Trends in Viral Vector-Based Vaccines for Tuberculosis: A Patent Review (2010–2023)
Abstract
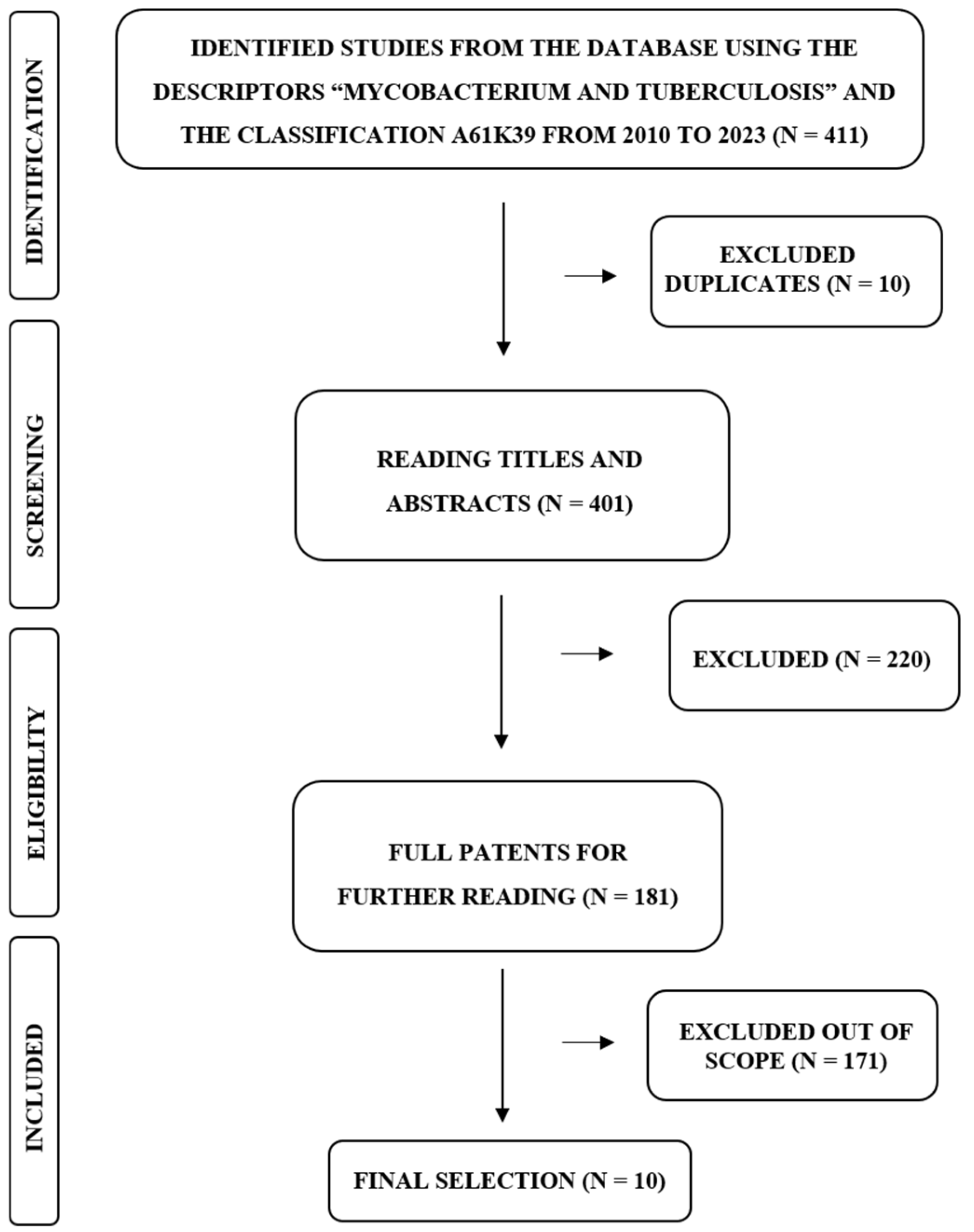
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Main Patented Strategies for TB Vaccine between 2010 and 2023: Few Patents Based on Nucleic Acid, Viral Vector, or Inactivation
3.2. Patents Based on Viral Vector for TB between 2010 and 2023
3.2.1. Viral Vectors
3.2.2. Mtb Antigens
3.2.3. Experimental Models
3.2.4. Current Stage of Patent’s Development: Information on Preclinical and Clinical Trials
3.3. Strength and Limitations of Viral Vector-Based Strategies
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russell, D.G. Mycobacterium tuberculosis: Here Today, and Here Tomorrow. Nat. Rev. Mol. Cell Biol. 2001, 2, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Mycobacterium tuberculosis Pathogenesis and Molecular Determinants of Virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023.
- Bourzac, K. Infectious Disease: Beating the Big Three. Nature 2014, 507, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; McNabb, S.J.; Hilsenrath, P.; Bae, S.; Lykens, K.; Vecino, E.; Munguia, G.; Miller, T.L.; Drewyer, G.; Weis, S.E. Pulmonary Impairment after Tuberculosis and Its Contribution to TB Burden. BMC Public Health 2010, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Vekemans, J.; Brennan, M.J.; Hatherill, M.; Schrager, L.; Fritzell, B.; Rutkowski, K.; De Vos, B.; Zignol, M.; Thiry, G.; Ginsberg, A.M.; et al. Preferred Product Characteristics for Therapeutic Vaccines to Improve Tuberculosis Treatment Outcomes: Key Considerations from World Health Organization Consultations. Vaccine 2020, 38, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Schünemann, H.J.; Harausz, E.; González-Angulo, L.; Lienhardt, C.; Jaramillo, E.; Weyer, K. World Health Organization Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Eur. Respir. J. 2017, 49, 1602308. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; Griffiths, U.K.; Sumner, T.; Laurence, Y.V.; Gheorghe, A.; Vassall, A.; Glaziou, P.; White, R.G. Impact and Cost-Effectiveness of New Tuberculosis Vaccines in Low- and Middle-Income Countries. Proc. Natl. Acad. Sci. USA 2014, 111, 15520–15525. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.M.; Dantas, O.M.S.; Ximenes, R.; Barreto, M.L. BCG vaccine against tuberculosis: Its protective effect and vaccination policies. Rev. Saude Publica 2007, 41 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef]
- Rowland, R.; McShane, H. Tuberculosis Vaccines in Clinical Trials. Expert Rev. Vaccines 2011, 10, 645–658. [Google Scholar]
- Aronson, N.E.; Santosham, M.; Comstock, G.W.; Howard, R.S.; Moulton, L.H.; Rhoades, E.R.; Harrison, L.H. Long-Term Efficacy of BCG Vaccine in American Indians and Alaska Natives: A 60-Year Follow-up Study. JAMA 2004, 291, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, I.; Pimpin, L.; Ariti, C.; Beynon, R.; Mangtani, P.; Sterne, J.A.C.; Fine, P.E.M.; Smith, P.G.; Lipman, M.; Elliman, D.; et al. Systematic Review and Meta-Analysis of the Current Evidence on the Duration of Protection by Bacillus Calmette-Guérin Vaccination against Tuberculosis. Health Technol. Assess. 2013, 17, 1–372, v–vi. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E. Vaccine Development Against Tuberculosis Over the Last 140 Years: Failure as Part of Success. Front. Microbiol. 2021, 12, 750124. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Flynn, J.L. Sterilizing Immunity: New Opportunities for Rational TB Vaccine Design. J. Exp. Med. 2021, 218, e20210454. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.-B.; Niu, S. In Silico Analysis of Epitope-Based Vaccine Candidate against Tuberculosis Using Reverse Vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Kaufmann, S.H.E. Novel Vaccination Strategies against Tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 4, a018523. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Kaufmann, S.H.E. Next-Generation Vaccines Based on Bacille Calmette-Guérin. Front. Immunol. 2018, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ye, Z.; Li, L.; Yang, L.; Gong, W. Next-Generation TB Vaccines: Progress, Challenges, and Prospects. Vaccines 2023, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.P.; Rodriguez, D.; Santos, C.C.; Amaral, E.P.; Rofatto, H.K.; Junqueira-Kipnis, A.P.; Gonçalves, E.D.C.; D’Império-Lima, M.R.; Hirata, M.H.; Silva, C.L.; et al. Recombinant BCG Expressing LTAK63 Adjuvant Induces Superior Protection against Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 2109. [Google Scholar] [CrossRef]
- Carvalho Dos Santos, C.; Rodriguez, D.; Kanno Issamu, A.; Cezar De Cerqueira Leite, L.; Pereira Nascimento, I. Recombinant BCG Expressing the LTAK63 Adjuvant Induces Increased Early and Long-Term Immune Responses against Mycobacteria. Hum. Vaccines Immunother. 2020, 16, 673–683. [Google Scholar] [CrossRef]
- Defendi, H.G.T.; da Silva Madeira, L.; Borschiver, S. Analysis of the COVID-19 Vaccine Development Process: An Exploratory Study of Accelerating Factors and Innovative Environments. J. Pharm. Innov. 2022, 17, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Cobelens, F.; Suri, R.K.; Helinski, M.; Makanga, M.; Weinberg, A.L.; Schaffmeister, B.; Deege, F.; Hatherill, M. TB Vaccine Roadmap Stakeholder Group Accelerating Research and Development of New Vaccines against Tuberculosis: A Global Roadmap. Lancet Infect. Dis. 2022, 22, e108–e120. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral Vector Vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef] [PubMed]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The Use of Viral Vectors in Vaccine Development. NPJ Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Squeglia, F.; Kramarska, E.; Barra, G.; Choi, H.-G.; Kim, H.-J.; Ruggiero, A.; Berisio, R. A Structural View at Vaccine Development against M. Tuberculosis. Cells 2023, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Charostad, J.; Shokouh, S.J.H.; Barati, M. An overview of history, evolution, and manufacturing of various generations of vaccines. J. Arch. Mil. Med. 2017, 5, e12315. [Google Scholar] [CrossRef]
- Nascimento Junior, J.A.C.; Santos, A.M.; Quintans-Júnior, L.J.; Walker, C.I.B.; Borges, L.P.; Serafini, M.R. SARS, MERS and SARS-CoV-2 (COVID-19) Treatment: A Patent Review. Expert Opin. Ther. Pat. 2020, 30, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and Efficacy of MVA85A, a New Tuberculosis Vaccine, in Infants Previously Vaccinated with BCG: A Randomised, Placebo-Controlled Phase 2b Trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Thanthrige-Don, N.; Afkhami, S.; Lai, R.; Damjanovic, D.; Zganiacz, A.; Feng, X.; Yao, X.-D.; Rosenthal, K.L.; Medina, M.F.; et al. Novel Chimpanzee Adenovirus-Vectored Respiratory Mucosal Tuberculosis Vaccine: Overcoming Local Anti-Human Adenovirus Immunity for Potent TB Protection. Mucosal Immunol. 2015, 8, 1373–1387. [Google Scholar] [CrossRef]
- Afkhami, S.; LeClair, D.A.; Haddadi, S.; Lai, R.; Toniolo, S.P.; Ertl, H.C.; Cranston, E.D.; Thompson, M.R.; Xing, Z. Spray Dried Human and Chimpanzee Adenoviral-Vectored Vaccines Are Thermally Stable and Immunogenic in Vivo. Vaccine 2017, 35, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, S.; Lai, R.; D’agostino, M.R.; Vaseghi-Shanjani, M.; Zganiacz, A.; Yao, Y.; Jeyanathan, M.; Xing, Z. Single-Dose Mucosal Immunotherapy With Chimpanzee Adenovirus-Based Vaccine Accelerates Tuberculosis Disease Control and Limits Its Rebound After Antibiotic Cessation. J. Infect. Dis. 2019, 220, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Belnoue, E.; Vogelzang, A.; Nieuwenhuizen, N.E.; Krzyzaniak, M.A.; Darbre, S.; Kreutzfeldt, M.; Wagner, I.; Merkler, D.; Lambert, P.-H.; Kaufmann, S.H.E.; et al. Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 2700. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wong, K.-W.; Zhao, H.-M.; Wen, H.-L.; Ji, P.; Ma, H.; Wu, K.; Lu, S.-H.; Li, F.; Li, Z.-M.; et al. Sendai Virus Mucosal Vaccination Establishes Lung-Resident Memory CD8 T Cell Immunity and Boosts BCG-Primed Protection against TB in Mice. Mol. Ther. 2017, 25, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gu, L.; Li, C.-L.; Shu, T.; Lowrie, D.B.; Fan, X.-Y. The Profile of T Cell Responses in Bacille Calmette-Guérin-Primed Mice Boosted by a Novel Sendai Virus Vectored Anti-Tuberculosis Vaccine. Front. Immunol. 2018, 9, 1796. [Google Scholar] [CrossRef]
- Buzitskaya, Z.; Stosman, K.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Abylai Sansyzbay, A.; Shurygina, A.-P.; Aleksandrov, A.; Sivak, K.; Stukova, M. A New Intranasal Influenza Vector-Based Vaccine TB/FLU-04L Against Tuberculosis: Preclinical Safety Studies. Drug Res. 2022, 72, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Shurygina, A.-P.; Zabolotnykh, N.; Vinogradova, T.; Khairullin, B.; Kassenov, M.; Nurpeisova, A.; Sarsenbayeva, G.; Sansyzbay, A.; Vasilyev, K.; Buzitskaya, J.; et al. Preclinical Evaluation of TB/FLU-04L-An Intranasal Influenza Vector-Based Boost Vaccine against Tuberculosis. Int. J. Mol. Sci. 2023, 24, 7439. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.G.; Zak, D.E.; Xu, G.; Ford, J.C.; Marshall, E.E.; Malouli, D.; Gilbride, R.M.; Hughes, C.M.; Ventura, A.B.; Ainslie, E.; et al. Prevention of Tuberculosis in Rhesus Macaques by a Cytomegalovirus-Based Vaccine. Nat. Med. 2018, 24, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sayedahmed, E.E.; Singh, V.K.; Mishra, A.; Dorta-Estremera, S.; Nookala, S.; Canaday, D.H.; Chen, M.; Wang, J.; Sastry, K.J.; et al. A Recombinant Bovine Adenoviral Mucosal Vaccine Expressing Mycobacterial Antigen-85B Generates Robust Protection against Tuberculosis in Mice. Cell Rep. Med. 2021, 2, 100372. [Google Scholar] [CrossRef]
- Davison, A.J.; Benkő, M.; Harrach, B. Genetic Content and Evolution of Adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med. 2021, 42, 800–821. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.-H.; Lowrie, D.B.; Fan, X.-Y. Research Advances for Virus-Vectored Tuberculosis Vaccines and Latest Findings on Tuberculosis Vaccine Development. Front. Immunol. 2022, 13, 895020. [Google Scholar] [CrossRef] [PubMed]
- Weklak, D.; Pembaur, D.; Koukou, G.; Jönsson, F.; Hagedorn, C.; Kreppel, F. Genetic and Chemical Capsid Modifications of Adenovirus Vectors to Modulate Vector-Host Interactions. Viruses 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, Z.C.; Krendelchtchikov, A.; Krendelchtchikova, V.; Wu, H.; Matthews, Q.L. Using Multivalent Adenoviral Vectors for HIV Vaccination. PLoS ONE 2013, 8, e60347. [Google Scholar] [CrossRef]
- Tang, X.; Yang, Y.; Xia, X.; Zhang, C.; Yang, X.; Song, Y.; Dai, X.; Wang, M.; Zhou, D. Recombinant Adenoviruses Displaying Matrix 2 Ectodomain Epitopes on Their Fiber Proteins as Universal Influenza Vaccines. J. Virol. 2017, 91, e02462-16. [Google Scholar] [CrossRef]
- Sedegah, M.; Tamminga, C.; McGrath, S.; House, B.; Ganeshan, H.; Lejano, J.; Abot, E.; Banania, G.J.; Sayo, R.; Farooq, F.; et al. Adenovirus 5-Vectored P. Falciparum Vaccine Expressing CSP and AMA1. Part A: Safety and Immunogenicity in Seronegative Adults. PLoS ONE 2011, 6, e24586. [Google Scholar] [CrossRef]
- Fuchs, J.D.; Bart, P.-A.; Frahm, N.; Morgan, C.; Gilbert, P.B.; Kochar, N.; DeRosa, S.C.; Tomaras, G.D.; Wagner, T.M.; Baden, L.R.; et al. Safety and Immunogenicity of a Recombinant Adenovirus Serotype 35-Vectored HIV-1 Vaccine in Adenovirus Serotype 5 Seronegative and Seropositive Individuals. J. AIDS Clin. Res. 2015, 6, 461. [Google Scholar] [CrossRef]
- Cicin-Sain, L.; Sylwester, A.W.; Hagen, S.I.; Siess, D.C.; Currier, N.; Legasse, A.W.; Fischer, M.B.; Koudelka, C.W.; Axthelm, M.K.; Nikolich-Zugich, J.; et al. Cytomegalovirus-Specific T Cell Immunity Is Maintained in Immunosenescent Rhesus Macaques. J. Immunol. 2011, 187, 1722–1732. [Google Scholar] [CrossRef]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly Targeted Human Cytomegalovirus-Specific CD4+ and CD8+ T Cells Dominate the Memory Compartments of Exposed Subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef]
- Gerna, G.; Lilleri, D.; Fornara, C.; Bruno, F.; Gabanti, E.; Cane, I.; Furione, M.; Revello, M.G. Differential Kinetics of Human Cytomegalovirus Load and Antibody Responses in Primary Infection of the Immunocompetent and Immunocompromised Host. J. Gen. Virol. 2015, 96, 360–369. [Google Scholar] [CrossRef]
- Zeng, J.; Jaijyan, D.K.; Yang, S.; Pei, S.; Tang, Q.; Zhu, H. Exploring the Potential of Cytomegalovirus-Based Vectors: A Review. Viruses 2023, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Gbedande, K.; Ibitokou, S.A.; Ong, M.L.; Degli-Esposti, M.A.; Brown, M.G.; Stephens, R. Boosting Live Malaria Vaccine with Cytomegalovirus Vector Can Prolong Immunity through Innate and Adaptive Mechanisms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Méndez, A.C.; Rodríguez-Rojas, C.; Del Val, M. Vaccine Vectors: The Bright Side of Cytomegalovirus. Med. Microbiol. Immunol. 2019, 208, 349–363. [Google Scholar] [CrossRef]
- Beverley, P.C.L.; Ruzsics, Z.; Hey, A.; Hutchings, C.; Boos, S.; Bolinger, B.; Marchi, E.; O’Hara, G.; Klenerman, P.; Koszinowski, U.H.; et al. A Novel Murine Cytomegalovirus Vaccine Vector Protects against Mycobacterium tuberculosis. J. Immunol. 2014, 193, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Galon, J.; Dieu-Nosjean, M.-C.; Tartour, E.; Sautès-Fridman, C.; Fridman, W.-H. Immune Infiltration in Human Tumors: A Prognostic Factor That Should Not Be Ignored. Oncogene 2010, 29, 1093–1102. [Google Scholar] [CrossRef]
- Hansen, S.G.; Vieville, C.; Whizin, N.; Coyne-Johnson, L.; Siess, D.C.; Drummond, D.D.; Legasse, A.W.; Axthelm, M.K.; Oswald, K.; Trubey, C.M.; et al. Effector Memory T Cell Responses Are Associated with Protection of Rhesus Monkeys from Mucosal Simian Immunodeficiency Virus Challenge. Nat. Med. 2009, 15, 293–299. [Google Scholar] [CrossRef]
- Valencia, S.; Gill, R.B.; Dowdell, K.C.; Wang, Y.; Hornung, R.; Bowman, J.J.; Lacayo, J.C.; Cohen, J.I. Comparison of Vaccination with Rhesus CMV (RhCMV) Soluble gB with a RhCMV Replication-Defective Virus Deleted for MHC Class I Immune Evasion Genes in a RhCMV Challenge Model. Vaccine 2019, 37, 333–342. [Google Scholar] [CrossRef]
- Ynga-Durand, M.A.; Dekhtiarenko, I.; Cicin-Sain, L. Vaccine Vectors Harnessing the Power of Cytomegaloviruses. Vaccines 2019, 7, 152. [Google Scholar] [CrossRef]
- Arbeitskreis Blut; Untergruppe «Bewertung Blutassoziierter Krankheitserreger». Influenza Virus. Transfus. Med. Hemother. 2009, 36, 32–39. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; Rimmelzwaan, G.F. Viral Vector-Based Influenza Vaccines. Hum. Vaccines Immunother. 2016, 12, 2881–2901. [Google Scholar] [CrossRef]
- Neumann, G.; Watanabe, T.; Ito, H.; Watanabe, S.; Goto, H.; Gao, P.; Hughes, M.; Perez, D.R.; Donis, R.; Hoffmann, E.; et al. Generation of Influenza A Viruses Entirely from Cloned cDNAs. Proc. Natl. Acad. Sci. USA 1999, 96, 9345–9350. [Google Scholar] [CrossRef] [PubMed]
- Stukova, M.A.; Sereinig, S.; Zabolotnyh, N.V.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.I.; Katinger, H.; Kiselev, O.I.; Egorov, A. Vaccine Potential of Influenza Vectors Expressing Mycobacterium tuberculosis ESAT-6 Protein. Tuberculosis 2006, 86, 236–246. [Google Scholar] [CrossRef]
- Sereinig, S.; Stukova, M.; Zabolotnyh, N.; Ferko, B.; Kittel, C.; Romanova, J.; Vinogradova, T.; Katinger, H.; Kiselev, O.; Egorov, A. Influenza Virus NS Vectors Expressing the Mycobacterium tuberculosis ESAT-6 Protein Induce CD4+ Th1 Immune Response and Protect Animals against Tuberculosis Challenge. Clin. Vaccine Immunol. 2006, 13, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Ferko, B.; Stasakova, J.; Sereinig, S.; Romanova, J.; Katinger, D.; Niebler, B.; Katinger, H.; Egorov, A. Hyperattenuated Recombinant Influenza A Virus Nonstructural-Protein-Encoding Vectors Induce Human Immunodeficiency Virus Type 1 Nef-Specific Systemic and Mucosal Immune Responses in Mice. J. Virol. 2001, 75, 8899–8908. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The Viruses and Their Replication. In Fields Virology, 5th ed.; Lippincott, Williams, and Wilkins: Philadelphia, PA, USA, 2007; pp. 1449–1496. [Google Scholar]
- Nakanishi, M.; Otsu, M. Development of Sendai Virus Vectors and Their Potential Applications in Gene Therapy and Regenerative Medicine. Curr. Gene Ther. 2012, 12, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Noda, K. Newborn Virus Pneumonitis, Type Sendai. III. Report: Pathological Studies on the 9 Autopsy Cases and the Mice Inoculated with the New-Found Virus. Yokohama Med. Bull. 1953, 4, 281–287. [Google Scholar] [PubMed]
- Hurwitz, J.L. Development of Recombinant Sendai Virus Vaccines for Prevention of Human Parainfluenza and Respiratory Syncytial Virus Infections. Pediatr. Infect. Dis. J. 2008, 27, S126–S128. [Google Scholar] [CrossRef]
- Hallam, S.J.; Koma, T.; Maruyama, J.; Paessler, S. Review of Mammarenavirus Biology and Replication. Front. Microbiol. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; de la Torre, J.C. Human Pathogenic Arenaviruses (Arenaviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 507–517. ISBN 9780128145166. [Google Scholar]
- Emonet, S.F.; Garidou, L.; McGavern, D.B.; de la Torre, J.C. Generation of Recombinant Lymphocytic Choriomeningitis Viruses with Trisegmented Genomes Stably Expressing Two Additional Genes of Interest. Proc. Natl. Acad. Sci. USA 2009, 106, 3473–3478. [Google Scholar] [CrossRef]
- Flatz, L.; Hegazy, A.N.; Bergthaler, A.; Verschoor, A.; Claus, C.; Fernandez, M.; Gattinoni, L.; Johnson, S.; Kreppel, F.; Kochanek, S.; et al. Development of Replication-Defective Lymphocytic Choriomeningitis Virus Vectors for the Induction of Potent CD8+ T Cell Immunity. Nat. Med. 2010, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Homann, D.; Teyton, L.; Oldstone, M.B. Differential Regulation of Antiviral T-Cell Immunity Results in Stable CD8+ but Declining CD4+ T-Cell Memory. Nat. Med. 2001, 7, 913–919. [Google Scholar] [CrossRef]
- Flatz, L.; Cheng, C.; Wang, L.; Foulds, K.E.; Ko, S.-Y.; Kong, W.-P.; Roychoudhuri, R.; Shi, W.; Bao, S.; Todd, J.-P.; et al. Gene-Based Vaccination with a Mismatched Envelope Protects against Simian Immunodeficiency Virus Infection in Nonhuman Primates. J. Virol. 2012, 86, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Zadeh Babaki, M.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 Complex as a Powerful Mycobacterium tuberculosis Immunogene: Biology, Immune-Pathogenicity, Applications in Diagnosis, and Vaccine Design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Gordhan, B.G.; Downing, K.J.; Sung, N.; Vostroktunova, G.; Machowski, E.E.; Tsenova, L.; Young, M.; Kaprelyants, A.; Kaplan, G.; et al. The Resuscitation-Promoting Factors of Mycobacterium tuberculosis Are Required for Virulence and Resuscitation from Dormancy but Are Collectively Dispensable for Growth in Vitro. Mol. Microbiol. 2008, 67, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-D.; Guinn, K.M.; Harrell, M.I.; Liao, R.; Voskuil, M.I.; Tompa, M.; Schoolnik, G.K.; Sherman, D.R. Rv3133c/dosR Is a Transcription Factor That Mediates the Hypoxic Response of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Madan, R.; Pandit, K.; Bhati, L.; Kumar, H.; Kumari, N.; Singh, S. Mining the Mycobacterium tuberculosis Proteome for Identification of Potential T-Cell Epitope Based Vaccine Candidates. Microb. Pathog. 2021, 157, 104996. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-J.; Bell, H.; Hsieh, C.-L.; Ptak, C.P.; Chang, Y.-F. Novel Mycobacteria Antigen 85 Complex Binding Motif on Fibronectin. J. Biol. Chem. 2012, 287, 1892–1902. [Google Scholar] [CrossRef] [PubMed]
- Belisle, J.T.; Vissa, V.D.; Sievert, T.; Takayama, K.; Brennan, P.J.; Besra, G.S. Role of the Major Antigen of Mycobacterium tuberculosis in Cell Wall Biogenesis. Science 1997, 276, 1420–1422. [Google Scholar] [CrossRef]
- Houben, E.N.G.; Korotkov, K.V.; Bitter, W. Take Five—Type VII Secretion Systems of Mycobacteria. Biochim. Biophys. Acta 2014, 1843, 1707–1716. [Google Scholar] [CrossRef]
- Gröschel, M.I.; Sayes, F.; Simeone, R.; Majlessi, L.; Brosch, R. ESX Secretion Systems: Mycobacterial Evolution to Counter Host Immunity. Nat. Rev. Microbiol. 2016, 14, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bisht, M.K.; Mukhopadhyay, S. Secretory Proteins of Mycobacterium tuberculosis and Their Roles in Modulation of Host Immune Responses: Focus on Therapeutic Targets. FEBS J. 2022, 289, 4146–4171. [Google Scholar] [CrossRef] [PubMed]
- Ruhwald, M.; de Thurah, L.; Kuchaka, D.; Zaher, M.R.; Salman, A.M.; Abdel-Ghaffar, A.-R.; Shoukry, F.A.; Michelsen, S.W.; Soborg, B.; Blauenfeldt, T.; et al. Introducing the ESAT-6 Free IGRA, a Companion Diagnostic for TB Vaccines Based on ESAT-6. Sci. Rep. 2017, 7, 45969. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, S.; Fang, R.; Li, X.; Xing, J.; Li, Z.; Song, N. Enhancing TB Vaccine Efficacy: Current Progress on Vaccines, Adjuvants and Immunization Strategies. Vaccines 2023, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Kana, B.D.; Mizrahi, V. Resuscitation-Promoting Factors as Lytic Enzymes for Bacterial Growth and Signaling. FEMS Immunol. Med. Microbiol. 2010, 58, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S.D.; Mueller, H.; Kunitz, F.; Neher, A.; Hoffmann, H.; Franken, K.L.C.M.; Repsilber, D.; Ottenhoff, T.H.M.; Kaufmann, S.H.E.; Jacobsen, M. Identification of T-Cell Antigens Specific for Latent Mycobacterium tuberculosis Infection. PLoS ONE 2009, 4, e5590. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Saraav, I.; Sharma, S. Immunogenic Potential of Latency Associated Antigens against Mycobacterium tuberculosis. Vaccine 2014, 32, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Kashino, S.S.; Ovendale, P.; Izzo, A.; Campos-Neto, A. Unique Model of Dormant Infection for Tuberculosis Vaccine Development. Clin. Vaccine Immunol. 2006, 13, 1014–1021. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Lyadova, I.V.; Kondratieva, T.K.; Majorov, K.B.; Scheglov, I.V.; Orlova, M.O.; Apt, A.S. Neutrophil Responses to Mycobacterium tuberculosis Infection in Genetically Susceptible and Resistant Mice. Infect. Immun. 2005, 73, 1744–1753. [Google Scholar] [CrossRef]
- Soldevilla, P.; Vilaplana, C.; Cardona, P.-J. Mouse Models for Mycobacterium tuberculosis Pathogenesis: Show and Do Not Tell. Pathogens 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavón, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the Kinetics of Th1, Th2 Cells and Pathology in a Murine Model of Experimental Pulmonary Tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar] [PubMed]
- Nuermberger, E.L. Preclinical Efficacy Testing of New Drug Candidates. Microbiol. Spectr. 2017, 5, 1–22. [Google Scholar] [CrossRef] [PubMed]
- De Groote, M.A.; Gilliland, J.C.; Wells, C.L.; Brooks, E.J.; Woolhiser, L.K.; Gruppo, V.; Peloquin, C.A.; Orme, I.M.; Lenaerts, A.J. Comparative Studies Evaluating Mouse Models Used for Efficacy Testing of Experimental Drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Bini, E.I.; Mata Espinosa, D.; Marquina Castillo, B.; Barrios Payán, J.; Colucci, D.; Cruz, A.F.; Zatarain, Z.L.; Alfonseca, E.; Pardo, M.R.; Bottasso, O.; et al. The Influence of Sex Steroid Hormones in the Immunopathology of Experimental Pulmonary Tuberculosis. PLoS ONE 2014, 9, e93831. [Google Scholar] [CrossRef] [PubMed]
- Dibbern, J.; Eggers, L.; Schneider, B.E. Sex Differences in the C57BL/6 Model of Mycobacterium tuberculosis Infection. Sci. Rep. 2017, 7, 10957. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, D.; Mehra, S.; Didier, P.J.; Lackner, A.A. The non-human primate model of tuberculosis. J. Med. Primatol. 2012, 41, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.W.; Vervenne, R.A.W.; Kondova, I.; van Kralingen, K.W.; Remarque, E.J.; Braskamp, G.; van der Werff, N.M.; Kersbergen, A.; Ottenhoff, T.H.M.; Heidt, P.J.; et al. MVA.85A Boosting of BCG and an Attenuated, phoP Deficient M. Tuberculosis Vaccine Both Show Protective Efficacy against Tuberculosis in Rhesus Macaques. PLoS ONE 2009, 4, e5264. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Dietrich, J.; Tan, E.; Abalos, R.M.; Burgos, J.; Bigbee, C.; Bigbee, M.; Milk, L.; Gideon, H.P.; Rodgers, M.; et al. The Multistage Vaccine H56 Boosts the Effects of BCG to Protect Cynomolgus Macaques against Active Tuberculosis and Reactivation of Latent Mycobacterium tuberculosis Infection. J. Clin. Investig. 2012, 122, 303–314. [Google Scholar] [CrossRef]
- Foreman, T.W.; Mehra, S.; Lackner, A.A.; Kaushal, D. Translational Research in the Nonhuman Primate Model of Tuberculosis. ILAR J. 2017, 58, 151–159. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal Vaccines—Fortifying the Frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Uddbäck, I.; Cartwright, E.K.; Schøller, A.S.; Wein, A.N.; Hayward, S.L.; Lobby, J.; Takamura, S.; Thomsen, A.R.; Kohlmeier, J.E.; Christensen, J.P. Long-Term Maintenance of Lung Resident Memory T Cells Is Mediated by Persistent Antigen. Mucosal Immunol. 2021, 14, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Lichty, B.D. Use of Recombinant Virus-Vectored Tuberculosis Vaccines for Respiratory Mucosal Immunization. Tuberculosis 2006, 86, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.A.; Carter, S.P.; Wilson, G.J.; Jones, G.; Brown, E.; Hewinson, R.G.; Vordermeier, M. Vaccination against tuberculosis in badgers and cattle: An overview of the challenges, developments and current research priorities in Great Britain. Vet. Rec. 2014, 175, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.; Whelan, A.; Clifford, D.; Salguero, F.J.; Xing, Z.; Gilbert, S.; McShane, H.; Hewinson, R.G.; Vordermeier, M.; Villarreal-Ramos, B. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine 2014, 32, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Smaill, F.; Jeyanathan, M.; Smieja, M.; Medina, M.F.; Thanthrige-Don, N.; Zganiacz, A.; Yin, C.; Heriazon, A.; Damjanovic, D.; Puri, L.; et al. A Human Type 5 Adenovirus-Based Tuberculosis Vaccine Induces Robust T Cell Responses in Humans despite Preexisting Anti-Adenovirus Immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C.J. Chimpanzee Adenovirus Antibodies in Humans, Sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef]
- Colloca, S.; Barnes, E.; Folgori, A.; Ammendola, V.; Capone, S.; Cirillo, A.; Siani, L.; Naddeo, M.; Grazioli, F.; Esposito, M.L.; et al. Vaccine Vectors Derived from a Large Collection of Simian Adenoviruses Induce Potent Cellular Immunity across Multiple Species. Sci. Transl. Med. 2012, 4, 115ra2. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, A.; Jayashankar, L.; Mittal, S.K. Bovine Adenoviral Vector-Based H5N1 Influenza Vaccine Overcomes Exceptionally High Levels of Pre-Existing Immunity against Human Adenovirus. Mol. Ther. 2008, 16, 965–971. [Google Scholar] [CrossRef]
- Bangari, D.S.; Mittal, S.K. Current Strategies and Future Directions for Eluding Adenoviral Vector Immunity. Curr. Gene Ther. 2006, 6, 215–226. [Google Scholar] [CrossRef]
- Palese, P. RNA Virus Vectors: Where Are We and Where Do We Need to Go? Proc. Natl. Acad. Sci. USA 1998, 95, 12750–12752. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-Existing Immunity against Ad Vectors: Humoral, Cellular, and Innate Response, What’s Important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, M. Viral Vectors for Veterinary Vaccines. Adv. Vet. Med. 1999, 41, 145–161. [Google Scholar] [PubMed]
- Callaway, E. “Make Ebola a Thing of the Past”: First Vaccine against Deadly Virus Approved. Nature 2019, 575, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; Pavithra, K.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-Generation DNA Vaccine Platforms and Delivery Systems: Advances, Challenges and Prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Mata-Espinosa, D.; Lara-Espinosa, J.V.; Barrios-Payán, J.; Hernández-Pando, R. The Use of Viral Vectors for Gene Therapy and Vaccination in Tuberculosis. Pharmaceuticals 2023, 16, 1475. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, D. Recent Advance in the Development of Tuberculosis Vaccines in Clinical Trials and Virus-like Particle-Based Vaccine Candidates. Front. Immunol. 2023, 14, 1238649. [Google Scholar] [CrossRef]
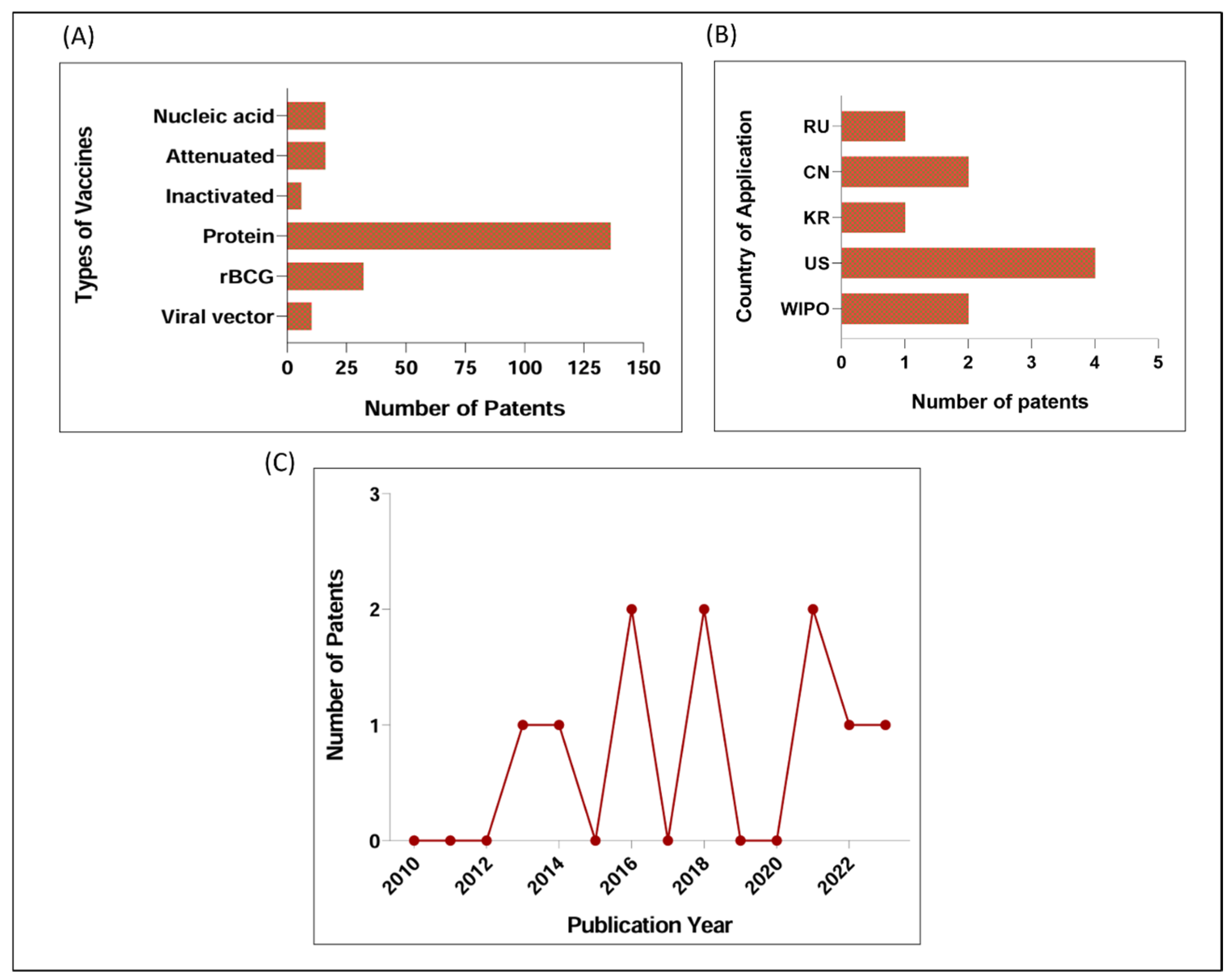
| Patent Number | Country Year | Viral Vector | Antigen(s) | Methods | Information about Clinical Trials and/or Current Stage of Development |
|---|---|---|---|---|---|
| WO2013123579 (A1) | WIPO 2013 | Adenovirus-based (chimpanzee adenovirus—AdC) | Antigenic fragments and combinations of Ag85A, Ag85B, TB 10.4, Rv2660c, Rv1773c | BALB/C mice; intranasal route of immunization; evaluation of activated CD8 T cell and IFN-γ-producing cells in the BAL and lung; intratracheal Mtb challenge | Preclinical phase, protective efficacy comparable to or better than BCG [31,32,33]; it was developed by the same group that developed the AdHu5Ag85, which is currently in phase I clinical trials (NCT02337270) |
| US2016024476 (A1); US9809801 (B2) | USA 2014 | Genetically modified arenaviruses (Lymphocytic choriomeningitis virus) | Ag85A, Ag85B, Ag85C, ESAT-6 family (TB10.3, TB12.9 or TB10.4) | C57BL/6 mice; intravenous and subcutaneous routes of immunization; evaluation of CD8+ T cells were measured in peripheral blood, antigen-specific IFN-γ and TNF-α CD8+ and CD4 T cells; replication-deficiency of viral vectors and infectivity assays; Mtb challenge was not performed or not shown | Preclinical phase, protective efficacy comparable to or better than BCG [34] |
| US10828359 (B2); US2018085449 (A1) | USA 2016 | Sendai virus | Antigenic fragments and combinations of Ag85A and Ag85B | BALB/c mice; intranasal and intramuscular routes of immunization; T- cell response and IFN-γ secreted in the lung or spleen; tested alone or as boosting vaccine for BCG; aerosol challenge with H37Rv Mtb strain | Preclinical phase, protective efficacy comparable to or better than BCG [35,36] |
| CN108018298 (A) | CN 2016 | Adenovirus-based (AdHu5) | Lipidated Ag85A | Mice; route of immunization is not clear; antigen-specific IgG antibody titers in mouse serum after immunization; Mtb challenge was not performed or not shown | Preclinial phase; no reference found |
| KR102135334 (B1); KR20200076335 (A) | KR 2018 | Adenovirus-based (AdHu5) | Ag85B, ESAT6, MPT64, Rv2660, and a signal peptide of secretion (tPA) | C57BL/6 mice; evaluated as a booster for BCG vaccine, subcutaneous route of immunization; evaluation of polyfunctional T cells, IFN-γ secretion and humoral response (IgG); challenge with a highly pathogenic Mtb strain (HN878), protective efficacy comparable to or better than BCG | Preclinial phase; no reference found |
| RU2678175 (C1) | RU 2018 | Recombinant influenza virus (influenza A) | ESAT-6 and Ag85A | Related to improving production of TB/FLU-04L (composition already described in another patent); C57BL/6 mice; intranasal route of immunization; evaluation of CD4 and CD8 T cells; Mtb challenge was not performed or not shown | Preclinical phase, protective efficacy comparable to or better than BCG [37,38]; a clinical trial phase I was registered for the TB/FLU-04L (NCT02501421) in 2013 |
| US2021403951 (A1) | USA 2021 | Rhesus and Human Cytomegalovirus (recombinant RhCMV or HCMV) | Ag85A-Ag85B-Rv3407, Rv1733-Rv2626c, RpfA-RpfC-RpfD, Ag85B-ESAT-6, and Ag85A-ESAT-6-Rv3407-Rv2626c-RpfA-RpfD | NHP (e.g., Rhesus Macaques); subcutaneous route of immunization; evaluation of CD4 and CD8 T cells in PBMC and BAL; challenge with Erdman Mtb strain; various efficacy criteria evaluated as CT scan analysis, necropsy score, and necropsy Mtb cultures; and 9 extrapulmonary tissues | Preclinical phase, protective efficacy comparable to or better than BCG [39] |
| CN112899295 (A) | CN 2021 | Adenovirus-based (not identified) | Ag85B-ESAT-6 and Rv2031c-Rv2626c | Mice; intranasal route of immunization; evaluation of IgA levels in BAL, antigen-specific antibodies in peripheral blood, spleen lymphocyte proliferation, and tissue memory T cells in BAL; Mtb challenge was not performed or not shown | Preclinical; no reference found |
| WO2022192163 (A1) | WIPO 2022 | Adenovirus-based (AdHu5 and bovine adenovirus—BAd) | Ag85B epitope alone or in fusion with autophagy-inducing peptide C5 | C57BL/6 mice; intranasal route of immunization; tested alone or as boosting vaccine for BCG; evaluation of effector and memory T cells after challenge; aerosol challenge with Mtb Erdman strain | Preclinical phase, protective efficacy comparable to or better than BCG [40] |
| US2023365631 (A1) | USA 2023 | Adenovirus-based (AdHu5, chimpanzee adenovirus) and others, such as, poxvirus, RhCMV, HCMV | Ag85B, Ag85A, Rv3407 | C57BL/6 and CB6F1 mice; subcutaneous route of immunization; tested as boosting vaccine for BCG or rBCG; evaluation of T cell responses; Mtb challenge was not performed or not shown for adenovirus-based construct | Preclinical phase; no reference found |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, L.C.; Fernandes, A.M.S.; Alves, I.A.; Serafini, M.R.; Silva, L.d.S.e.; de Freitas, H.F.; Leite, L.C.C.; Santos, C.C. Trends in Viral Vector-Based Vaccines for Tuberculosis: A Patent Review (2010–2023). Vaccines 2024, 12, 876. https://doi.org/10.3390/vaccines12080876
Santos LC, Fernandes AMS, Alves IA, Serafini MR, Silva LdSe, de Freitas HF, Leite LCC, Santos CC. Trends in Viral Vector-Based Vaccines for Tuberculosis: A Patent Review (2010–2023). Vaccines. 2024; 12(8):876. https://doi.org/10.3390/vaccines12080876
Chicago/Turabian StyleSantos, Lana C., Antônio Márcio Santana Fernandes, Izabel Almeida Alves, Mairim Russo Serafini, Leandra da Silva e Silva, Humberto Fonseca de Freitas, Luciana C. C. Leite, and Carina C. Santos. 2024. "Trends in Viral Vector-Based Vaccines for Tuberculosis: A Patent Review (2010–2023)" Vaccines 12, no. 8: 876. https://doi.org/10.3390/vaccines12080876








