Preclinical Safety Assessment of the EBS-LASV Vaccine Candidate against Lassa Fever Virus
Abstract
1. Introduction
2. Materials and Methods
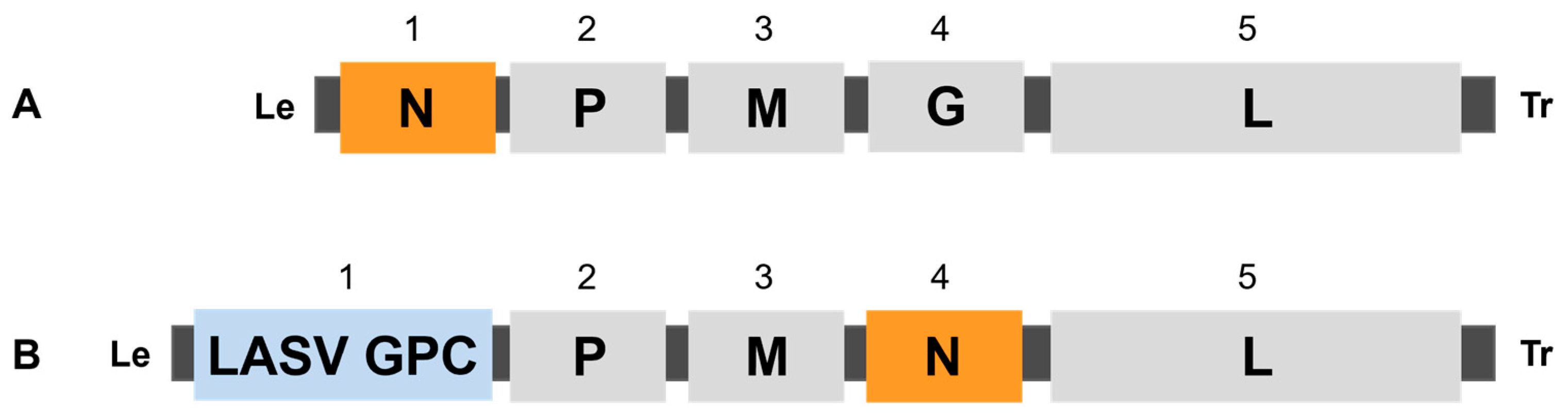
2.1. Generation and Characterization of EBS-LASV Vaccine Candidate
2.2. Test Article and Test Article Preparation
2.3. Animals
2.4. Mouse Neurovirulence Study
2.5. Mouse Biodistribution Study
2.6. Plaque Assay
2.7. GLP Repeat-Dose Rabbit Toxicology Study
2.7.1. Experimental Design and Dose Administration
2.7.2. Clinical and Injection Site Observations
2.7.3. Clinical Pathology
2.7.4. C-Reactive Protein (CRP) Evaluation
2.7.5. Vaccine Shedding
2.7.6. Immunogenicity Evaluation
2.7.7. Statistical Analysis
3. Results
3.1. Mouse Neurovirulence Study
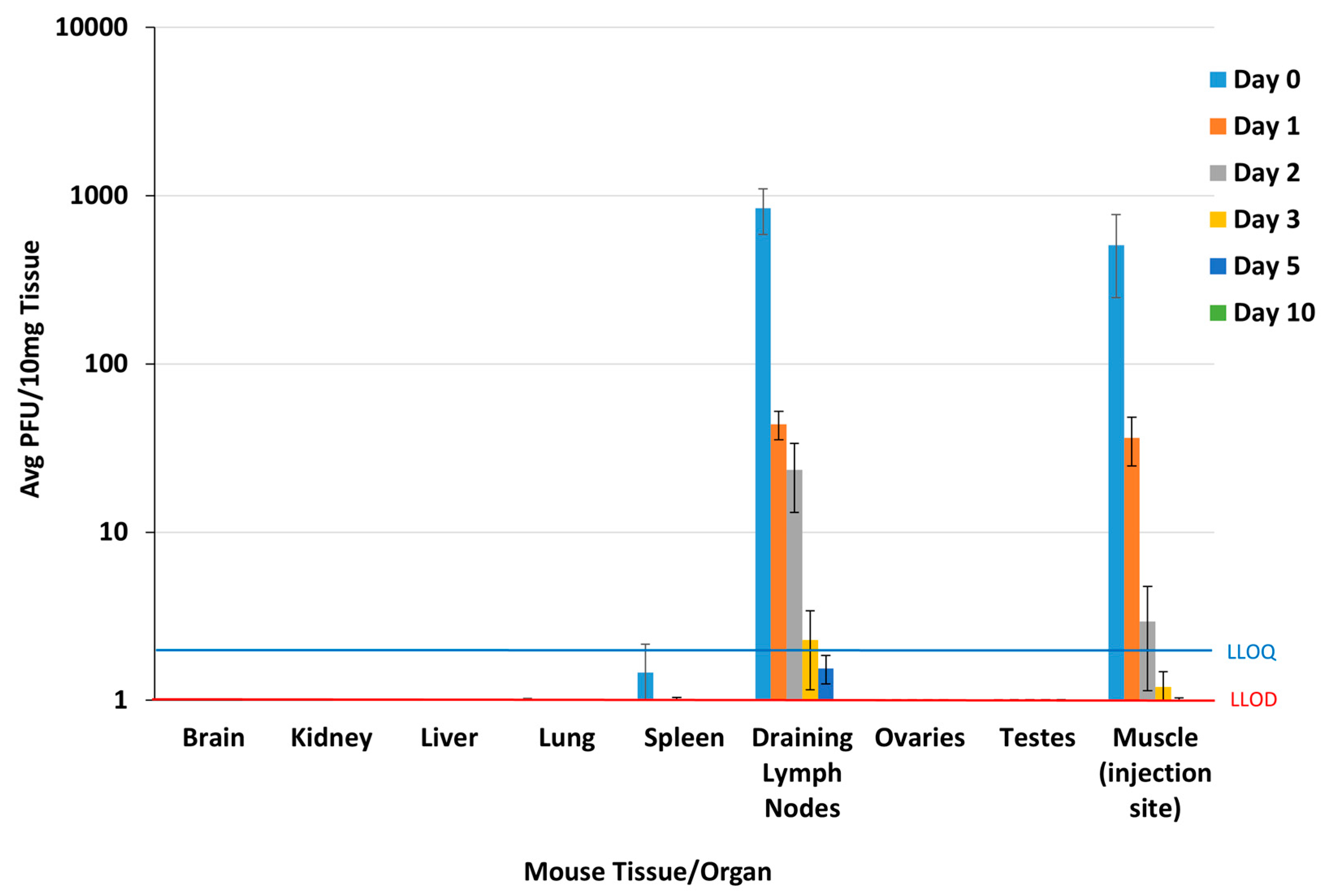
3.2. Mouse Biodistribution Study
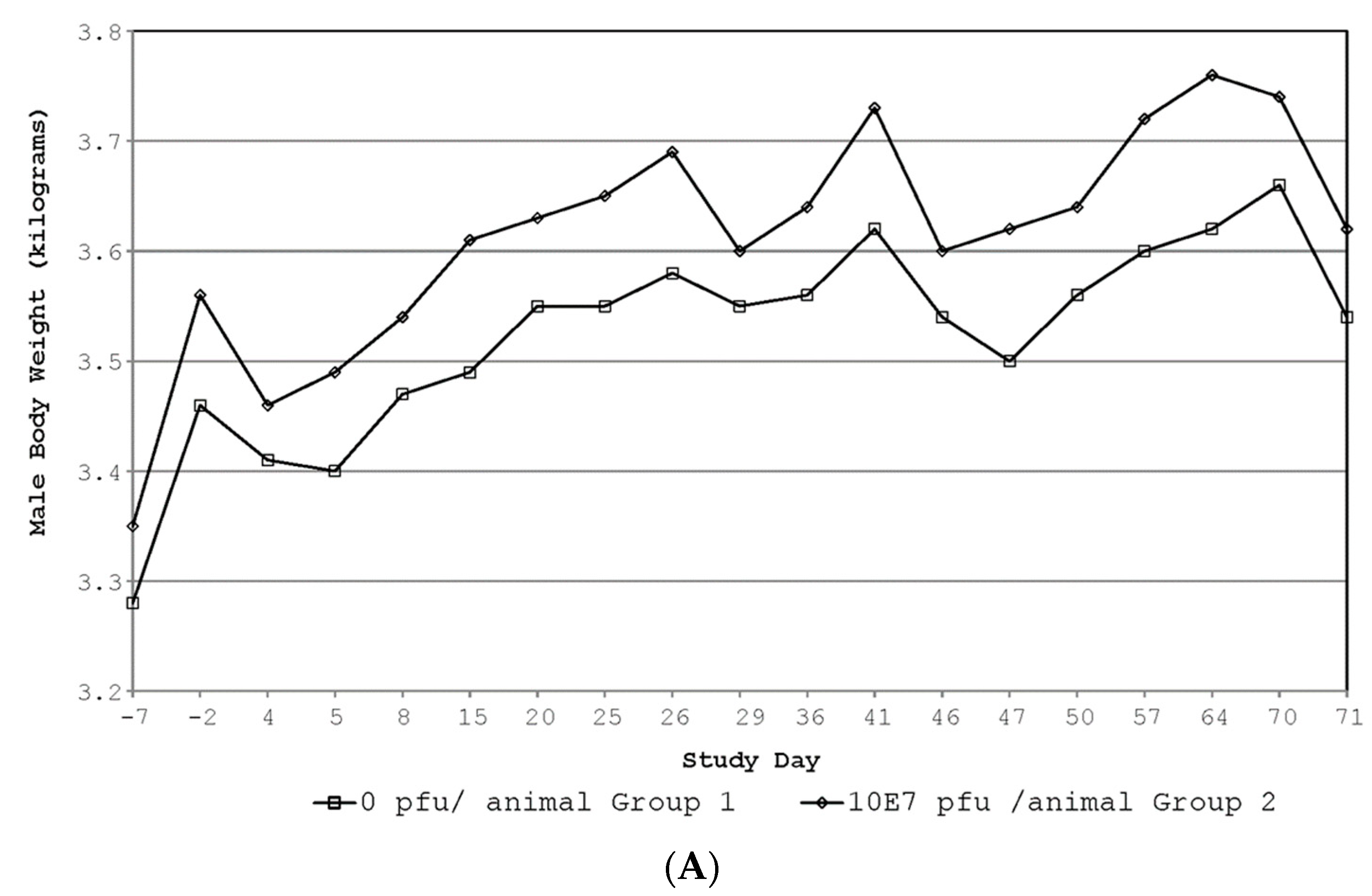
3.3. GLP Rabbit Toxicology Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houlihan, C.; Behrens, R. Lassa fever. BMJ 2017, 358, j2986. [Google Scholar] [CrossRef]
- WHO. Geographic Distribution of Lassa Fever in West African Affected Countries (1969 to 2018): World Health Organization. 2018. Available online: https://www.who.int/health-topics/lassa-fever/#tab=tab_1 (accessed on 20 August 2020).
- CDC. Lassa Fever: Centers for Disease Control and Prevention (United States of America). In Content Source: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP), Viral Special Pathogens Branch (VSPB). 31 January 2019. Available online: https://www.cdc.gov/vhf/lassa/index.html (accessed on 20 August 2020).
- WHO. Lassa Fever World Health Organization. 31 July 2017. Available online: https://www.who.int/health-topics/lassa-fever#tab=tab_1 (accessed on 20 August 2017).
- Price, M.E.; Fisher-Hoch, S.P.; Craven, R.B.; McCormick, J.B. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ 1988, 297, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.K.; Hendry, R.M.; Singh, V.; Rose, J.K.; Seligman, S.J.; Klug, B.; Kochhar, S.; Mac, L.M.; Carbery, B.; Chen, R.T. Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2016, 34, 6597–6609. [Google Scholar] [CrossRef] [PubMed]
- NIAID. Evaluating the Safety of and Immune Response to the VSV-Indiana HIV Vaccine in Healthy, HIV-Uninfected Adults ClinicalTrials.gov: National Institute of Allergy and Infectious Diseases (NIAID). 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT01438606 (accessed on 24 July 2024).
- Clarke, D.K.; Nasar, F.; Lee, M.; Johnson, J.E.; Wright, K.; Calderon, P.; Guo, M.; Natuk, R.; Cooper, D.; Hendry, R.M.; et al. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 2007, 81, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.K.; Xu, R.; Matassov, D.; Latham, T.E.; Ota-Setlik, A.; Gerardi, C.S.; Luckay, A.; Witko, S.E.; Hermida, L.; Higgins, T.; et al. Safety and immunogenicity of a highly attenuated rVSVN4CT1-EBOVGP1 Ebola virus vaccine: A randomised, double-blind, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020, 20, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.D.; Frank, I.; Elizaga, M.L.; Allen, M.; Frahm, N.; Kochar, N.; Li, S.; Edupuganti, S.; Kalams, S.A.; Tomaras, G.D.; et al. First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090). Open Forum Infect. Dis. 2015, 2, ofv082. [Google Scholar] [CrossRef]
- Wolf, J.; Jannat, R.; Dubey, S.; Troth, S.; Onorato, M.T.; Coller, B.-A.; Hanson, M.E.; Simon, J.K. Development of Pandemic Vaccines: ERVEBO Case Study. Vaccines 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- FDA. Package Insert—ERVEBO® 2019. Available online: https://www.fda.gov/media/133748/download (accessed on 24 July 2024).
- EMA. ERVEBO® Ebola Zaire Vaccine, (rVSVΔG-ZEBOV-GP, Live). 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo (accessed on 24 July 2024).
- Ozduman, K.; Wollmann, G.; Ahmadi, S.A.; van den Pol, A.N. Peripheral immunization blocks lethal actions of vesicular stomatitis virus within the brain. J. Virol. 2009, 83, 11540–11549. [Google Scholar] [CrossRef] [PubMed]
- Sur, J.H.; Allende, R.; Doster, A.R. Vesicular stomatitis virus infection and neuropathogenesis in the murine model are associated with apoptosis. Vet. Pathol. 2003, 40, 512–520. [Google Scholar] [CrossRef][Green Version]
- Clarke, D.K.; Nasar, F.; Chong, S.; Johnson, J.E.; Coleman, J.W.; Lee, M.; Witko, S.E.; Kotash, C.S.; Abdullah, R.; Megati, S.; et al. Neurovirulence and immunogenicity of attenuated recombinant vesicular stomatitis viruses in nonhuman primates. J. Virol. 2014, 88, 6690–6701. [Google Scholar] [CrossRef]
- Wertz, G.W.; Perepelitsa, V.P.; Ball, L.A. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc. Natl. Acad. Sci. USA 1998, 95, 3501–3506. [Google Scholar] [CrossRef]
- Monath, T.P.; Nichols, R.; Tussey, L.; Scappaticci, K.; Pullano, T.G.; Whiteman, M.D.; Vasilakis, N.; Rossi, S.L.; Campos, R.K.; Azar, S.R.; et al. Recombinant vesicular stomatitis vaccine against Nipah virus has a favorable safety profile: Model for assessment of live vaccines with neurotropic potential. PLoS Pathog. 2002, 18, e1010658. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Wright, K.J.; Calderon, P.C.; Guo, M.; Nasar, F.; Johnson, J.E.; Coleman, J.W.; Lee, M.; Kotash, C.; Yurgelonis, I.; et al. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J. Virol. 2008, 82, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Tang-Huau, T.; Feldmann, H.; Rosenke, K. Animal models for Lassa virus infection. Curr. Opin. Virol. 2019, 37, 112–117. [Google Scholar] [CrossRef]
- Flanagan, E.B.; Schoeb, T.R.; Wertz, G.W. Vesicular stomatitis viruses with rearranged genomes have altered invasiveness and neurolapathogenesis in mice. J. Virol. 2003, 77, 5740–5748. [Google Scholar] [CrossRef] [PubMed]
- Forger, J.M., 3rd; Bronson, R.T.; Huang, A.S.; Reiss, C.S. Murine infection by vesicular stomatitis virus: Initial characterization of the H-2d system. J. Virol. 1991, 65, 4950–4958. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B.; Olitsky, P.K. Influence of Host Factors on Neuroinvasiveness of Vesicular Stomatitis Virus: Iii. Effect of Age and Pathway of Infection on the Character and Localization of Lesions in the Central Nervous System. J. Exp. Med. 1938, 67, 201–228. [Google Scholar] [CrossRef]
- Cross, R.W.; Xu, R.; Matassov, D.; Hamm, S.; Latham, T.E.; Gerardi, C.S.; Nowak, R.M.; Geisbert, J.B.; Ota-Setlik, A.; Agans, K.N.; et al. Quadrivalent VesiculoVax vaccine protects nonhuman primates from viral-induced hemorrhagic fever and death. J. Clin. Investig. 2020, 130, 539–551. [Google Scholar] [CrossRef]
- Auperin, D.D.; McCormick, J.B. Nucleotide sequence of the Lassa virus (Josiah strain) S genome RNA and amino acid sequence comparison of the N and GPC proteins to other arenaviruses. Virology 1989, 168, 421–425. [Google Scholar] [CrossRef]
- Witko, S.E.; Kotash, C.S.; Nowak, R.M.; Johnson, J.E.; Boutilier, L.A.; Melville, K.J.; Heron, S.G.; Clarke, D.K.; Abramovitz, A.S.; Hendry, R.M.; et al. An efficient helper-virus-free method for rescue of recombinant paramyxoviruses and rhadoviruses from a cell line suitable for vaccine development. J. Virol. Methods 2006, 135, 91–101. [Google Scholar] [CrossRef]
- Simon, I.D.; Publicover, J.; Rose, J.K. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: Vector spread correlates with induction of immune responses and persistence of genomic RNA. J. Virol. 2007, 81, 2078–2082. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use (CHMP). Note for Guidance on Repeated Dose Toxicity; CPMP/SWP/1042/99corr; Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2010. [Google Scholar]
- ICH Harmonised Tripartite Guideline M3 (R2). Nonclinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals and Marketing Authorization for Pharmaceuticals: FDA Rockville Maryland January 2010. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m3r2-nonclinical-safety-studies-conduct-human-clinical-trials-and-marketing-authorization (accessed on 24 July 2024).
- National Research Council. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. (In English) [Google Scholar]
- Canadian Council on Animal Care (CCAC). Guide to the Care and Use of Experimental Animals, 2nd ed.; CCAC: Ottawa, ON, Canada, 2020; Volume 1. [Google Scholar]
- Green, M.D.; Hussain Al-Humadi, N. Preclinical Toxicology of Vaccines. In A Comprehensive Guide to Toxicology in Preclinical Drug Development; Elsevier: Amsterdam, The Netherlands, 2013; pp. 619–645. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7161391/ (accessed on 24 July 2024).
- Johnson, J.E.; Coleman, J.W.; Kalyan, N.K.; Calderon, P.; Wright, K.J.; Obregon, J.; Ogin-Wilson, E.; Natuk, R.J.; Clarke, D.K.; Udem, S.A.; et al. In vivo biodistribution of a highly attenuated recombinant vesicular stomatitis virus expressing HIV-1 Gag following intramuscular, intranasal, or intravenous inoculation. Vaccine 2009, 27, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Witko, S.E.; Johnson, J.E.; Kalyan, N.K.; Felber, B.K.; Pavlakis, G.N.; Sidhu, M.K.; Hendry, R.M.; Udem, S.A.; Parks, C.L. Refined methods for propagating vesicular stomatitis virus vectors that are defective for G protein expression. J. Virol. Methods 2010, 164, 43–50. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Nonclinical Evaluation of Vaccines; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2014; Available online: https://www.who.int/publications/m/item/TRS-987-annex2 (accessed on 24 July 2024).
- Pan African Clinical Trials Registry. A Study to Assess a New Lassa Virus Vaccine in Healthy Volunteers. Available online: https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=14618 (accessed on 17 August 2021).


| Group | Test Material | Dose Route | Dose Volume (mL) 1 | Dosing Days | Number of Rabbits 2 | |||
|---|---|---|---|---|---|---|---|---|
| Main Study | Recovery Study | |||||||
| Males | Females | Males | Females | |||||
| 1 | 0.9% sodium chloride for injection, USP | IM | 0.5 | 1, 22, 43 | 5 | 5 | 5 | 5 |
| 2 | EBS-LASV (5.3 × 107 PFU 3/rabbit) | IM | 0.5 | 1, 22, 43 | 5 | 5 | 5 | 5 |
| Inoculum | IC Dose (PFU) | No. Surviving 24 h | No. Surviving 14 Days | % Survival |
|---|---|---|---|---|
| EBS-LASV | 1 × 107 | 10 | 10 | 100 |
| 1 × 106 | 10 | 10 | 100 | |
| 1 × 105 | 10 | 10 | 100 | |
| 1 × 104 | 10 | 10 | 100 | |
| 1 × 103 | 10 | 10 | 100 | |
| rVSV-HIVGag5 | 1 × 102 | 10 | 0 | 0 |
| PBS | N/A | 10 | 10 | 100 |
| Timepoint (Study Day) | N Per Sex | Body Temp (°C) | |||
|---|---|---|---|---|---|
| Saline Control | EBS-LASV | ||||
| Male | Female | Male | Female | ||
| 1 (PR) | 10 | 38.91 ± 0.22 | 39.02 ± 0.41 | 38.83 ± 0.27 | 38.95 ± 0.16 |
| 1 (2–4 HR) | 10 | 38.73 ± 0.29 | 39.02 ± 0.20 | 38.98 ± 0.28 | 39.16 ± 0.18 |
| 22 (2–4 HR) | 10 | 39.00 ± 0.35 | 39.05 ± 0.36 | 39.13 ± 0.14 | 39.51 ± 0.30 a |
| 43 (2–4 HR) | 10 | 38.96 ± 0.37 | 39.28 ± 0.22 | 39.24 ± 0.32 | 39.31 ± 0.21 |
| 46 | 5 | 38.38 ± 0.33 | 38.38 ± 0.20 | 38.56 ± 0.40 | 38.70 ± 0.23 |
| 71 | 5 | 37.98 ± 0.43 | 38.68 ± 0.15 | 38.34 ± 0.34 | 38.86 ± 0.26 |
| Timepoint (Study Day) | N Per Sex | Saline Control | EBS-LASV | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Fibrinogen (mg/dL) | |||||
| −7 | 10 | 308.2 ± 26.7 | 358.2 ± 70.2 | 365.7 ± 80.3 b | 287.5 ± 36.4 b |
| 1 d | 10 | 291.3 ± 29.8 | 307.4 ± 37.6 | 325.3 ± 51.0 | 280.1 ± 58.4 |
| 3 | 10 | 308.0 ± 28.8 | 330.8 ± 66.3 | 402.4 ± 52.3 a | 332.7 ± 61.7 |
| 8 | 10 | 299.1 ± 30.6 | 329.3 ± 61.6 | 342.0 ± 54.2 | 302.3 ± 37.5 |
| 22 d | 10 | 234.4 ± 46.7 | 263.2 ± 23.3 | 268.3 ± 20.7 | 242.3 ± 24.8 |
| 24 | 10 | 265.9 ± 18.8 | 263.0 ± 37.1 | 344.1 ± 42.1 a | 262.1 ± 29.2 |
| 29 | 10 | 283.5 ± 50.6 | 272.7 ± 42.4 | 309.9 ± 26.8 | 229.8 ± 25.7 b |
| 43 d | 10 | 287.3 ± 37.5 | 247.8 ± 16.8 | 309.4 ± 37.5 | 214.9 ± 23.5 a |
| 45 | 10 | 290.7 ± 40.7 | 262.0 ± 14.9 | 345.9 ± 32.7 a | 262.1 ± 22.3 |
| 50 | 5 | 284.4 ± 28.1 | 253.3 ± 16.9 | 334.6 ± 14.8 a | 274.3 ± 13.0 |
| 71 | 5 | 281.6 ± 47.6 | 257.0 ± 23.9 | 284.0 ± 26.7 | 213.8 ± 20.7 b |
| CRP (µg/mL) | |||||
| −7 | 10 | 2.47 ± 1.38 | 17.08 ± 23.38 | 3.81 ± 2.54 | 5.52 ± 3.98 |
| 2 | 10 | 4.73 ± 5.02 | 33.48 ± 30.96 | 60.07 ± 29.80 c | 89.27± 41.50 a |
| 3 | 10 | 2.67 ± 2.03 | 18.96 ± 22.65 | 28.90 ± 15.90 c | 31.18 ± 19.72 |
| 4 | 10 | 4.11 ± 1.73 | 33.46 ± 41.32 | 5.77 ± 3.16 | 7.28 ± 3.10 |
| 23 | 10 | 2.14 ± 1.13 | 10.71 ± 12.28 | 27.72 ± 20.38 c | 28.31 ± 16.95 b |
| 25 | 10 | 31.05 ± 78.84 | 6.98 ± 4.59 | 3.67 ± 2.89 | 3.19 ± 1.44 b |
| 44 | 10 | 1.98 ± 1.04 | 6.47 ± 4.43 | 14.12 ± 8.94 c | 22.47 ± 20.24 c |
| 46 | 10 | 2.78 ± 1.16 | 3.42 ± 1.83 | 2.71 ± 1.04 | 3.23 ± 1.80 |
| 71 | 5 | 2.06 ± 2.34 | 4.00 ± 3.47 | 0.98 ± 0.58 | 2.03 ± 1.24 |
| Organ | Mean Absolute Organ Weights (g) | |||
|---|---|---|---|---|
| Saline Control | EBS-LASV | |||
| Male | Female | Male | Female | |
| Study Day 46 (Main Study Group) | ||||
| Mean body weight (kg) | 3.46 ± 0.21 | 3.22 ± 0.19 | 3.52 ± 0.18 | 3.80 ± 0.19 b |
| brain | 9.74 ± 0.38 | 9.69 ± 0.29 | 9.69 ± 0.37 | 10.04 ± 0.43 e |
| epididymis | 2.32 ± 0.45 | N/A | 2.56 ± 0.24 | N/A |
| adrenal | 0.40 ± 0.09 | 0.33 ± 0.10 | 0.45 ± 0.13 | 0.30 ± 0.06 |
| pituitary | 0.031 ± 0.005 | 0.029 ± 0.004 | 0.027 ± 0.003 | 0.045 ± 0.004 b,d |
| prostate | 0.62 ± 0.09 | N/A | 0.65 ± 0.10 | N/A |
| thyroid | 0.24 ± 0.05 | 0.25 ± 0.04 | 0.29 ± 0.02 | 0.34± 0.04 b |
| heart | 7.28 ± 0.63 | 6.01 ± 0.43 | 7.76 ± 0.74 | 6.48 ± 0.26 |
| kidney | 17.36 ± 1.55 | 13.90 ± 1.43 | 16.49 ± 1.20 | 16.36 ± 0.97 c |
| liver | 77.24 ± 8.87 | 55.16 ± 10.60 | 73.40 ± 6.50 | 71.52 ± 4.83 c |
| lung | 9.95 ± 0.72 | 10.23 ± 1.90 | 11.35 ± 3.26 | 10.73 ± 1.19 |
| ovary | N/A | 0.46 ± 0.25 | N/A | 0.29 ± 0.08 |
| spleen | 1.03 ± 0.07 | 1.48 ± 0.44 | 1.40 ± 0.27 a,e | 1.76 ± 0.46 |
| testis | 5.86 ± 1.02 | N/A | 6.23 ± 0.54 | N/A |
| thymus | 2.92 ± 0.70 | 2.89 ± 0.74 | 3.54 ± 0.55 | 3.68 ± 1.38 |
| uterus | N/A | 6.87 ± 2.64 | N/A | 7.49 ± 3.53 |
| Study Day 71 (Recovery Study Group) | ||||
| Mean body weight (kg) | 3.54 ± 0.17 | 3.54 ± 0.44 | 3.62 ± 0.11 | 4.14 ± 0.32 c |
| brain | 10.09 ± 0.48 | 9.76 ± 0.85 | 10.73 ± 0.28 c | 10.57 ± 0.47 |
| epididymis | 2.73 ± 0.39 | N/A | 2.98 ± 0.63 | N/A |
| adrenal | 0.45 ± 0.07 | 0.33 ± 0.04 | 0.47 ± 0.06 | 0.33 ± 0.05 |
| pituitary | 0.030 ± 0.000 | 0.038 ± 0.001 | 0.030 ± 0.002 | 0.041 ± 0.003 |
| prostate | 0.78 ± 0.19 | N/A | 0.70 ± 0.15 | N/A |
| thyroid | 0.33 ± 0.07 | 0.27 ± 0.04 | 0.27 ± 0.05 | 0.36 ± 0.06 c |
| heart | 7.74 ± 1.01 | 6.95 ± 0.61 | 7.96 ± 0.30 | 7.58 ± 0.44 |
| kidney | 15.46 ± 1.56 | 15.95 ± 2.94 | 16.91 ± 1.26 | 17.28 ± 2.01 |
| liver | 76.24 ± 23.96 | 63.08 ± 8.14 | 67.18 ± 4.45 | 71.99 ± 7.24 |
| lung | 12.27 ± 4.16 | 10.92 ± 1.10 | 10.63 ± 0.40 | 12.10 ± 2.20 |
| ovary | N/A | 0.25 ± 0.08 | N/A | 0.30 ± 0.08 |
| spleen | 1.21 ± 0.33 | 1.66 ± 0.62 | 1.47 ± 0.25 | 2.14 ± 0.66 |
| testis | 5.89 ± 0.46 | N/A | 6.38 ± 1.38 | N/A |
| thymus | 3.15 ± 0.86 | 3.53 ± 0.82 | 3.54 ± 0.81 | 6.08 ± 1.39 b,e |
| uterus | N/A | 6.21 ± 1.46 | N/A | 8.52 ± 2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matassov, D.; DeWald, L.E.; Hamm, S.; Nowak, R.M.; Gerardi, C.S.; Latham, T.E.; Xu, R.; Luckay, A.; Chen, T.; Tremblay, M.; et al. Preclinical Safety Assessment of the EBS-LASV Vaccine Candidate against Lassa Fever Virus. Vaccines 2024, 12, 858. https://doi.org/10.3390/vaccines12080858
Matassov D, DeWald LE, Hamm S, Nowak RM, Gerardi CS, Latham TE, Xu R, Luckay A, Chen T, Tremblay M, et al. Preclinical Safety Assessment of the EBS-LASV Vaccine Candidate against Lassa Fever Virus. Vaccines. 2024; 12(8):858. https://doi.org/10.3390/vaccines12080858
Chicago/Turabian StyleMatassov, Demetrius, Lisa Evans DeWald, Stefan Hamm, Rebecca M. Nowak, Cheryl S. Gerardi, Theresa E. Latham, Rong Xu, Amara Luckay, Tracy Chen, Marc Tremblay, and et al. 2024. "Preclinical Safety Assessment of the EBS-LASV Vaccine Candidate against Lassa Fever Virus" Vaccines 12, no. 8: 858. https://doi.org/10.3390/vaccines12080858
APA StyleMatassov, D., DeWald, L. E., Hamm, S., Nowak, R. M., Gerardi, C. S., Latham, T. E., Xu, R., Luckay, A., Chen, T., Tremblay, M., Shearer, J., Wynn, M., Eldridge, J. H., Warfield, K., & Spurgers, K. (2024). Preclinical Safety Assessment of the EBS-LASV Vaccine Candidate against Lassa Fever Virus. Vaccines, 12(8), 858. https://doi.org/10.3390/vaccines12080858







