Intestinal Microbiota and Its Effect on Vaccine-Induced Immune Amplification and Tolerance
Abstract
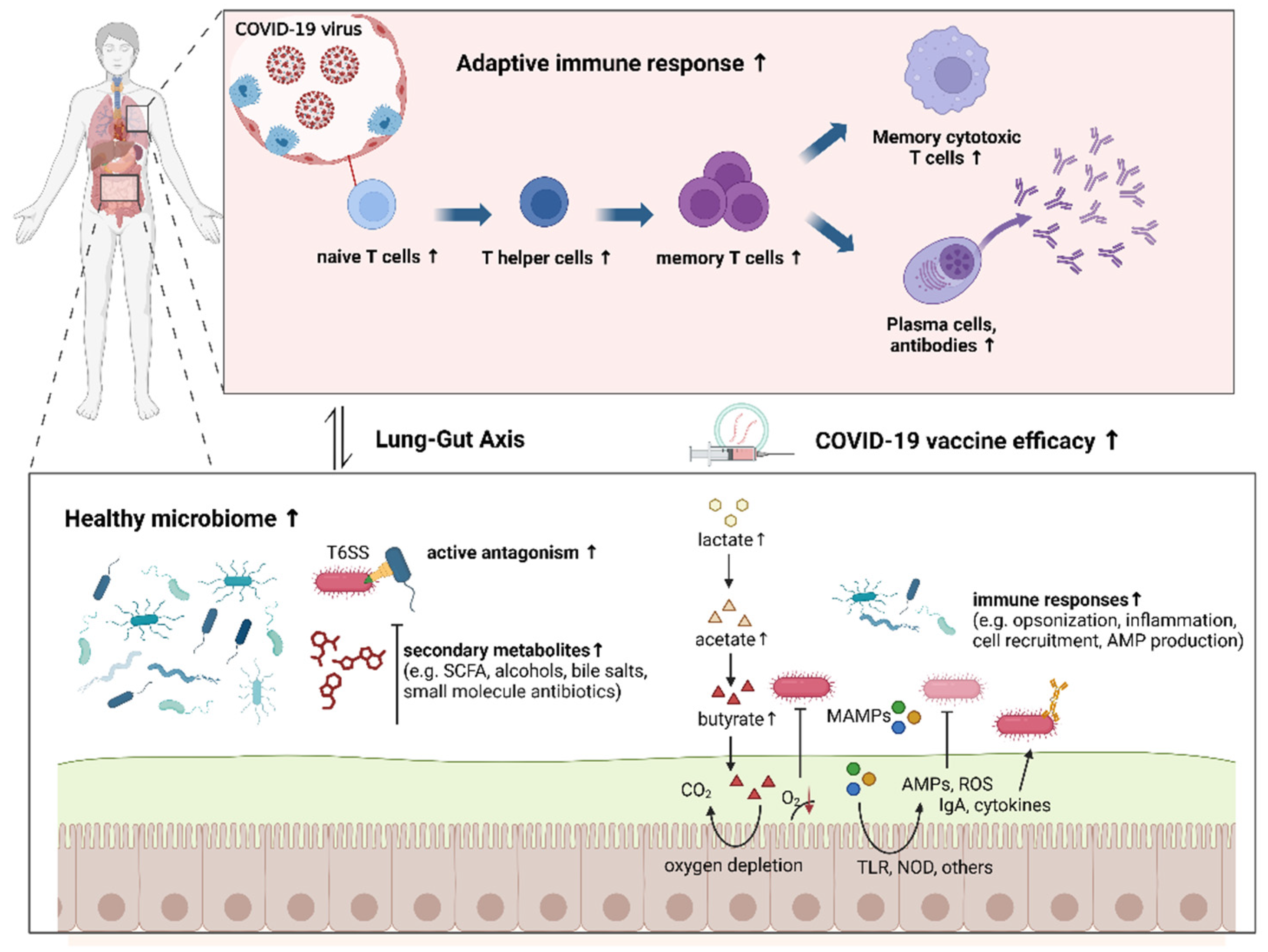
:1. Introduction
2. The Influence of Intestinal Microbiota on the Immune System and Host Immune Responses
2.1. Intestinal Microbiota and Its Potential Relationship with Immune Responses
2.2. Intestinal Microbiota Mediate Immune Tolerance and Diseases
3. Intestinal Microbiota and Vaccines
3.1. Oral Vaccines and Intestinal Microbiota
3.2. Parenteral Vaccines and Intestinal Microbiota
4. Intestinal Microbiota to Vaccine Effect: Immune Amplification or Tolerance
4.1. Amplification of Vaccine Efficacy by Intestinal Microbiota
4.2. Intestinal Microbiota and Immune Tolerance Response to Vaccine
5. Leveraging the Intestinal Microbiome for Vaccine Design
5.1. Fundamental Principles of Vaccine Sequence Design
5.2. Utilizing Intestinal Microbiota Features in the Vaccine Design
6. Potential of the Vaccine Utilizing Microbiota-Derived Outer Membrane Vesicles
7. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kayser, V.; Ramzan, I. Vaccines and vaccination: History and emerging issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Franza, L.; Massaro, M.G.; Borriello, R.; De Vito, F.; Gambassi, G. The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines 2020, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, U.; Garner-Spitzer, E.; Wagner, A. Primary vaccine failure to routine vaccines: Why and what to do? Hum. Vaccines Immunother. 2016, 12, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Kubba, A.K.; Taylor, P.; Graneek, B.; Strobel, S. Non-responders to hepatitis B vaccination: A review. Commun. Dis. Public Health 2003, 6, 106–112. [Google Scholar] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Tomalka, J.A.; Suthar, M.S.; Deeks, S.G.; Sekaly, R.P. Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses. Nat. Immunol. 2022, 23, 360–370. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, C.D.; Noz, M.P.; Joosten, L.A.; Netea, M.G.; Riksen, N.P.; Keating, S.T. Epigenetics and Trained Immunity. Antioxid. Redox Signal. 2018, 29, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. Gut microbiome, Vitamin D, ACE2 interactions are critical factors in immune-senescence and inflammaging: Key for vaccine response and severity of COVID-19 infection. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 13–26. [Google Scholar] [CrossRef]
- Ng, H.-Y.; Liao, Y.; Zhang, R.; Chan, K.-H.; To, W.-P.; Hui, C.-H.; Seto, W.-K.; Leung, W.K.; Hung, I.F.N.; Lam, T.T.Y.; et al. The Predictive Value of Gut Microbiota Composition for Sustained Immunogenicity following Two Doses of CoronaVac. Int. J. Mol. Sci. 2024, 25, 2583. [Google Scholar] [CrossRef]
- Oh, S.; Seo, H. Dietary intervention with functional foods modulating gut microbiota for improving the efficacy of COVID-19 vaccines. Heliyon 2023, 9, e15668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Röwekamp, I.; Maschirow, L.; Rabes, A.; Vernengo, F.F.; Hamann, L.; Heinz, G.A.; Mashreghi, M.-F.; Caesar, S.; Milek, M.; Fonseca, A.C.F.; et al. IL-33 controls IL-22-dependent antibacterial defense by modulating the microbiota. Proc. Natl. Acad. Sci. USA 2024, 121, e2310864121. [Google Scholar] [CrossRef]
- Zheng, H.T.; Zhao, Q.Y.; Ding, Y.; Ma, S.X.; Chen, W.X.; Qiu, J.L.; Li, X.F.; Sun, X.X.; Zhang, Y.J.; Yuan, B.; et al. Investigation of the relationships among respiratory syncytial virus infection, T cell immune response and intestinal flora. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Shyanti, R.K.; Greggs, J.; Malik, S.; Mishra, M. Gut dysbiosis impacts the immune system and promotes prostate cancer. Immunol. Lett. 2024, 268, 106883. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Mohanty, S.; Sharma, S.; Tripathi, P. Possible role of gut microbes and host’s immune response in gut–lung homeostasis. Front. Immunol. 2022, 13, 954339. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, Y.; You, J. Antigen transfer and its effect on vaccine-induced immune amplification and tolerance. Theranostics 2022, 12, 5888–5913. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-J.H.; Zegarra-Ruiz, D.F.; Diehl, G.E. Intestinal Microbes in Autoimmune and Inflammatory Disease. Front. Immunol. 2020, 11, 597966. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Rafizadeh, D.L.; Wang, L.; Bowlus, C.L.; Kumar, N.; Mishra, J.; Timilsina, S.; Ridgway, W.M.; Gershwin, M.E.; et al. The gut ecosystem and immune tolerance. J. Autoimmun. 2023, 141, 103114. [Google Scholar] [CrossRef]
- Lyu, M.; Suzuki, H.; Kang, L.; Gaspal, F.; Zhou, W.; Goc, J.; Zhou, L.; Zhou, J.; Zhang, W.; Artis, D.; et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 2022, 610, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Singh, S.; Singh, R.P.; Agrewala, J.N.; Kumar, R. Age-mediated gut microbiota dysbiosis promotes the loss of dendritic cells tolerance. Aging Cell 2023, 22, e13838. [Google Scholar] [CrossRef] [PubMed]
- Akagbosu, B.; Tayyebi, Z.; Shibu, G.; Paucar Iza, Y.A.; Deep, D.; Parisotto, Y.F.; Brown, C.C. Novel antigen-presenting cell imparts T(reg)-dependent tolerance to gut microbiota. Nature 2022, 610, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Sanidad, K.Z.; Rager, S.L.; Carrow, H.C.; Ananthanarayanan, A.; Callaghan, R.; Hart, L.R.; Li, T.; Ravisankar, P.; Brown, J.A.; Amir, M.; et al. Gut bacteria–derived serotonin promotes immune tolerance in early life. Sci. Immunol. 2024, 9, eadj4775. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Y.; Chen, Y.; Chen, D. The Role of the Microbiota in Graves’ Disease and Graves’ Orbitopathy. Front. Cell. Infect. Microbiol. 2021, 11, 739707. [Google Scholar] [CrossRef]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Canani, R.B. Specific gut microbiome signatures and the associated pro-inflamatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef]
- Méndez, C.S.; Bueno, S.M.; Kalergis, A.M. Contribution of Gut Microbiota to Immune Tolerance in Infants. J. Immunol. Res. 2021, 2021, 7823316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, Y.; Yang, Y.; Ren, L.; Li, J.; Wang, Y.; Shen, X.; He, F. Maternal gestational Bifidobacterium bifidum TMC3115 treatment shapes construction of offspring gut microbiota and development of immune system and induces immune tolerance to food allergen. Front. Cell. Infect. Microbiol. 2022, 12, 1045109. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Zhao, M.; Li, P.; Gao, C.; Fan, X.; Cai, G.; Lu, Q.; Chen, X. Fecal microbiota transplantation for the management of autoimmune diseases: Potential mechanisms and challenges. J. Autoimmun. 2023, 141, 103109. [Google Scholar] [CrossRef]
- Köhler, N.; Zeiser, R. Intestinal Microbiota Influence Immune Tolerance Post Allogeneic Hematopoietic Cell Transplantation and Intestinal GVHD. Front. Immunol. 2019, 9, 3179. [Google Scholar] [CrossRef]
- Chac, D.; Bhuiyan, T.R.; Saha, A.; Alam, M.M.; Salma, U.; Jahan, N.; Chowdhury, F.; Khan, A.I.; Ryan, E.T.; LaRocque, R.; et al. Gut Microbiota and Development of Vibrio cholerae-Specific Long-Term Memory B Cells in Adults after Whole-Cell Killed Oral Cholera Vaccine. Infect. Immun. 2021, 89, e0021721. [Google Scholar] [CrossRef]
- Magwira, C.A.; Taylor, M.B. Composition of gut microbiota and its influence on the immunogenicity of oral rotavirus vaccines. Vaccine 2018, 36, 3427–3433. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Hand, T.W. Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin. Sci. 2018, 132, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Nojima, M.; Hosono, O.; Tanaka, H.; Kimura, Y.; Satoh, T.; Kiyono, H. Oral MucoRice-CTB vaccine for safety and microbiota-dependent immunogenicity in humans: A phase 1 randomised trial. Lancet Microbe. 2021, 2, e429–e440. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Medeiros, M.M.; Ingham, A.C.; Nanque, L.M.; Correia, C.; Stegger, M.; Andersen, P.S.; Fisker, A.B.; Benn, C.S.; Lanaspa, M.; Silveira, H.; et al. Oral polio revaccination is associated with changes in gut and upper respiratory microbiomes of infants. Front. Microbiol. 2022, 13, 1016220. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.; Soliman, S.S.M.; Retuerto, M.; Quijano, J.C.; Orr, C.; Ghannoum, M.; Kandeel, F.; Husseiny, M.I. Changes in the gut microbiota of NOD mice in response to an oral Salmonella-based vaccine against type 1 diabetes. PLoS ONE 2023, 18, e0285905. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, K.; Kunisawa, J. Impact of the intestinal environment on the immune responses to vaccination. Vaccine 2020, 38, 6959–6965. [Google Scholar] [CrossRef]
- Arnold, I.C.; Hutchings, C.; Kondova, I.; Hey, A.; Powrie, F.; Beverley, P.; Tchilian, E. Helicobacter hepaticus infection in BALB/c mice abolishes subunit-vaccine-induced protection against M. tuberculosis. Vaccine 2015, 33, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.A.; Kang, A.; D’agostino, M.R.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut–lung axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef]
- Ng, H.Y.; Leung, W.K.; Cheung, K.S. Association between Gut Microbiota and SARS-CoV-2 Infection and Vaccine Immunogenicity. Microorganisms 2023, 11, 452. [Google Scholar] [CrossRef]
- Ray, S.; Narayanan, A.; Vesterbacka, J.; Blennow, O.; Chen, P.; Gao, Y.; Gabarrini, G.; Ljunggren, H.-G.; Buggert, M.; Manoharan, L.; et al. Impact of the gut microbiome on immunological responses to COVID-19 vaccination in healthy controls and people living with HIV. NPJ Biofilms Microbiomes 2023, 9, 104. [Google Scholar] [CrossRef]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Leung, J.S.M. Interaction between gut microbiota and COVID-19 and its vaccines. World J. Gastroenterol. 2022, 28, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, A.; Guerrini, M.M.; Minato, N.; Fagarasan, S. Microbiota—An amplifier of autoimmunity. Curr. Opin. Immunol. 2018, 55, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Takanashi, S.; Miyazaki, A.; Rajashekara, G.; Saif, L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019, 37, 16–25. [Google Scholar] [CrossRef] [PubMed]
- AL Nabhani, Z.; Dulauroy, S.; Marques, R.; Cousu, C.; Al Bounny, S.; Déjardin, F.; Sparwasser, T.; Bérard, M.; Cerf-Bensussan, N.; Eberl, G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019, 50, 1276–1288.e5. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, J.; Li, L. Recent five-year progress in the impact of gut microbiota on vaccination and possible mechanisms. Gut Pathog. 2023, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Georg, P.; Sander, L.E. Innate sensors that regulate vaccine responses. Curr. Opin. Immunol. 2019, 59, 31–41. [Google Scholar] [CrossRef]
- Ruane, D.; Chorny, A.; Lee, H.; Faith, J.; Pandey, G.; Shan, M.; Simchoni, N.; Rahman, A.; Garg, A.; Weinstein, E.G.; et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med. 2016, 213, 53–73. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.-G.; Seo, S.-U.; Kim, D.-J.; Kamada, N.; Prescott, D.; Chamaillard, M.; Philpott, D.J.; Rosenstiel, P.; Inohara, N.; et al. Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin. Nat. Med. 2016, 22, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Coppola, G.; Rio, P.; Caldarelli, M.; Borriello, R.; Gambassi, G.; Gasbarrini, A.; Cianci, R. Factors Influencing Microbiota in Modulating Vaccine Immune Response: A Long Way to Go. Vaccines 2023, 11, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Peng, Y.; Zhang, L.; Mok, C.K.; Zhao, S.; Li, A.; Tun, H.M. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 2022, 71, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Z.; Ravindran, R.; Chassaing, B.; Carvalho, F.A.; Maddur, M.S.; Bower, M.; Hakimpour, P.; Gill, K.P.; Nakaya, H.I.; Yarovinsky, F.; et al. TLR5-Mediated Sensing of Gut Microbiota Is Necessary for Antibody Responses to Seasonal Influenza Vaccination. Immunity 2014, 41, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.; Hylemon, P.B. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl. Environ. Microbiol. 2000, 66, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Daddi, L.; Dorsett, Y.; Geng, T.; Bokoliya, S.; Yuan, H.; Wang, P.; Zhou, Y. Baseline Gut Microbiome Signatures Correlate with Immunogenicity of SARS-CoV-2 mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 11703. [Google Scholar] [CrossRef] [PubMed]
- de Vrese, M.; Rautenberg, P.; Laue, C.; Koopmans, M.; Herremans, T.; Schrezenmeir, J. Probiotic bacteria stimulate virus–specific neutralizing antibodies following a booster polio vaccination. Eur. J. Nutr. 2005, 44, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, E.K.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2017, 215, 34–41. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool Microbiota and Vaccine Responses of Infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef] [PubMed]
- Lépine, A.F.P.; Konstanti, P.; Borewicz, K.; Resink, J.-W.; de Wit, N.J.; de Vos, P.; Smidt, H.; Mes, J.J. Combined dietary supplementation of long chain inulin and Lactobacillus acidophilus W37 supports oral vaccination efficacy against Salmonella Typhimurium in piglets. Sci. Rep. 2019, 9, 18017. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.-F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.-B. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Citron, M.; Xiao, J.; Norton, J.E.; Reens, A.L.; Topçuoğlu, B.D.; Maritz, J.M.; Lee, K.-J.; Freed, D.C.; Weber, T.M.; et al. Vaccine Hyporesponse Induced by Individual Antibiotic Treatment in Mice and Non-Human Primates Is Diminished upon Recovery of the Gut Microbiome. Vaccines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, A.; Astill, J.; Hodgins, D.C.; Parkinson, J.; Nagy, É.; Sharif, S. Commensal gut microbiota can modulate adaptive immune responses in chickens vaccinated with whole inactivated avian influenza virus subtype H9N2. Vaccine 2019, 37, 6640–6647. [Google Scholar] [CrossRef] [PubMed]
- Lynn, M.A.; Tumes, D.J.; Choo, J.M.; Sribnaia, A.; Blake, S.J.; Leong, L.E.X.; Young, G.P.; Marshall, H.S.; Wesselingh, S.L.; Rogers, G.B.; et al. Early-Life Antibiotic-Driven Dysbiosis Leads to Dysregulated Vaccine Immune Responses in Mice. Cell Host Microbe 2018, 23, 653–660.e5. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.-Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef] [PubMed]
- Kazemifard, N.; Dehkohneh, A.; Ghavami, S.B. Probiotics and probiotic-based vaccines: A novel approach for improving vaccine efficacy. Front. Med. 2022, 9, 940454. [Google Scholar] [CrossRef]
- Michael, H.; Langel, S.N.; Miyazaki, A.; Paim, F.C.; Chepngeno, J.; Alhamo, M.A.; Fischer, D.D.; Srivastava, V.; Kathayat, D.; Deblais, L.; et al. Malnutrition Decreases Antibody Secreting Cell Numbers Induced by an Oral Attenuated Human Rotavirus Vaccine in a Human Infant Fecal Microbiota Transplanted Gnotobiotic Pig Model. Front. Immunol. 2020, 11, 196. [Google Scholar] [CrossRef]
- Bremel, R.D.; Homan, E.J. Extensive T-Cell Epitope Repertoire Sharing among Human Proteome, Gastrointestinal Microbiome, and Pathogenic Bacteria: Implications for the Definition of Self. Front. Immunol. 2015, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.B.; Liao, H.-X.; Moody, M.A.; Kepler, T.B.; Alam, S.M.; Gao, F.; Wiehe, K.; Trama, A.M.; Jones, K.; Zhang, R.; et al. Diversion of HIV-1 vaccine–induced immunity by gp41-microbiota cross-reactive antibodies. Science 2015, 349, aab1253. [Google Scholar] [CrossRef]
- Velasquez, D.E.; Parashar, U.; Jiang, B. Decreased performance of live attenuated, oral rotavirus vaccines in low-income settings: Causes and contributing factors. Expert Rev. Vaccines 2018, 17, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Stražar, M.; Mourits, V.P.; Koeken, V.A.C.M.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Joosten, L.A.B.; van Crevel, R.; Vlamakis, H.; Netea, M.G.; Xavier, R.J. The influence of the gut microbiome on BCG-induced trained immunity. Genome Biol. 2021, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.N.; Ahmad, S.M.; Alam, M.J.; Khanam, A.; Kalanetra, K.M.; Taft, D.H.; Stephensen, C.B. Bifidobacterium Abundance in Early Infancy and Vaccine Response at 2 Years of Age. Pediatrics 2019, 143, e20181489. [Google Scholar] [CrossRef]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. Toward rational vaccine engineering. Adv. Drug Deliv. Rev. 2022, 183, 114142. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Castro, A.; Arsiwala, A.; Kane, R.S. Bionanotechnology for vaccine design. Curr. Opin. Biotechnol. 2018, 52, 80–88. [Google Scholar] [CrossRef]
- Mao, Y.; Xiao, X.; Zhang, J.; Mou, X.; Zhao, W. Designing a multi-epitope vaccine against Peptostreptococcus anaerobius based on an immunoinformatics approach. Synth. Syst. Biotechnol. 2023, 8, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Gastelum-Aviña, P.; Velazquez, C.; Espitia, C.; Lares-Villa, F.; Garibay-Escobar, A. A PE_PGRS33 protein of Mycobacterium tuberculosis: An ideal target for future tuberculosis vaccine design. Expert Rev. Vaccines 2015, 14, 699–711. [Google Scholar] [CrossRef]
- Cheng, P.; Xue, Y.; Wang, J.; Jia, Z.; Wang, L.; Gong, W. Evaluation of the consistence between the results of immunoinformatics predictions and real-world animal experiments of a new tuberculosis vaccine MP3RT. Front. Cell. Infect. Microbiol. 2022, 12, 1047306. [Google Scholar] [CrossRef]
- Rinaudo, C.D.; Telford, J.L.; Rappuoli, R.; Seib, K.L. Vaccinology in the genome era. J. Clin. Investig. 2009, 119, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Ulmer, J.B.; Rappuoli, R. Structure-based antigen design: A strategy for next generation vaccines. Trends Biotechnol. 2008, 26, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Rio, P.; Caldarelli, M.; Chiantore, M.; Ocarino, F.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Immune Cells, Gut Microbiota, and Vaccines: A Gender Perspective. Cells 2024, 13, 526. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Belkaid, Y. Do the Microbiota Influence Vaccines and Protective Immunity to Pathogens? Engaging Our Endogenous Adjuvants. Cold Spring Harb. Perspect. Biol. 2018, 10, a028860. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H. Influence of Microbiota on Vaccine Effectiveness: “Is the Microbiota the Key to Vaccine-induced Responses?”. J. Microbiol. 2023, 61, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Ting, J.P. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Huan, T.; Rahman, G.; Zhi, H.; Xu, Z.; Oh, T.G.; Guo, J.; Coulter, S.; Tripathi, A.; Martino, C.; et al. Paired microbiome and metabolome analyses associate bile acid changes with colorectal cancer progression. Cell Rep. 2023, 42, 112997. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.-G.; et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef]
- Bessell, C.A.; Isser, A.; Havel, J.J.; Lee, S.; Bell, D.R.; Hickey, J.W.; Chaisawangwong, W.; Bieler, J.G.; Srivastava, R.; Kuo, F.; et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. J. Clin. Investig. 2020, 5, 135597. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Perez-Shibayama, C.; De Martin, A.; Ronchi, F.; van der Borght, K.; Niederer, R.; Onder, L.; Lütge, M.; Novkovic, M.; Nindl, V.; et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019, 366, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Baumann, R.; Gao, X.; Mendoza, M.; Singh, S.; Sand, I.K.; Baranzini, S.E. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell 2022, 185, 3467–3486.e16. [Google Scholar] [CrossRef] [PubMed]
- Bagavant, H.; Araszkiewicz, A.M.; Ingram, J.K.; Cizio, K.; Merrill, J.T.; Arriens, C.; Guthridge, J.M.; James, J.A.; Deshmukh, U.S. Immune Response to Enterococcus gallinarum in Lupus Patients Is Associated with a Subset of Lupus-Associated Autoantibodies. Front. Immunol. 2021, 12, 635072. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Szeto, C.H.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Wei, L.; Dong, C.; Zhu, W.; Wang, B.-Z. mRNA Vaccine Nanoplatforms and Innate Immunity. Viruses 2024, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, S.; Yao, Y.; Xia, Y.; Yang, X.; Li, K.; Sun, P.; Liu, C.; Sun, W.; Bai, H.; et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci. Rep. 2016, 6, 37242. [Google Scholar] [CrossRef] [PubMed]
- Fantappiè, L.; de Santis, M.; Chiarot, E.; Carboni, F.; Bensi, G.; Jousson, O.; Margarit, I.; Grandi, G. Antibody-mediated immunity induced by engineered Escherichia coli OMVs carrying heterologous antigens in their lumen. J. Extracell. Vesicles 2014, 3, 24015. [Google Scholar] [CrossRef]
- Del Tordello, E.; Danilchanka, O.; McCluskey, A.J.; Mekalanos, J.J. Type VI secretion system sheaths as nanoparticles for antigen display. Proc. Natl. Acad. Sci. USA 2016, 113, 3042–3047. [Google Scholar] [CrossRef]
- Singh, A.; Khan, A.; Ghosh, T.; Mondal, S.; Mallick, A.I. Gut Microbe-Derived Outer Membrane Vesicles: A Potential Platform to Control Cecal Load of Campylobacter jejuni. ACS Infect. Dis. 2021, 7, 1186–1199. [Google Scholar] [CrossRef]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef]
- Turner, L.; Praszkier, J.; Hutton, M.L.; Steer, D.; Ramm, G.; Kaparakis-Liaskos, M.; Ferrero, R.L. Increased Outer Membrane Vesicle Formation in a Helicobacter pylori tolB Mutant. Helicobacter 2015, 20, 269–283. [Google Scholar] [CrossRef]
- Fiocca, R.; Necchi, V.; Sommi, P.; Ricci, V.; Telford, J.; Cover, T.L.; Solcia, E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999, 188, 220–226. [Google Scholar] [CrossRef]
- Deo, P.; Chow, S.H.; Hay, I.D.; Kleifeld, O.; Costin, A.; Elgass, K.D.; Jiang, J.-H.; Ramm, G.; Gabriel, K.; Dougan, G.; et al. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog. 2018, 14, e1006945. [Google Scholar] [CrossRef]

| Microbial Species | Effect to Vaccines | References |
|---|---|---|
| Actinobacteria | Improved response to BCG, HepB, IPV and OPV vaccination. | Huda and Lépine et al. [71,73] |
| Firmicutes bacteria | Associated with higher humoral responses to ORV in infants and cellular responses to Salmonella Typhi in adults. | Harris et al. [70] |
| Bifidobacterium adolescentis; Butyricimonas virosa, Adlercreutzia equolifaciens; Asaccharobacter celatus | Enhance the efficacy of CoronaVac vaccine. | Siew et al. [63] |
| Eubacterium rectale; Roseburia faecis; Bacteroides | Enhance the efficacy of BNT162b2 vaccine. | Siew et al. [63] |
| Ruminococcus torques; Eubacterium ventriosum; Streptococcus salivarius | Associated with high responses to CoronaVac in overweight or obese population. | Siew et al. [63] |
| Escherichia coli | Enhancing the immune response of Influenza Vaccine through the role of TLR5 receptor. | Jason et al. [64] |
| Desulfobacterota, Bilophila | Enhance the immunogenicity of COVID-19 mRNA vaccine (Moderna and BNT162b2) by synthesizing endotoxin with immunostimulatory effect. | Daddi et al. [67] |
| Streptococcus bovis, Clostridium cluster XI; Proteobacteria | Positively correlated with the enhancement of immune response to ORV vaccine. | Harris et al. [69] |
| Clostridiales | Associated with high abundance of long-term IgG and IgA memory B cells in oral cholera vaccine. | Denise et al. [36] |
| Bifidobacterium animalis ssp. Lactis; Lactobacillus paracasei | Increased the specific antibody level of influenza vaccine after vaccination. | Rizzardini et al. [74] |
| Bifidobacterium infantis; Bifidobacterium breve | Increased titer of anti-poliovirus IgA. | Mullié et al. [73] |
| Lactobacillus acidophilus | Enhanced production of IgA and IgM after OPV vaccination, increased vaccine efficacy against Salmonella Typhimurium strains. | Michael and Lépine et al. [68,72] |
| Microbial Species | Effect to Vaccines | References |
|---|---|---|
| Proteobacteria | Associated with lower responses to BCG, HepB, IPV and OPV vaccination. | Huda et al. [71] |
| Bacteroides | high abundance of Bacteroides is related to the lower humoral response to ORV. | Harris et al. [70] |
| Burkholderia | A large number of TCEM similar to human immunoglobulin V region can reduce the efficacy of the vaccine. | Michael et al. [81] |
| Escherichia coli | Induce the production of non-neutralizing antibodies against HIV gp41 protein. | Williams et al. [82] |
| Enterobacterales | Associated with low abundance of long-term IgG and IgA memory B cells in oral cholera vaccine. | Denise et al. [36] |
| Pseudomonadales | Lower specific T cell response and serum IgG levels were associated with oral OPV vaccination or intramuscular tetanus-hepatitis B vaccine and intradermal BCG vaccine. | Huda et al. [71] |
| Streptococcus | Decrease the efficacy of COVID-19 vaccine (BNT162b2). | Siew et al. [63] |
| Bacteroides and Ruminococcus | Decrease the efficacy of COVID-19 vaccine (CoronaVac). | Siew et al. [63] |
| Clostridium; Enterobacter; Pseudomonadales | The serum titers of tetanus toxoid and HepB vaccine specific IgG and IgA decreased. | Huda et al. [85] |
| Microbial Species | Relevant Components and Mechanisms for Optimizing Vaccine Design | References |
|---|---|---|
| Mycobacteria and Burkholderia spp. | TCEMs have been identified and can be leveraged for the development of targeted vaccines. | Robert et al. [81] |
| Mycobacterium tuberculosis | The PE domain of the PE_PGRS33 protein can induce a protective cellular immune response and is a potential vaccine target. It can also be used as the N-terminus of fusion proteins for constructing recombinant vaccines. | Paola et al. [89] |
| Enterococci | A peptide segment of the TMP1 protein from Enterococci bacteriophage cross-reacts with tumor-associated antigens, making it a potential candidate for developing anti-cancer vaccines. | Aurélie et al. [99] |
| Bifidobacterium breve | The SVYRYYGL peptide cross-reacts with melanoma antigens and can be utilized for the development of melanoma vaccines. | Catherine et al. [100] |
| Bacteroides | The β-galactosidase in Bacteroides contains peptide segments that cross-react with human cardiac myosin heavy chain protein, making it a potential candidate for myocarditis vaccine research. | Cristina et al. [101] |
| Akkermansia muciniphila | Certain peptide segments are associated with multiple sclerosis and can be utilized for the development of potential vaccines. | Sergio et al. [102] |
| Enterococcus gallinarum | Higher titers of anti-Enterococcus gallinarum antibodies are significantly associated with the presence of anti-dsDNA and anti-Sm autoantibodies. | Harini et al. [103] |
| Peptostreptococcus anaerobius | This anaerobic bacterium is enriched in patients with colorectal cancer and may serve as a potential vaccine target. | Long et al. [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, J.; Yang, Y.; Chen, X.; Chen, L.; Wu, Y. Intestinal Microbiota and Its Effect on Vaccine-Induced Immune Amplification and Tolerance. Vaccines 2024, 12, 868. https://doi.org/10.3390/vaccines12080868
Liu Y, Zhou J, Yang Y, Chen X, Chen L, Wu Y. Intestinal Microbiota and Its Effect on Vaccine-Induced Immune Amplification and Tolerance. Vaccines. 2024; 12(8):868. https://doi.org/10.3390/vaccines12080868
Chicago/Turabian StyleLiu, Yixin, Jianfeng Zhou, Yushang Yang, Xiangzheng Chen, Longqi Chen, and Yangping Wu. 2024. "Intestinal Microbiota and Its Effect on Vaccine-Induced Immune Amplification and Tolerance" Vaccines 12, no. 8: 868. https://doi.org/10.3390/vaccines12080868




