Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems
Abstract
1. Introduction
2. Results
2.1. Expansion of the mRNA Application
2.1.1. The Application of mRNA in Infectious Diseases
2.1.2. The Application of mRNA in Cancer Therapy
2.1.3. mRNA Technology in Other Diseases
2.2. Antigen Selection and Design
2.2.1. Antigen Selection: The Key to Success in Vaccine Development
2.2.2. Epitope Optimization Enhances the Adaptive Immune Responses
2.3. mRNA Sequence Optimization
2.3.1. Codon Optimization Contributes to Antigen Expression
2.3.2. The Shadowed UTRs Determine the Fate of mRNA
2.3.3. The Long and Structured Poly(A) Tail in mRNA Vaccines
2.4. In Vitro Transcription
2.4.1. Modified Nucleotides Protect mRNA from Immune Systems
2.4.2. Cap Analogs Empower Co-Transcriptional Capping
2.4.3. Multiple Measures to Minimize the Production of dsRNA
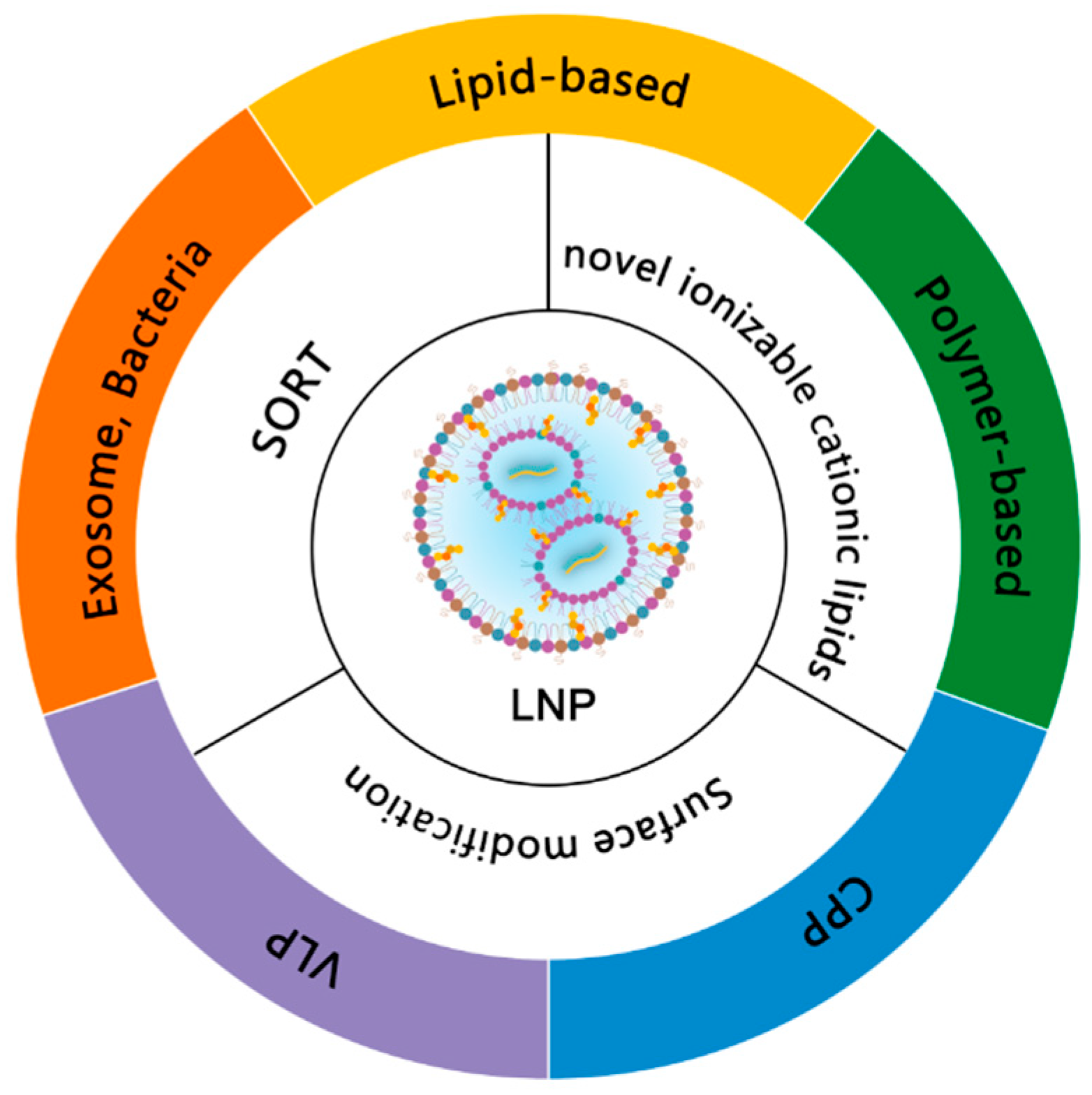
2.5. Delivery Systems for mRNA Vaccines
2.5.1. Lipid-Based mRNA Delivery Systems
2.5.2. Lipids for Advanced LNPs
2.5.3. Polymer-Based mRNA Delivery Systems
2.5.4. Other Promising mRNA Delivery Systems
2.5.5. Surface Modification Expands the Applications of LNPs
2.6. Adjuvants
2.7. Security Issues
2.7.1. Cationic Lipid Oxidation Injuries in the Safety of LNPs
2.7.2. Anti-PEG Antibody: Potential Barriers for mRNA Application
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Badizadegan, K.; Kalkowska, D.A.; Thompson, K.M. Polio by the Numbers-A Global Perspective. J. Infect. Dis. 2022, 226, 1309–1318. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e2417. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Zhang, N.N.; Zhang, R.R.; Zhang, Y.F.; Ji, K.; Xiong, X.C.; Qin, Q.S.; Gao, P.; Lu, X.S.; Zhou, H.Y.; Song, H.F.; et al. Rapid development of an updated mRNA vaccine against the SARS-CoV-2 Omicron variant. Cell Res. 2022, 32, 401–403. [Google Scholar] [CrossRef]
- Cargill, T.; Barnes, E. Therapeutic vaccination for treatment of chronic hepatitis B. Clin. Exp. Immunol. 2021, 205, 106–118. [Google Scholar] [CrossRef]
- Boilesen, D.R.; Nielsen, K.N.; Holst, P.J. Novel Antigenic Targets of HPV Therapeutic Vaccines. Vaccines 2021, 9, 1262. [Google Scholar] [CrossRef]
- Cohen, K.W.; De Rosa, S.C.; Fulp, W.J.; deCamp, A.C.; Fiore-Gartland, A.; Mahoney, C.R.; Furth, S.; Donahue, J.; Whaley, R.E.; Ballweber-Fleming, L.; et al. A first-in-human germline-targeting HIV nanoparticle vaccine induced broad and publicly targeted helper T cell responses. Sci. Transl. Med. 2023, 15, eadf3309. [Google Scholar] [CrossRef]
- Lin, M.J.; Svensson-Arvelund, J.; Lubitz, G.S.; Marabelle, A.; Melero, I.; Brown, B.D.; Brody, J.D. Cancer vaccines: The next immunotherapy frontier. Nat. Cancer 2022, 3, 911–926. [Google Scholar] [CrossRef]
- Boettler, T.; Csernalabics, B.; Salié, H.; Luxenburger, H.; Wischer, L.; Salimi Alizei, E.; Zoldan, K.; Krimmel, L.; Bronsert, P.; Schwabenland, M.; et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J. Hepatol. 2022, 77, 653–659. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Fan, M.; Lai, F.T.T.; Cheng, F.W.T.; Tsie, N.T.Y.; Li, X.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; Wong, I.C.K.; et al. Risk of carditis after three doses of vaccination with mRNA (BNT162b2) or inactivated (CoronaVac) COVID-19 vaccination: A self-controlled cases series and a case-control study. Lancet Reg. Health-West. Pac. 2023, 35, 100745. [Google Scholar] [CrossRef]
- Lederer, K.; Castano, D.; Gomez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e1285. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Akahata, W.; Sekida, T.; Nogimori, T.; Ode, H.; Tamura, T.; Kono, K.; Kazami, Y.; Washizaki, A.; Masuta, Y.; Suzuki, R.; et al. Safety and immunogenicity of SARS-CoV-2 self-amplifying RNA vaccine expressing an anchored RBD: A randomized, observer-blind phase 1 study. Cell Rep. Med. 2023, 4, 101134. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Fierro, C.; Brune, D.; Shaw, M.; Schwartz, H.; Knightly, C.; Lin, J.; Carfi, A.; Natenshon, A.; Kalidindi, S.; Reuter, C.; et al. Safety and Immunogenicity of a Messenger RNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results From a Phase 1, Randomized, Clinical Trial. J. Infect. Dis. 2024, jiae114. [Google Scholar] [CrossRef]
- Bollman, B.; Nunna, N.; Bahl, K.; Hsiao, C.J.; Bennett, H.; Butler, S.; Foreman, B.; Burgomaster, K.E.; Aleshnick, M.; Kong, W.P.; et al. An optimized messenger RNA vaccine candidate protects non-human primates from Zika virus infection. NPJ Vaccines 2023, 8, 58. [Google Scholar] [CrossRef]
- Li, M.; Fang, E.; Wang, Y.; Shi, L.; Li, J.; Peng, Q.; Li, X.; Zhao, D.; Liu, X.; Liu, X.; et al. An mRNA vaccine against rabies provides strong and durable protection in mice. Front. Immunol. 2023, 14, 1288879. [Google Scholar] [CrossRef]
- Xie, Z.; Lin, Y.C.; Steichen, J.M.; Ozorowski, G.; Kratochvil, S.; Ray, R.; Torres, J.L.; Liguori, A.; Kalyuzhniy, O.; Wang, X.; et al. mRNA-LNP HIV-1 trimer boosters elicit precursors to broad neutralizing antibodies. Science 2024, 384, eadk0582. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, X.; Yu, Y.; Huang, L.; Amor, N.P.; Guo, K.; Weng, C.; Zhao, W.; Yang, A.; Hu, J.; et al. A therapeutic hepatitis B mRNA vaccine with strong immunogenicity and persistent virological suppression. NPJ Vaccines 2024, 9, 22. [Google Scholar] [CrossRef]
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.; Moreno, A.C.R.; Aps, L.; Venceslau-Carvalho, A.A.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- Qi, H.; Sun, Z.; Gao, T.; Yao, Y.; Wang, Y.; Li, W.; Wang, X.; Wang, X.; Liu, D.; Jiang, J.D. Genetic fusion of CCL11 to antigens enhances antigenicity in nucleic acid vaccines and eradicates tumor mass through optimizing T-cell response. Mol. Cancer 2024, 23, 46. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Maruggi, G.; Chiarot, E.; Giovani, C.; Buccato, S.; Bonacci, S.; Frigimelica, E.; Margarit, I.; Geall, A.; Bensi, G.; Maione, D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 2017, 35, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-encoded MIF ortholog confers protective immunity against malaria infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Bailey, D.; Zielinski, J.; Apte, A.; Musenge, F.; Karp, R.; Burke, S.; Garcon, F.; Mishra, A.; Gurumurthy, S.; et al. Intratumoral IL12 mRNA Therapy Promotes TH1 Transformation of the Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 6284–6298. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Bai, A.; Bailey, D.; Ichikawa, K.; Zielinski, J.; Karp, R.; Apte, A.; Arnold, K.; Zacharek, S.J.; Iliou, M.S.; et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 2019, 11, eaat9143. [Google Scholar] [CrossRef]
- Bähr-Mahmud, H.; Ellinghaus, U.; Stadler, C.R.; Fischer, L.; Lindemann, C.; Chaturvedi, A.; Diekmann, J.; Wöll, S.; Biermann, I.; Hebich, B.; et al. Preclinical characterization of an mRNA-encoded anti-Claudin 18.2 antibody. Oncoimmunology 2023, 12, 2255041. [Google Scholar] [CrossRef]
- Stadler, C.R.; Ellinghaus, U.; Fischer, L.; Bähr-Mahmud, H.; Rao, M.; Lindemann, C.; Chaturvedi, A.; Scharf, C.; Biermann, I.; Hebich, B.; et al. Preclinical efficacy and pharmacokinetics of an RNA-encoded T cell-engaging bispecific antibody targeting human claudin 6. Sci. Transl. Med. 2024, 16, eadl2720. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, X.Q.; Ho, W.; Tang, M.; Li, Z.; Bu, L.; Xu, X. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat. Commun. 2023, 14, 4223. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.N.; Carvalho Costa, P.A.; Scalzo Júnior, S.R.A.; Ferreira, H.A.S.; Prazeres, P.; Campos, C.L.V.; Rodrigues Alves, M.T.; Alves da Silva, N.J.; de Castro Santos, A.L.; Guimarães, L.C.; et al. Ionizable Lipid Nanoparticle-Mediated TRAIL mRNA Delivery in the Tumor Microenvironment to Inhibit Colon Cancer Progression. Int. J. Nanomed. 2024, 19, 2655–2673. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Koeberl, D.; Schulze, A.; Sondheimer, N.; Lipshutz, G.S.; Geberhiwot, T.; Li, L.; Saini, R.; Luo, J.; Sikirica, V.; Jin, L.; et al. Interim analyses of a first-in-human phase 1/2 mRNA trial for propionic acidaemia. Nature 2024, 628, 872–877. [Google Scholar] [CrossRef]
- Garcia-Ocana, A.; Takane, K.K.; Syed, M.A.; Philbrick, W.M.; Vasavada, R.C.; Stewart, A.F. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000, 275, 1226–1232. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, P.; Shiota, C.; Zhang, T.; Coudriet, G.M.; Fischbach, S.; Prasadan, K.; Fusco, J.; Ramachandran, S.; Witkowski, P.; et al. Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes. Cell Stem Cell 2018, 22, 78–90.e74. [Google Scholar] [CrossRef]
- Melamed, J.R.; Yerneni, S.S.; Arral, M.L.; LoPresti, S.T.; Chaudhary, N.; Sehrawat, A.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Weissman, D.; et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv. 2023, 9, eade1444. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2022, 20, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, D.Y.; Zhang, H.N.; Jiang, H.W.; Tian, X.; Ma, M.L.; Qi, H.; Meng, Q.F.; Guo, S.J.; Wu, Y.; et al. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ni, W.; Liang, S.; Dong, L.; Xiang, M.; Cai, Z.; Niu, D.; Zhang, Q.; Wang, D.; Zheng, Y.; et al. Vaccination with S(pan), an antigen guided by SARS-CoV-2 S protein evolution, protects against challenge with viral variants in mice. Sci. Transl. Med. 2023, 15, eabo3332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Multi-epitope vaccines: A promising strategy against tumors and viral infections. Cell. Mol. Immunol. 2018, 15, 182–184. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Almajhdi, F.N.; Waqas, M.; Ullah, I.; Salim, M.A.; Khan, N.A.; Ali, A. Contriving multi-epitope vaccine ensemble for monkeypox disease using an immunoinformatics approach. Front. Immunol. 2022, 13, 1004804. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; Feng, S.; Chai, B.; Lu, S.; Zhao, G.; Chen, D.; Yu, W.; Ren, L.; Shi, H.; Lu, J.; et al. An mRNA-based T-cell-inducing antigen strengthens COVID-19 vaccine against SARS-CoV-2 variants. Nat. Commun. 2023, 14, 2962. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Jin, H.T.; Hur, S.Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.W.; Kim, S.; Woo, J.W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Ma, L.; Lv, K.; Han, L.; Chen, Y.; Zhou, R.; Zhou, H.; Chen, H.; Wang, Y.; et al. Development of an mRNA-based therapeutic vaccine mHTV-03E2 for high-risk HPV-related malignancies. Mol. Ther. 2024, 32, 2340–2356. [Google Scholar] [CrossRef]
- Graham, B.S.; Gilman, M.S.A.; McLellan, J.S. Structure-Based Vaccine Antigen Design. Annu. Rev. Med. 2019, 70, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Theiler, J.; Korber, B. Graph-based optimization of epitope coverage for vaccine antigen design. Stat. Med. 2018, 37, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Hanson, G.; Coller, J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2018, 19, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, A.A.; Reid, D.W.; Licata, M.C.; Jones, A.K.; Cheng, Y.M.; Li, M.; Hsiao, C.J.; Pepin, C.S.; Metkar, M.; Levdansky, Y.; et al. Attenuating ribosome load improves protein output from mRNA by limiting translation-dependent mRNA decay. Cell Rep. 2024, 43, 114098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Bonam, S.R.; McKay, L.G.A.; Plante, J.A.; Walker, J.; Zhao, Y.; Huang, C.; Chen, J.; Xu, C.; Li, Y.; et al. Monovalent SARS-CoV-2 mRNA vaccine using optimal UTRs and LNPs is highly immunogenic and broadly protective against Omicron variants. Proc. Natl. Acad. Sci. USA 2023, 120, e2311752120. [Google Scholar] [CrossRef] [PubMed]
- Horste, E.L.; Fansler, M.M.; Cai, T.; Chen, X.; Mitschka, S.; Zhen, G.; Lee, F.C.Y.; Ule, J.; Mayr, C. Subcytoplasmic location of translation controls protein output. Mol. Cell 2023, 83, 4509–4523.e4511. [Google Scholar] [CrossRef] [PubMed]
- Gebre, M.S.; Rauch, S.; Roth, N.; Yu, J.; Chandrashekar, A.; Mercado, N.B.; He, X.; Liu, J.; McMahan, K.; Martinot, A.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 2022, 601, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Schön, J.; Hoffmann, D.; Thran, M.; Thess, A.; Mueller, S.O.; Petsch, B.; Rauch, S. Optimised Non-Coding Regions of mRNA SARS-CoV-2 Vaccine CV2CoV Improves Homologous and Heterologous Neutralising Antibody Responses. Vaccines 2022, 10, 1251. [Google Scholar] [CrossRef]
- He, S.; Gao, B.; Sabnis, R.; Sun, Q. RNAdegformer: Accurate prediction of mRNA degradation at nucleotide resolution with deep learning. Brief. Bioinform. 2023, 24, bbac581. [Google Scholar] [CrossRef]
- Chu, Y.; Yu, D.; Li, Y.; Huang, K.; Shen, Y.; Cong, L.; Zhang, J.; Wang, M. A 5′ UTR language model for decoding untranslated regions of mRNA and function predictions. Nat. Mach. Intell. 2024, 6, 449–460. [Google Scholar] [CrossRef]
- Ning, H.; Liu, G.; Li, L.; Liu, Q.; Huang, H.; Xie, Z. Rational design of microRNA-responsive switch for programmable translational control in mammalian cells. Nat. Commun. 2023, 14, 7193. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, S.; Qiu, X.; Jiang, J.; Zhang, L.; Wang, P.; Si, Y.; Wu, Y.; He, M.; Xiong, Q.; et al. Engineered poly(A)-surrogates for translational regulation and therapeutic biocomputation in mammalian cells. Cell Res. 2024, 34, 31–46. [Google Scholar] [CrossRef]
- Passmore, L.A.; Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 2022, 23, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Trepotec, Z.; Geiger, J.; Plank, C.; Aneja, M.K.; Rudolph, C. Segmented poly(A) tails significantly reduce recombination of plasmid DNA without affecting mRNA translation efficiency or half-life. Rna 2019, 25, 507–518. [Google Scholar] [CrossRef]
- Li, C.Y.; Liang, Z.; Hu, Y.; Zhang, H.; Setiasabda, K.D.; Li, J.; Ma, S.; Xia, X.; Kuang, Y. Cytidine-containing tails robustly enhance and prolong protein production of synthetic mRNA in cell and in vivo. Mol. Ther. Nucleic Acids 2022, 30, 300–310. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Guo, J.; Aditham, A.; Zhou, Y.; Tian, J.; Luo, S.; Ren, J.; Hsu, A.; Huang, J.; et al. Branched chemically modified poly(A) tails enhance the translation capacity of mRNA. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Ahuad Guerrero, R.A.; Arana-Arri, E.; Aroca Martinez, G.J.; Bonten, M.; Chandler, R.; Corral, G.; De Block, E.J.L.; Ecker, L.; Gabor, J.J.; et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): A randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022, 22, 329–340. [Google Scholar] [CrossRef]
- Komori, M.; Morey, A.L.; Quiñones-Molina, A.A.; Fofana, J.; Romero, L.; Peters, E.; Matsuda, K.; Gummuluru, S.; Smith, J.F.; Akahata, W.; et al. Incorporation of 5 methylcytidine alleviates innate immune response to self-amplifying RNA vaccine. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N(1)-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [Google Scholar] [CrossRef]
- Aboshi, M.; Matsuda, K.; Kawakami, D.; Kono, K.; Kazami, Y.; Sekida, T.; Komori, M.; Morey, A.L.; Suga, S.; Smith, J.F.; et al. Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine. iScience 2024, 27, 108964. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.E.; Kirsch, J.R.; Kenney, D.; Chavez, E.; Shih, T.Y.; Douam, F.; Wong, W.W.; Grinstaff, M.W. Complete substitution with modified nucleotides suppresses the early interferon response and increases the potency of self-amplifying RNA. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ramanathan, A.; Robb, G.B.; Chan, S.H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. [Google Scholar] [CrossRef] [PubMed]
- Despic, V.; Jaffrey, S.R. mRNA ageing shapes the Cap2 methylome in mammalian mRNA. Nature 2023, 614, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A. RNA 2’-O-methylation modification and its implication in COVID-19 immunity. Cell Death Discov. 2020, 6, 118. [Google Scholar] [CrossRef] [PubMed]
- Grudzien-Nogalska, E.; Stepinski, J.; Jemielity, J.; Zuberek, J.; Stolarski, R.; Rhoads, R.E.; Darzynkiewicz, E. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007, 431, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Ujita, A.; Hill, E.; Yousif-Rosales, S.; Smith, C.; Ko, N.; McReynolds, T.; Cabral, C.R.; Escamilla-Powers, J.R.; Houston, M.E. Cap 1 Messenger RNA Synthesis with Co-transcriptional CleanCap(®) Analog by In Vitro Transcription. Curr. Protoc. 2021, 1, e39. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, M.; Senthilvelan, A.; Kore, A.R. Recent Advances in Modified Cap Analogs: Synthesis, Biochemical Properties, and mRNA Based Vaccines. Chem. Rec. 2022, 22, e202200005. [Google Scholar] [CrossRef]
- Inagaki, M.; Abe, N.; Li, Z.; Nakashima, Y.; Acharyya, S.; Ogawa, K.; Kawaguchi, D.; Hiraoka, H.; Banno, A.; Meng, Z.; et al. Cap analogs with a hydrophobic photocleavable tag enable facile purification of fully capped mRNA with various cap structures. Nat. Commun. 2023, 14, 2657. [Google Scholar] [CrossRef]
- Warminski, M.; Trepkowska, E.; Smietanski, M.; Sikorski, P.J.; Baranowski, M.R.; Bednarczyk, M.; Kedzierska, H.; Majewski, B.; Mamot, A.; Papiernik, D.; et al. Trinucleotide mRNA Cap Analogue N6-Benzylated at the Site of Posttranscriptional (m6)A(m) Mark Facilitates mRNA Purification and Confers Superior Translational Properties In Vitro and In Vivo. J. Am. Chem. Soc. 2024, 146, 8149–8163. [Google Scholar] [CrossRef]
- Klöcker, N.; Weissenboeck, F.P.; van Dülmen, M.; Špaček, P.; Hüwel, S.; Rentmeister, A. Photocaged 5’ cap analogues for optical control of mRNA translation in cells. Nat. Chem. 2022, 14, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jacobus, E.J.; Stirling, D.C.; Krumm, S.; Flight, K.E.; Cunliffe, R.F.; Mottl, J.; Singh, C.; Mosscrop, L.G.; Santiago, L.A.; et al. Reducing cell intrinsic immunity to mRNA vaccine alters adaptive immune responses in mice. Mol. Ther. Nucleic Acids 2023, 34, 102045. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Gal, J.; Creisson, A.; Chamorey, E. Myocarditis and COVID-19 mRNA vaccines: A mechanistic hypothesis involving dsRNA. Futur. Virol. 2021, 17, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Dousis, A.; Ravichandran, K.; Hobert, E.M.; Moore, M.J.; Rabideau, A.E. An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts. Nat. Biotechnol. 2023, 41, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Lampaya, V.; Larraga, A.; Magallón, H.; Casabona, D. Purification of linearized template plasmid DNA decreases double-stranded RNA formation during IVT reaction. Front. Mol. Biosci. 2023, 10, 1248511. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Yadav, V.; Wang, E.; Chang, W.; Tau, L.; Lindenmuth, B.E.; Wang, S.X. Double-stranded RNA reduction by chaotropic agents during in vitro transcription of messenger RNA. Mol. Ther. Nucleic Acids 2022, 29, 618–624. [Google Scholar] [CrossRef]
- Baiersdorfer, M.; Boros, G.; Muramatsu, H.; Mahiny, A.; Vlatkovic, I.; Sahin, U.; Kariko, K. A Facile Method for the Removal of dsRNA Contaminant from In Vitro-Transcribed mRNA. Mol. Ther. Nucleic Acids 2019, 15, 26–35. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent Advances in Lipid Nanoparticles for Delivery of mRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for mRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Hafez, I.M.; Baoukina, S.; Belliveau, N.M.; Zhigaltsev, I.V.; Afshinmanesh, E.; Tieleman, D.P.; Hansen, C.L.; Hope, M.J.; Cullis, P.R. Lipid Nanoparticles Containing siRNA Synthesized by Microfluidic Mixing Exhibit an Electron-Dense Nanostructured Core. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 18440–18450. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef]
- Liu, K.; Nilsson, R.; Lázaro-Ibáñez, E.; Duàn, H.; Miliotis, T.; Strimfors, M.; Lerche, M.; Salgado Ribeiro, A.R.; Ulander, J.; Lindén, D.; et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat. Commun. 2023, 14, 4007. [Google Scholar] [CrossRef]
- Han, X.; Zhang, H.; Butowska, K.; Swingle, K.L.; Alameh, M.G.; Weissman, D.; Mitchell, M.J. An ionizable lipid toolbox for RNA delivery. Nat. Commun. 2021, 12, 7233. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Liu, Z.; Yuan, W. Lipopolyplex for Therapeutic Gene Delivery and Its Application for the Treatment of Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 68. [Google Scholar] [CrossRef]
- Yang, R.; Deng, Y.; Huang, B.; Huang, L.; Lin, A.; Li, Y.; Wang, W.; Liu, J.; Lu, S.; Zhan, Z.; et al. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct. Target. Ther. 2021, 6, 213. [Google Scholar] [CrossRef]
- Qiu, M.; Tang, Y.; Chen, J.; Muriph, R.; Ye, Z.; Huang, C.; Evans, J.; Henske, E.P.; Xu, Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119. [Google Scholar] [CrossRef]
- Du, S.; Li, W.; Zhang, Y.; Xue, Y.; Hou, X.; Yan, J.; Cheng, J.; Deng, B.; McComb, D.W.; Lin, J.; et al. Cholesterol-Amino-Phosphate (CAP) Derived Lipid Nanoparticles for Delivery of Self-Amplifying RNA and Restoration of Spermatogenesis in Infertile Mice. Adv. Sci. 2023, 10, e2300188. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Newby, A.N.; Arral, M.L.; Yerneni, S.S.; LoPresti, S.T.; Doerfler, R.; Petersen, D.M.S.; Montoya, C.; Kim, J.S.; Fox, B.; et al. Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2307810121. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Safford, H.C.; Geisler, H.C.; Hamilton, A.G.; Thatte, A.S.; Billingsley, M.M.; Joseph, R.A.; Mrksich, K.; Padilla, M.S.; Ghalsasi, A.A.; et al. Ionizable Lipid Nanoparticles for In Vivo mRNA Delivery to the Placenta during Pregnancy. J. Am. Chem. Soc. 2023, 145, 4691–4706. [Google Scholar] [CrossRef] [PubMed]
- Ralvenius, W.T.; Andresen, J.L.; Huston, M.M.; Penney, J.; Bonner, J.M.; Fenton, O.S.; Langer, R.; Tsai, L.H. Nanoparticle-Mediated Delivery of Anti-PU.1 siRNA via Localized Intracisternal Administration Reduces Neuroinflammation. Adv. Mater. 2024, 36, e2309225. [Google Scholar] [CrossRef] [PubMed]
- Dilliard, S.A.; Cheng, Q.; Siegwart, D.J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl. Acad. Sci. USA 2021, 118, e2109256118. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Benedicto, E.; Tian, Z.; Chatterjee, S.; Orlando, D.; Kim, M.; Guerrero, E.D.; Wang, X.; Siegwart, D.J. Spleen SORT LNP Generated in situ CAR T Cells Extend Survival in a Mouse Model of Lymphoreplete B Cell Lymphoma. Angew. Chem. Int. Ed. 2023, 62, e202310395. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Chatterjee, S.; Sun, Y.; Dilliard, S.A.; Moore, S.; Xiao, Y.; Bian, X.; Yamada, K.; Sung, Y.C.; Levine, R.M.; et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat. Nanotechnol. 2024. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, S.; Sun, J.; Liu, L.; Ma, X.; Li, J.; Fan, B.; Yang, C.; Zhao, Y.; Liu, S.; et al. A Synergistic Lipid Nanoparticle Encapsulating mRNA Shingles Vaccine Induces Potent Immune Responses and Protects Guinea Pigs from Viral Challenges. Adv. Mater. 2024, 36, e2310886. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.; Wu, S.; Lin, L.; He, S.; Yan, X.; Shi, L.; Liu, S. One-Component Cationic Lipids for Systemic mRNA Delivery to Splenic T Cells. Angew. Chem. Int. Ed. 2024, 63, e202405444. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Wang, W.; Liu, X.; Deng, H. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc. Natl. Acad. Sci. USA 2023, 120, e2311276120. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gomez, H.R.; DeVries, A.; Castillo, P.; von Roemeling, C.; Qdaisat, S.; Stover, B.D.; Xie, C.; Weidert, F.; Zhao, C.; Moor, R.; et al. RNA aggregates harness the danger response for potent cancer immunotherapy. Cell 2024, 187, 2521–2535.e2521. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, L.; Vanover, D.; Bruno, N.C.; Peck, H.E.; Zurla, C.; Murray, J.; Noel, R.K.; O’Farrell, L.; Araínga, M.; Orr-Burks, N.; et al. Species-agnostic polymeric formulations for inhalable messenger RNA delivery to the lung. Nat. Mater. 2023, 22, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.Y.; Witten, J.; Raji, I.O.; Eweje, F.; MacIsaac, C.; Meng, S.; Oladimeji, F.A.; Hu, Y.; Manan, R.S.; Langer, R.; et al. Combinatorial development of nebulized mRNA delivery formulations for the lungs. Nat. Nanotechnol. 2024, 19, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shao, S.; Piao, Y.; Xiang, J.; Wei, X.; Zhang, Z.; Zhou, Z.; Tang, J.; Qiu, N.; Xu, X.; et al. Esterase-Labile Quaternium Lipidoid Enabling Improved mRNA-LNP Stability and Spleen-Selective mRNA Transfection. Adv. Mater. 2023, 35, e2303614. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yu, F.; Hsu, J.C.; Shi, J.; Cai, W. Soybean Oil-Derived Lipids for Efficient mRNA Delivery. Adv. Mater. 2024, 36, e2302901. [Google Scholar] [CrossRef]
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12, e2202688. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gaudin, A.; Zhang, J.; Agarwal, T.; Song, E.; Kauffman, A.C.; Tietjen, G.T.; Wang, Y.; Jiang, Z.; Cheng, C.J.; et al. A “top-down” approach to actuate poly(amine-co-ester) terpolymers for potent and safe mRNA delivery. Biomaterials 2018, 176, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Blanchard, E.L.; Vanover, D.; Bawage, S.S.; Tiwari, P.M.; Rotolo, L.; Beyersdorf, J.; Peck, H.E.; Bruno, N.C.; Hincapie, R.; Michel, F.; et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Nat. Biotechnol. 2021, 39, 717–726. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Li, J.; Van Guyse, J.F.R.; Hayashi, K.; Vummaleti, S.V.C.; Kaur, S.; Mochida, Y.; Fukushima, S.; Kataoka, K. Effective mRNA Protection by Poly(l-ornithine) Synergizes with Endosomal Escape Functionality of a Charge-Conversion Polymer toward Maximizing mRNA Introduction Efficiency. Macromol. Rapid Commun. 2022, 43, e2100754. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Zhu, Y.; McKay, P.F.; Bouton, C.R.; Yeow, J.; Tang, J.; Hu, K.; Samnuan, K.; Grigsby, C.L.; Shattock, R.J.; et al. Big Is Beautiful: Enhanced saRNA Delivery and Immunogenicity by a Higher Molecular Weight, Bioreducible, Cationic Polymer. ACS Nano 2020, 14, 5711–5727. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, H.; Oba, M.; Uchida, S. Cell-Penetrating Peptides: Emerging Tools for mRNA Delivery. Pharmaceutics 2021, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef]
- An, M.; Raguram, A.; Du, S.W.; Banskota, S.; Davis, J.R.; Newby, G.A.; Chen, P.Z.; Palczewski, K.; Liu, D.R. Engineered virus-like particles for transient delivery of prime editor ribonucleoprotein complexes in vivo. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e216. [Google Scholar] [CrossRef]
- Liu, M.; Hu, S.; Yan, N.; Popowski, K.D.; Cheng, K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat. Nanotechnol. 2024, 19, 565–575. [Google Scholar] [CrossRef]
- You, Y.; Tian, Y.; Yang, Z.; Shi, J.; Kwak, K.J.; Tong, Y.; Estania, A.P.; Cao, J.; Hsu, W.H.; Liu, Y.; et al. Intradermally delivered mRNA-encapsulating extracellular vesicles for collagen-replacement therapy. Nat. Biomed. Eng. 2023, 7, 887–900. [Google Scholar] [CrossRef]
- Gu, W.; Luozhong, S.; Cai, S.; Londhe, K.; Elkasri, N.; Hawkins, R.; Yuan, Z.; Su-Greene, K.; Yin, Y.; Cruz, M.; et al. Extracellular vesicles incorporating retrovirus-like capsids for the enhanced packaging and systemic delivery of mRNA into neurons. Nat. Biomed. Eng. 2024, 8, 415–426. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; An, Y.; Sun, Y.; Li, J.; Zheng, M.; Zou, Y.; Shi, B. Non-invasive PTEN mRNA brain delivery effectively mitigates growth of orthotopic glioblastoma. Nano Today 2023, 49, 101790. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Sousa, M.M.G.; de Macêdo, L.S.; de França Neto, P.L.; de Moura, I.A.; Espinoza, B.C.F.; Invenção, M.; de Pinho, S.S.; da Gama, M.; de Freitas, A.C. RNA Vaccines: Yeast as a Novel Antigen Vehicle. Vaccines 2023, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Jawalagatti, V.; Kirthika, P.; Lee, J.H. Oral mRNA Vaccines Against Infectious Diseases- A Bacterial Perspective [Invited]. Front. Immunol. 2022, 13, 884862. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, M.M.; Gong, N.; Mukalel, A.J.; Thatte, A.S.; El-Mayta, R.; Patel, S.K.; Metzloff, A.E.; Swingle, K.L.; Han, X.; Xue, L.; et al. In Vivo mRNA CAR T Cell Engineering via Targeted Ionizable Lipid Nanoparticles with Extrahepatic Tropism. Small 2024, 20, e2304378. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, J.; Sui, D.; Yang, Q.; Wang, T.; Xu, Z.; Li, X.; Gao, X.; Yan, X.; Liu, X.; et al. Simultaneous dendritic cells targeting and effective endosomal escape enhance sialic acid-modified mRNA vaccine efficacy and reduce side effects. J. Control. Release 2023, 364, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, N.; Xue, L.; Billingsley, M.M.; El-Mayta, R.; Shepherd, S.J.; Alameh, M.G.; Weissman, D.; Mitchell, M.J. Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat. Commun. 2023, 14, 75. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 2023, 8, 162. [Google Scholar] [CrossRef]
- Han, X.; Alameh, M.G.; Butowska, K.; Knox, J.J.; Lundgreen, K.; Ghattas, M.; Gong, N.; Xue, L.; Xu, Y.; Lavertu, M.; et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 2023, 18, 1105–1114. [Google Scholar] [CrossRef]
- Ma, Y.; Fenton, O.S. A Unified Strategy to Improve Lipid Nanoparticle Mediated mRNA Delivery Using Adenosine Triphosphate. J. Am. Chem. Soc. 2023, 145, 19800–19811. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Li, J.; Meng, J.; Jiang, Z.; Yang, C.; Wen, Y.; Liu, S.; Cheng, X.; Mi, S.; et al. All-Trans-Retinoic Acid-Adjuvanted mRNA Vaccine Induces Mucosal Anti-Tumor Immune Responses for Treating Colorectal Cancer. Adv. Sci. 2024, 11, e2309770. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Chen, K.; Zhu, R.; Zhang, Z.; Huang, H.; Qin, S.; Zheng, Q.; He, Z.; He, X.; Xiao, W.; et al. Manganese-coordinated mRNA vaccines with enhanced mRNA expression and immunogenicity induce robust immune responses against SARS-CoV-2 variants. Sci. Adv. 2022, 8, eabq3500. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Gosselin, E.A.; Sengupta, D.; Concepcion, H.; Arinze, C.; Chow, J.; Nikiforov, A.; Komoroski, V.; MacFarlane, C.; Sullivan, C.; et al. mRNAs encoding self-DNA reactive cGAS enhance the immunogenicity of lipid nanoparticle vaccines. mBio 2023, 14, e0250623. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, S.; Selmi, A.; Diken, M.; Sebastian, M.; Osterloh, P.; Schild, H.; Huber, C.; Türeci, O.; Sahin, U. Increased antigen presentation efficiency by coupling antigens to MHC class I trafficking signals. J. Immunol. 2008, 180, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wojtak, K.; Perales-Puchalt, A.; Weiner, D.B. Novel Synthetic DNA Immunogens Targeting Latent Expressed Antigens of Epstein-Barr Virus Elicit Potent Cellular Responses and Inhibit Tumor Growth. Vaccines 2019, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, A.Y.; Raji, I.; Atyeo, C.; Raimondo, T.M.; Gordon, A.G.R.; Rhym, L.H.; Samad, T.; MacIsaac, C.; Witten, J.; et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sun, Z.; Yao, Y.; Chen, L.; Su, X. Immunogenicity of the Xcl1-SARS-CoV-2 Spike Fusion DNA Vaccine for COVID-19. Vaccines 2022, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef]
- Takano, T.; Morikawa, M.; Adachi, Y.; Kabasawa, K.; Sax, N.; Moriyama, S.; Sun, L.; Isogawa, M.; Nishiyama, A.; Onodera, T.; et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell Rep. Med. 2022, 3, 100631. [Google Scholar] [CrossRef]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J.; White, P. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 6777. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, B.; Li, K.; Liu, Y.; Li, C.; Zheng, L.; Zhang, M.; Yang, T.; Guo, S.; Dong, X.; et al. Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia. Sci. Adv. 2022, 8, eabm1418. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, A.; Patrizii, P.; Teti, A.; Sutera, F.M.; Baran-Rachwalska, P.; Burns, C.; Nandi, U.; Welsh, M.; Torabi-Pour, N.; Dehsorkhi, A.; et al. Novel hybrid silicon-lipid nanoparticles deliver a siRNA to cure autosomal dominant osteopetrosis in mice. Implications for gene therapy in humans. Mol. Ther. Nucleic Acids 2023, 33, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Friis, K.P.; Gracin, S.; Oag, S.; Leijon, A.; Sand, E.; Lindberg, B.; Lázaro-Ibáñez, E.; Lindqvist, J.; Whitehead, K.A.; Bak, A. Spray dried lipid nanoparticle formulations enable intratracheal delivery of mRNA. J. Control. Release 2023, 363, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Zhou, Q.A. PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective. Bioconjugate Chem. 2023, 34, 941–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Yuan, C.; Xu, X.; Zhou, W.; Huang, Y.; Lu, H.; Zheng, Y.; Luo, G.; Shang, J.; et al. Polyethylene glycol (PEG)-associated immune responses triggered by clinically relevant lipid nanoparticles in rats. NPJ Vaccines 2023, 8, 169. [Google Scholar] [CrossRef]
- Kang, D.D.; Hou, X.; Wang, L.; Xue, Y.; Li, H.; Zhong, Y.; Wang, S.; Deng, B.; McComb, D.W.; Dong, Y. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 2024, 37, 86–93. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Sun, W.; Qi, H. Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems. Vaccines 2024, 12, 873. https://doi.org/10.3390/vaccines12080873
Wu Z, Sun W, Qi H. Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems. Vaccines. 2024; 12(8):873. https://doi.org/10.3390/vaccines12080873
Chicago/Turabian StyleWu, Zhongyan, Weilu Sun, and Hailong Qi. 2024. "Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems" Vaccines 12, no. 8: 873. https://doi.org/10.3390/vaccines12080873
APA StyleWu, Z., Sun, W., & Qi, H. (2024). Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems. Vaccines, 12(8), 873. https://doi.org/10.3390/vaccines12080873






