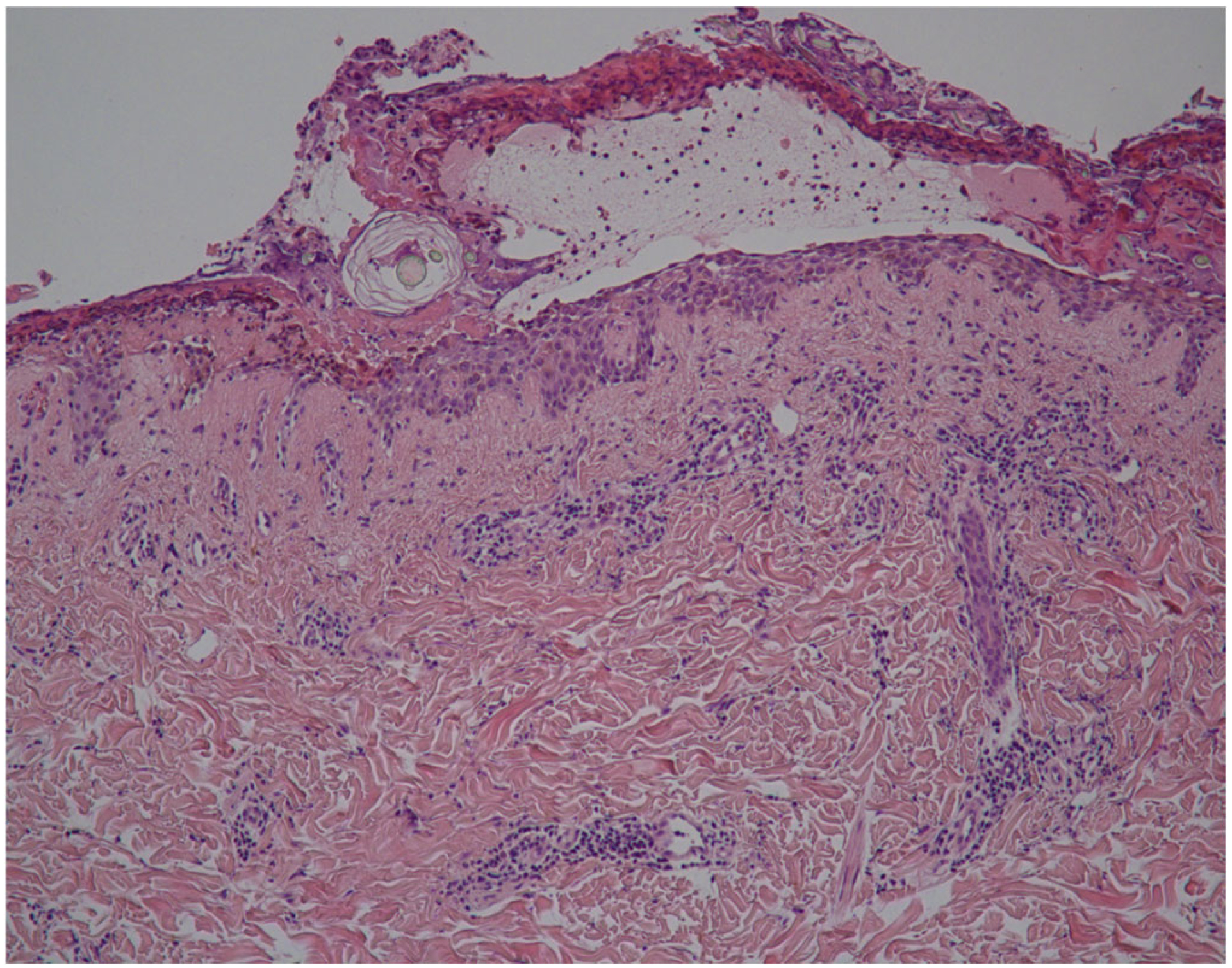
| Authors | Country | Number of Cases | Age | Sex | Vaccine Regimen | Onset Milestones | Concomitant Drugs | Histopathology (DIF) | Treatment | Clinical Response |
|---|---|---|---|---|---|---|---|---|---|---|
| Cojocaru et al. [2] | Romania | 1 | 34 | F | 2 doses of BNT162b2 | 5 days after 2nd dose | None | Acantholysis at the granular layer (epidermal reticular deposition of IgG and C3, with the absence of IgM, IgA, and fibrinogen | Oral corticosteroid | Complete response after 4 weeks |
| Pham et al. [24] | Vietnam | 2 | 53 | F | Mixed-3 doses of BBIBP-CorV followed by 1 dose of AZD1222 | 3 weeks after 4th AZD1222 dose in the mixed regimen | Amlodipine | Acantholysis above the stratum basalis, dermal lymphocyte, and neutrophil infiltration (intraepidermal IgG and C3) | Corticosteroid and rituximab | Almost complete response in 3 weeks |
| 30 | F | 2 doses of mRNA-1273 | 2 months after 2nd mRNA-1273 dose | None | Acantholysis above the stratum basalis (intraepidermal IgG and C3) | Topical and systemic corticosteroid | Almost complete response in 4 weeks | |||
| Corrá et al. [27] | Italy | 2 | 80 | M | 3 doses of BNT162b2 | 17 days after 3rd dose | Amiloride, hydrochlorothiazide, esomeprazole | Subcorneal acantholysis with neutrophilic infiltration within the blister (PT1: negative; PT2: intercellular IgG deposits) | Oral corticosteroid, rituximab, mycophenolate | Probably good clinical response |
| 66 | F | 2 doses of BNT162b2 | 4 weeks after 2nd dose | Rabeprazole, ticlopidine, atorvastatin, amlodipine, hydrochlorothiazide | Subcorneal acantholysis with neutrophilic infiltration within the blister (PT1: negative; PT2: intercellular IgG deposits) | Oral corticosteroid, rituximab, mycophenolate | Probably good clinical response | |||
| Pourani et al. [25] | Iran | 1 | 75 | M | 3 doses of BBIBP-CorV | 2 weeks after 3rd dose | None | Superficial epidermal bullae, mild spongiosis, superficial dermal perivascular inflammation (intraepidermal IgG and C3) | Topical corticosteroid, rituximab | Significant response in 4 weeks |
| Lua et al. [26] | Singapore | 1 | 83 | M | 2 doses of BNT162b2 | 2 days after 2nd dose | N/A | Subacute spongiotic dermatitis (C3 dermal-epidermal junction and intercellular deposition) | Prednisolone | Good clinical response |
| Hali et al. [28] | Morocco | 1 | 50 | F | 2 doses of BNT162b2 | 15 days after 2nd dose | None | Superficial epidermal blistering process, intact basal layer, intraepidermal eosinophils (intracellular IgG and C3) | Oral corticosteroid | Complete response in 3 weeks |
| Yıldırıcı et al. [29] | Turkey | 1 | 65 | M | 2 doses of BNT162b2 (6 weeks apart) | 1 month after 1st dose; 2 weeks after 2nd dose | Nebivolol, valsartan-hydrochlorothiazide | Intraepidermal acantholytic blister, abundant neutrophils, and scarce eosinophils (intercellular IgG and C3) | Oral corticosteroid, azathioprine | Marked response in 2 weeks |
| Rouatbi et al. [30] | Tunisia | 2 | 70 | M | Mixed-2 doses of CoronaVac followed by 1 dose of BNT162b2 | 7 days after BNT162b2 dose | N/A | Intraepidermal acantholytic blister (intercellular IgG and C3 within the epidermis) | Prednisone, Clobetasol | Good clinical response in 3 weeks |
| 48 | M | 1 dose of AZD1222 | 5 days after 1st dose | None | Superficiel cleft within the epidermis with acantholysis (intercellular IgG and C3 within the epidermis) | Prednisone, Clobetasol | Good clinical response in 2 weeks | |||
| Gui et al. [31] | California | 1 | 67 | F | 2 doses of mRNA-1273 | 2 weeks after 2nd dose | N/A | Intraepidermal acantholysis (intercellular IgG and C3) | Prednisone, Clobetasol and Mupirocin | Almost complete response in 8 weeks |
| Alami et al. [18] | Morocco | 1 | 44 | M | 2 doses of BBIBP-CorV | 7 days after 1st dose | None | Acantholysis with superficial intra-epidermal cleavage (intercellular IgG) | Prednisone, Azathioprine | No good clinical response |
| Reis et al. [32] | Portugal | 1 | 35 | F | 2 doses of BNT162b2 (6 weeks apart) | 2 weeks after 2nd dose | None | Acantholitic keratinocytes and detachment of the stratum corneum (intercellular deposition of IgG and C3) | Prednisolone, Clobetasol | Good clinical response in 4 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălăceanu-Gurău, B.; Dumitrascu, A.; Giurcăneanu, C.; Tatar, R.; Gurău, C.-D.; Orzan, O.A. A Comprehensive Review on the Intricate Interplay between COVID-19 Immunization and the New Onset of Pemphigus Foliaceus. Vaccines 2024, 12, 857. https://doi.org/10.3390/vaccines12080857
Bălăceanu-Gurău B, Dumitrascu A, Giurcăneanu C, Tatar R, Gurău C-D, Orzan OA. A Comprehensive Review on the Intricate Interplay between COVID-19 Immunization and the New Onset of Pemphigus Foliaceus. Vaccines. 2024; 12(8):857. https://doi.org/10.3390/vaccines12080857
Chicago/Turabian StyleBălăceanu-Gurău, Beatrice, Adrian Dumitrascu, Călin Giurcăneanu, Raluca Tatar, Cristian-Dorin Gurău, and Olguța Anca Orzan. 2024. "A Comprehensive Review on the Intricate Interplay between COVID-19 Immunization and the New Onset of Pemphigus Foliaceus" Vaccines 12, no. 8: 857. https://doi.org/10.3390/vaccines12080857





