Vaccination with Tozimameran Induces T-Cell Activation, but Not Senescent or Exhaustive Alterations, in Kidney Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Schedule of the Study
- (a)
- Anti-SARS-CoV-2 neutralizing antibody (NAb) levels, utilizing chemiluminescence immunoassay (CLIA)
- (b)
- Viral-specific interferon gamma (IFN-γ) production, measured with enzyme-linked immunosorbent spot (ELISpot)
- (c)
- T-lymphocyte subpopulation concentrations, with the use of flow cytometry.
2.3. Laboratory Methods
2.3.1. Flow Cytometry
- (i)
- recent thymic emigrants (CD3+CD4+CD31+ or CD3+CD8+CD31+),
- (ii)
- naïve T-cells (CD3+CD4+CD45RA+CCR7+ or CD3+CD8+CD45RA+CCR7+),
- (iii)
- TCM (CD3+CD4+CD45RA−CCR7+ or CD3+CD8+CD45RA−CCR7+),
- (iv)
- TEM (CD3+CD4+CD45RA−CCR7− or CD3+CD8+CD45RA−CCR7−)
- (v)
- TEMRA (CD3+CD4+CD45RA+CCR7− or CD3+CD8+CD45RA+CCR7−).
- (vi)
- CD3+CD4+CD28+, CD3+CD8+CD28+ and CD3+CD4+CD28−, CD3+CD8+CD28−
- (vii)
- CD3+CD4+PD1+, CD3+CD8+PD1+
2.3.2. Chemiluminescence Immunoassay
2.3.3. Enzyme-Linked Immunosorbent Spot
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
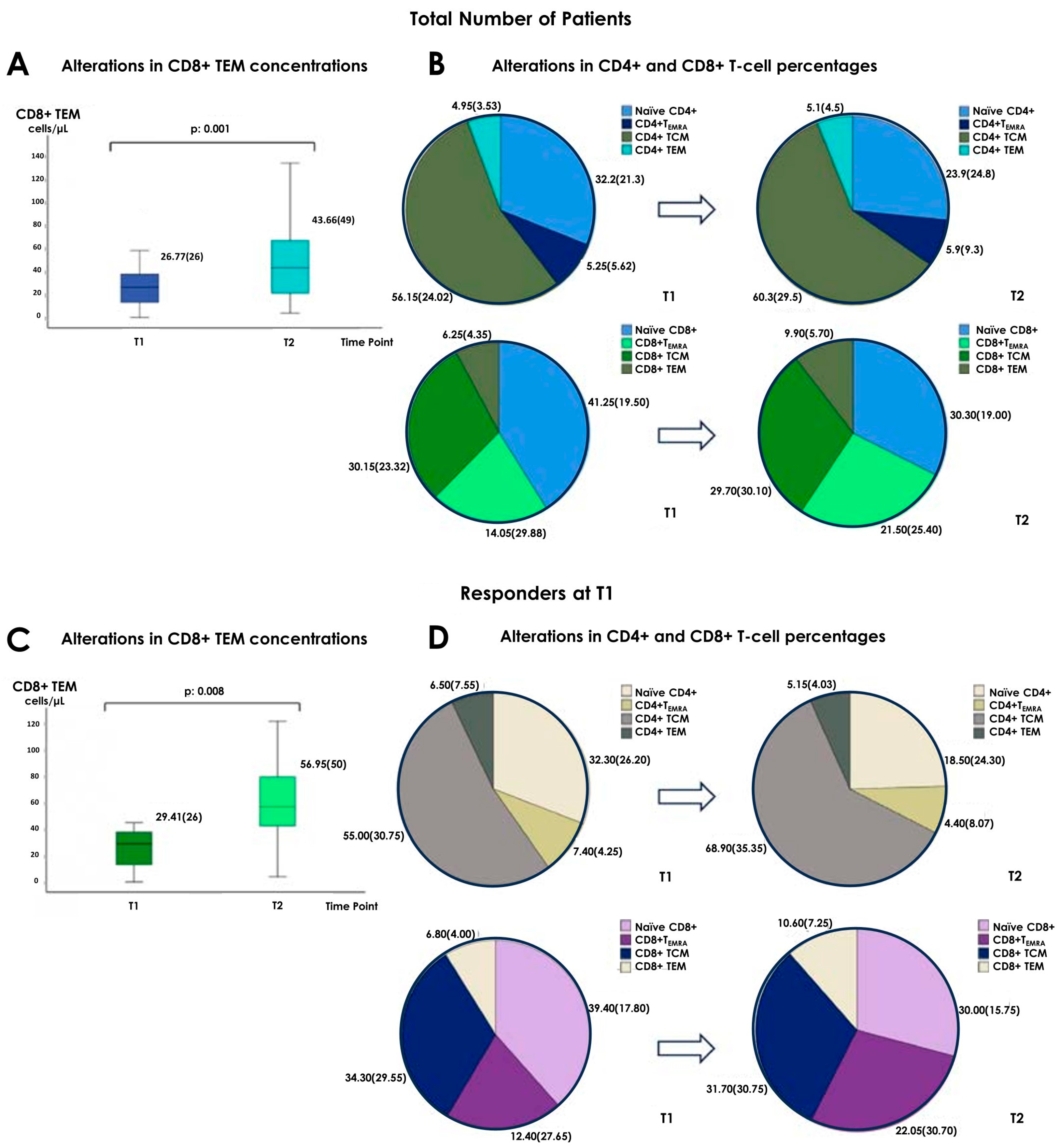
3.2. Alterations in the Concentrations of Cellular Subpopulations
3.3. Alterations in the Percentages of Cellular Subpopulations
3.4. Differences between Responders and Non-Responders at T1 and T2
3.5. Study of the Effects of the Third Tozinameran Dose on Cellular Subpopulations’ Compositions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Larbi, A. Markers of T cell senescence in humans. Int. J. Mol. Sci. 2017, 18, 1742. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Legendre, M.; Bamoulid, J.; Saas, P.; Courivaud, C.; Crepin, T. End-stage renal disease-related accelerated immune senescence: Is rejuvenation of the immune system a therapeutic goal? Front. Med. 2021, 8, 720402. [Google Scholar] [CrossRef]
- Hartzell, S.; Bin, S.; Cantarelli, C.; Haverly, M.; Manrique, J.; Angeletti, A.; Manna, G.; Murphy, B.; Zhang, W.; Levitsky, J.; et al. Kidney Failure Associates with T Cell Exhaustion and Imbalanced Follicular Helper T Cells. Front. Immunol. 2020, 11, 583702. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Chen, R.; Cao, X.; Zou, J.; Ji, J.; Ding, X.; Yu, X. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC Nephrol. 2019, 20, 301. [Google Scholar] [CrossRef]
- Ceprian, N.; Valera, G.; Caro, J.; Yuste, C.; Serroukh, N.; González de Pablos, I.; Oliva, C.; Figuer, A.; Praga, M.; Alique, M.; et al. Effect of kidney transplantation on accelerated immunosenescence and vascular changes induced by chronic kidney disease. Front. Med. 2021, 8, 705159. [Google Scholar] [CrossRef] [PubMed]
- Bouwmans, P.; Messchendorp, A.L.; Sanders, J.S.; Hilbrands, L.; Reinders, M.E.J.; Vart, P.; Bemelman, F.J.; Abrahams, A.C.; van den Dorpel, M.A.; Ten Dam, M.A.; et al. Long-term efficacy and safety of SARS-CoV-2 vaccination in patients with chronic kidney disease, on dialysis or after kidney transplantation: A national prospective observational cohort study. BMC Nephrol. 2022, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Meijers, R.W.; Litjens, N.H.; de Wit, E.A.; Langerak, A.W.; Baan, C.C.; Betjes, M.G. Uremia-associated immunological aging is stably imprinted in the T-cell system and not reversed by kidney transplantation. Transpl. Int. 2014, 27, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- In ‘t Veld, A.E.; Eveleens Maarse, B.C.; Juachon, M.J.; Meziyerh, S.; de Vries, A.P.J.; van Rijn, A.L.; Feltkamp, M.C.W.; Moes, D.J.A.R.; Burggraaf, J.; Moerland, M. Immune responsiveness in stable kidney transplantation patients: Complete inhibition of T-cell proliferation but residual T-cell activity during maintenance immunosuppressive treatment. Clin. Transl. Sci. 2024, 17, e13860. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.; Fischer, S.; Müller, L.; Lübke, N.; Hillebrandt, J.; Andrée, M.; Schmitz, M.; Schmidt, C.; Küçükköylü, S.; Koster, L.; et al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360 2021, 2, 1491–1498. [Google Scholar] [CrossRef]
- Udomkarnjananun, S.; Gatechompol, S.; Leelahavanichkul, A.; Kerr, S.J. Cellular immune response of SARS-CoV-2 vaccination in kidney transplant recipients: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1220148. [Google Scholar] [CrossRef]
- Azim Majumder, M.A.; Razzaque, M.S. Repeated vaccination and ‘vaccine exhaustion’: Relevance to the COVID-19 crisis. Expert Rev. Vaccines 2022, 21, 1011–1014. [Google Scholar] [CrossRef]
- Benitez Fuentes, J.D.; Mohamed Mohamed, K.; de Luna Aguilar, A.; Jiménez García, C.; Guevara-Hoyer, K.; Fernandez-Arquero, M.; Rodríguez de la Peña, M.A.; Garciía Bravo, L.; Jiménez Ortega, A.F.; Flores Navarro, P.; et al. Evidence of exhausted lymphocytes after the third anti-SARS-CoV-2 vaccine dose in cancer patients. Front. Oncol. 2022, 12, 975980. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, X.; Zhang, X.; Yao, H.; Wu, N. Immune interference in effectiveness of influenza and COVID-19 vaccination. Front. Immunol. 2023, 14, 1167214. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2020, 21, 83–100. [Google Scholar] [CrossRef]
- Bert, F.; Scaioli, G.; Vola, L.; Accortanzo, D.; Lo Moro, G.; Siliquini, R. Booster doses of Anti COVID-19 Vaccines: An overview of implementation policies among OECD and EU countries. Int. J. Environ. Res. Public Health 2022, 19, 7233. [Google Scholar] [CrossRef]
- Dadras, O.; SeyedAlinaghi, S.; Karimi, A.; Shojaei, A.; Amiri, A.; Mahdiabadi, S.; Fakhfouri, A.; Razi, A.; Mojdeganlou, H.; Mojdeganlou, P.; et al. COVID-19 Vaccines’ Protection Over Time and the Need for Booster Doses; a Systematic Review. Arch. Acad. Emerg. Med. 2022, 10, e53. [Google Scholar] [CrossRef]
- Al Hajji, Y.; Taylor, H.; Starkey, T.; Lee, L.Y.; Tilby, M. Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: A systematic review. Br. J. Cancer 2022, 127, 1827–1836. [Google Scholar] [CrossRef]
- Fylaktou, A.; Stai, S.; Kasimatis, E.; Xochelli, A.; Nikolaidou, V.; Papadopoulou, A.; Myserlis, G.; Lioulios, G.; Asouchidou, D.; Giannaki, M.; et al. Humoral and cellular immunity are significantly affected in renal transplant recipients, following vaccination with BNT162B2. Vaccines 2023, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Fang, F.; Weyand, C.M.; Goronzy, J.J. The life cycle of a T cell after vaccination—Where does immune ageing strike? Clin. Exp. Immunol. 2016, 187, 71–81. [Google Scholar] [CrossRef]
- Gray, J.I.; Westerhof, L.M.; MacLeod, M.K. The roles of resident, Central and effector memory CD4 T-cells in protective immunity following infection or vaccination. Immunology 2018, 154, 574–581. [Google Scholar] [CrossRef]
- Henson, S.M.; Riddell, N.E.; Akbar, A.N. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 2012, 24, 476–481. [Google Scholar] [CrossRef]
- Carrasco, J.; Codelaine, D.; Van Pel, A.; Boon, T.; van der Bruggen, P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood 2006, 108, 2897–2905. [Google Scholar] [CrossRef]
- Wirth, T.C.; Xue, H.-H.; Rai, D.; Sabel, J.T.; Bair, T.; Harty, J.T.; Badovinac, V.P. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 2010, 33, 128–140. [Google Scholar] [CrossRef]
- Minato, N.; Hattori, M.; Hamazaki, Y. Physiology and pathology of T-cell aging. Int. Immunol. 2020, 32, 223–231. [Google Scholar] [CrossRef]

| Clinical Parameter | MED.(IQR) |
|---|---|
| Age (years) | 47 (16) |
| eGFR (mL/min/1.73 m2) | 54.1 (22.7) |
| Time interval from transplantation (years) | 6.9 (14.5) |
| Duration of potential previous hemodialysis (months) | 18 (45) |
| Tacrolimus levels (ng/mL) | 6 (2) |
| Cyclosporine levels (ng/mL) | 256 (186) |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at Specific Time Points (MED.(IQR)) | p Value | Cell Percentages (%) at Specific Time Points (MED.(IQR)) | p Value | ||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |||
| Naïve CD4+ T-cells | 189.87 (193) | 125.26 (105) | 0.245 (NS) | 32.3 (26.2) | 18.5 (24.3) | 0.030 |
| CD4+ TCM | 424.03 (303) | 380.42 (411) | 0.470 (NS) | 55 (30.75) | 68.9 (35.35) | 0.013 |
| CD4+ TEM | 45 (71) | 28.83 (40) | 0.683 (NS) | 7.65 (8.65) | 5.1 (3.55) | 0.485 (NS) |
| CD4+ TEMRA | 62.75 (70) | 22.54 (49) | 0.056 (NS) | 7.3 (4.38) | 4 (5.1) | 0.365 (NS) |
| Naïve CD8+ T-cells | 145.19 (172) | 132.04 (141) | 0.158 (NS) | 37.55 (15.38) | 30.4 (12.2) | 0.052 (NS) |
| CD8+ TCM | 138.12 (138) | 155.71 (148) | 0.245 (NS) | 28.45 (31.95) | 34.6 (28.10) | 0.423 (NS) |
| CD8+ TEM | 29.41 (26) | 56.95 (50) | 0.008 | 6.8 (4) | 10.6 (7.25) | 0.044 |
| CD8+ TEMRA | 72.33 (140) | 126.15 (168) | 0.022 | 14.95 (31.85) | 16.1 (19.65) | 0.088 (NS) |
| CD3+CD4+CD28+ T-cells | 703.54 (412) | 520.75 (594) | 0.551 (NS) | 85.8 (11.87) | 92.3 (10.05) | 0.717 (NS) |
| CD3+CD4+CD28− T-cells | 78.65 (145) | 67.56 (49) | 0.470 (NS) | 14.15 (12.25) | 7.4 (10) | 0.642 (NS) |
| CD3+CD8+CD28+ T-cells | 220.73 (165) | 176.73 (146) | 0.778 (NS) | 37.15 (20.8) | 36.7 (16.65) | 0.897 (NS) |
| CD3+CD8+CD28− T-cells | 221.63 (235) | 277.98 (298) | 0.594 (NS) | 62.75 (18.95) | 64 (17.25) | 1.0 (NS) |
| CD3+PD1+ T-cells | 41.33 (44) | 34.13 (51) | 0.826 (NS) | 2.8 (3.7) | 2.45 (2.6) | 0.679 (NS) |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at Specific Time Points [MED.(IQR)] | p Value | Cell Percentages (%) at Specific Time Points [MED.(IQR)] | p Value | ||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |||
| Naïve CD4+ T-cells | 256.10 (258) | 159.12 (235) | 0.020 | 32.2 (21.3) | 23.3 (25.3) | 0.006 |
| CD4+ TCM | 436.10 (399) | 545.81 (431) | 1.0 (NS) | 54.9 (24.02) | 61.9 (29.5) | 0.004 |
| CD4+ TEM | 47.92 (55) | 31.84 (46) | 0.773 (NS) | 5.8 (7.55) | 4.45 (3.9) | 0.991 (NS) |
| CD4+ TEMRA | 69.62 (88) | 53.55 (70) | 0.037 | 7.4 (5.17) | 3.7 (4.55) | 0.299 (NS) |
| Naïve CD8+ T-cells | 206.33 (247) | 182.27 (248) | 0.230 (NS) | 42.9 (23.25) | 37.75 (18.9) | 0.060 (NS) |
| CD8+ TCM | 134.69 (216) | 162.48 (155) | 0.581 (NS) | 24 (23.95) | 34.85 (26.57) | 0.837 (NS) |
| CD8+ TEM | 27.16 (27) | 44.63 (56) | 0.020 | 6.4 (5.85) | 9.65 (6.23) | 0.071 (NS) |
| CD8+ TEMRA | 93.94 (141) | 91.59 (121) | 0.361 (NS) | 19.5 (29.57) | 14.9 (13.45) | 0.336 (NS) |
| CD3+CD4+CD28+ T-cells | 753.93 (419) | 743.56 (587) | 0.845 (NS) | 90.9 (14.63) | 92.9 (5.47) | 0.340 (NS) |
| CD3+CD4+CD28− T-cells | 63.31 (179) | 63.23 (54) | 0.289 (NS) | 9.35 (15) | 7.1 (5.73) | 0.369 (NS) |
| CD3+CD8+CD28+ T-cells | 214.87 (201) | 227.15 (147) | 0.558 (NS) | 37.15 (29.52) | 38.55 (29) | 0.531 (NS) |
| CD3+CD8+CD28− T-cells | 284.14 (318) | 256.06 (233) | 0.453 (NS) | 62.75 (28.03) | 63 (30.4) | 0.513 (NS) |
| CD3+PD1+ T-cells | 41.33 (50) | 32.95 (48) | 0.558 (NS) | 2.65 (2.4) | 2.45 (2.2) | 0.822 (NS) |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at T1 MED.(IQR) | Cell Percentages (%) at T1 MED.(IQR) | ||||
|---|---|---|---|---|---|---|
| Responders | Non-Responders | p Value | Responders | Non-Responders | p Value | |
| Naïve CD4+ T-cells | 198.76 (254) | 250.25 (224) | 0.590 (NS) | 32.3 (26.2) | 32.1 (20.4) | 0.876 (NS) |
| CD4+ TCM | 372.6 (204) | 531.54 (441) | 0.184 (NS) | 55 (30.75) | 57.3 (25.1) | 0.397 (NS) |
| CD4+ TEM | 42.59 (56) | 35.94 (46) | 0.318 (NS) | 6.5 (7.55) | 3.8 (3.3) | 0.066 (NS) |
| CD4+ TEMRA | 69.62 (77) | 47.75 (81) | 0.909 (NS) | 7.4 (4.25) | 6.7 (6) | 0.639 (NS) |
| Naïve CD8+ T-cells | 154.72 (166) | 201.85 (255) | 0.782 (NS) | 39.4 (17.8) | 45.1 (19.3) | 0.196 (NS) |
| CD8+ TCM | 141.56 (165) | 138.03 (211) | 0.195 (NS) | 34.3 (29.55) | 28.3 (26.5) | 0.346 (NS) |
| CD8+ TEM | 30.24 (27) | 17.7 (28) | 0.988 (NS) | 6.8 (4) | 4.2 (4.8) | 0.138 (NS) |
| CD8+ TEMRA | 70.05 (162) | 96.12 (115) | 0.935 (NS) | 12.4 (27.65) | 18.8 (31) | 0.639 (NS) |
| CD3+CD4+CD28+ T-cells | 742.66 (489) | 843.4 (501) | 0.232 (NS) | 87.8 (13.5) | 93.5 (9.4) | 0.073 (NS) |
| CD3+CD4+CD28− T-cells | 70.18 (165) | 54.67 (142) | 0.463 (NS) | 12.5 (13.7) | 5.9 (10.7) | 0.052 (NS) |
| CD3+CD8+CD28+ T-cells | 209.3 (170) | 209.37 (235) | 0.988 (NS) | 37.3 (17.1) | 41.9 (32.3) | 0.931 (NS) |
| CD3+CD8+CD28− T-cells | 228.62 (221) | 226.21 (323) | 0.798 (NS) | 63.4 (15.4) | 59.9 (31.55) | 0.931 (NS) |
| CD3+PD1+ T-cells | 41.33 (44) | 32.49 (41) | 0.322 (NS) | 2.8 (3.7) | 2.45 (2) | 0.319 (NS) |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at T2 MED.(IQR) | Cell Percentages (%) at T2 MED.(IQR) | ||||
|---|---|---|---|---|---|---|
| Responders | Non-Responders | p Value | Responders | Non-Responders | p Value | |
| Naïve CD4+ T-cells | 165.19 (252) | 251.75 (405) | 0.680 (NS) | 23.3 (25.3) | 39.7 (27.28) | 0.160 (NS) |
| CD4+ TCM | 530.84 (375) | 394.16 (443) | 0.457 (NS) | 61.9 (29.5) | 46.95 (40.3) | 0.352 (NS) |
| CD4+ TEM | 36.54 (51) | 16.35 (73) | 0.151 (NS) | 5.2 (4.6) | 3.6 (3.75) | 0.352 (NS) |
| CD4+ TEMRA | 54.54 (78) | 44.78 (183) | 1.0 (NS) | 5.9 (8.2) | 9.65 (16) | 0.635 (NS) |
| Naïve CD8+ T-cells | 144.55 (156) | 159.03 (149) | 0.763 (NS) | 29.9 (19.2) | 34.5 (12.48) | 0.467 (NS) |
| CD8+ TCM | 153.18 (160) | 214.44 (176) | 0.640 (NS) | 29.5 (28.1) | 41.6 (27.4) | 0.467 (NS) |
| CD8+ TEM | 43.66 (55) | 31.5 (71) | 0.424 (NS) | 9.9 (5.6) | 9 (9.35) | 0.499 (NS) |
| CD8+ TEMRA | 123.01 (142) | 42.26 (80) | 0.094 (NS) | 22.7 (25.1) | 15.7 (26.87) | 0.407 (NS) |
| CD3+CD4+CD28+ T-cells | 756.34 (368) | 446.57 (925) | 0.239 (NS) | 93.8 (6) | 72.05 (30.73) | 0.005 |
| CD3+CD4+CD28− T-cells | 59.65 (66) | 161.19 (92) | 0.026 | 6.1 (5.5) | 20.7 (25) | 0.004 |
| CD3+CD8+CD28+ T-cells | 227.15 (166) | 131.44 (121) | 0.036 | 40 (30.4) | 31.55 (15.77) | 0.154 (NS) |
| CD3+CD8+CD28− T-cells | 256.06 (309) | 327.8 (343) | 0.979 (NS) | 60 (30.9) | 68.4 (12.85) | 0.127 (NS) |
| CD3+PD1+ T-cells | 32.95 (48) | 59.85 (72) | 0.624 (NS) | 2.45 (2.2) | 3.55 (2.2) | 0.269 (NS) |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at Specific Time Points [MED.(IQR)] | p Value | Cell Percentages (%) at Specific Time Points [MED.(IQR)] | p Value | ||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |||
| Naïve CD4+ T-cells | 251.67 (280) | 138.80 (213) | 0.158 | 30.10 (24.50) | 18.35 (21.28) | 0.048 |
| CD4+ TCM | 432.58 (242) | 465.76 (467) | 0.530 | 54.30 (29.73) | 68.90 (31.45) | 0.009 |
| CD4+ TEM | 53.86 (64) | 42.2 (59) | 0.754 | 7.65 (7.45) | 5.35 (4.90) | 0.572 |
| CD4+ TEMRA | 80.46 (73) | 43.35 (83) | 0.023 | 7.45 (3.58) | 4.40 (7.62) | 0.187 |
| Naïve CD8+ T-cells | 175.81 (198) | 144.55 (129) | 0.239 | 38.40 (20.27) | 29 (18.27) | 0.116 |
| CD8+ TCM | 138.12 (178) | 132.44 (144) | 0.239 | 34.25 (30.95) | 31.7 (26.78) | 0.802 |
| CD8+ TEM | 37.03 (22) | 61.7 (60) | 0.015 | 7.2 (4.15) | 10.60 (8.45) | 0.109 |
| CD8+ TEMRA | 80.43 (202) | 177.5 (192) | 0.050 | 12.75 (29.75) | 22.05 (34.28) | 0.221 |
| CD3+CD4+CD28+ T-cells | 774.71 (462) | 795.42 (495) | 0.754 | 87.8 (14.12) | 92.75 (6.92) | 0.594 |
| CD3+CD4+CD28− T-cells | 118 (181) | 66.18 (51) | 0.308 | 11.75 (14.85) | 7.10 (6.50) | 0.510 |
| CD3+CD8+CD28+ T-cells | 223.53 (186) | 198.18 (171) | 0.583 | 37.15 (16.05) | 36.15 (29.08) | 0.925 |
| CD3+CD8+CD28− T-cells | 330.91 (221) | 320.73 (287) | 0.875 | 63.6 (14.65) | 64.65 (29.55) | 0.975 |
| CD3+PD1+ T-cells | 44.72 (63) | 38.86 (50) | 0.814 | 2.8 (5.4) | 2.45 (2.8) | 0.510 |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at Specific Time Points [MED.(IQR)] | p Value | Cell Percentages (%) at Specific Time Points [MED.(IQR)] | p Value | ||
|---|---|---|---|---|---|---|
| T1 | T2 | T1 | T2 | |||
| Naïve CD4+ T-cells | 265.10 (222) | 202.77 (293) | 0.061 | 32.4 (21.98) | 25.10 (26.63) | 0.053 |
| CD4+ TCM | 553.26 (427) | 542.61 (317) | 0.532 | 56.05 (25.53) | 58.35 (31.20) | 0.191 |
| CD4+ TEM | 36.96 (45) | 33.11 (46) | 0.910 | 3.85 (3.35) | 4.45 (4.72) | 0.478 |
| CD4+ TEMRA | 47.78 (81) | 57.94 (75) | 0.307 | 6.8 (6.67) | 7.25 (9.95) | 0.955 |
| Naïve CD8+ T-cells | 205.41 (270) | 134.04 (259) | 0.570 | 44.9 (22.27) | 31.65 (26.78) | 0.191 |
| CD8+ TCM | 141.81 (229) | 158.31 (167) | 0.776 | 27.4 (22.10) | 26.7 (30.10) | 0.712 |
| CD8+ TEM | 18.47 (32) | 30.05 (28) | 0.394 | 3.75 (4.9) | 8.9 (7.9) | 0.379 |
| CD8+ TEMRA | 92.29 (120) | 108.23 (94) | 0.733 | 19.4 (30.30) | 22.10 (23.55) | 0.650 |
| CD3+CD4+CD28+ T-cells | 840.86 (389) | 743.56 (464) | 0.983 | 93.7 (7.72) | 95.85 (6.77) | 0.433 |
| CD3+CD4+CD28− T-cells | 52.83 (162) | 46.67 (79) | 0.647 | 5.25 (9.15) | 4.65 (6.13) | 0.557 |
| CD3+CD8+CD28+ T-cells | 212.18 (268) | 245.69 (184) | 0.231 | 42.45 (30.10) | 49.25 (31.07) | 0.327 |
| CD3+CD8+CD28− T-cells | 275.31 (399) | 210.79 (239) | 0.446 | 58.70 (29.72) | 50.15 (30.78) | 0.349 |
| CD3+PD1+ T-cells | 30.28 (46) | 31.73 (28) | 0.500 | 2.15 (2) | 2.4 (2) | 0.879 |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at T1 MED.(IQR) | Cell Percentages (%) at T1 MED.(IQR) | ||||
|---|---|---|---|---|---|---|
| Responders at Both T1 and T2 | Non Responders at T1—Responders at T2 | p Value | Responders at Both T1 and T2 | Non Responders at T1—Responders at T2 | p Value | |
| Naïve CD4+ cells | 251.67 (280) | 265.10 (222) | 0.891 | 30.10 (24.50) | 32.4 (21.98) | 0.722 |
| CD4+ TCM | 432.58 (242) | 553.26 (427) | 0.262 | 54.30 (29.73) | 56.05 (25.53) | 0.377 |
| CD4+ TEM | 53.86 (64) | 36.96 (45) | 0.092 | 7.65 (7.45) | 3.85 (3.35) | 0.014 |
| CD4+ TEMRA | 80.46 (73) | 47.78 (81) | 0.444 | 7.45 (3.58) | 6.8 (6.67) | 0.357 |
| Naïve CD8+ T-cells | 175.81 (198) | 205.41 (270) | 0.570 | 38.40 (20.27) | 44.9 (22.27) | 0.135 |
| CD8+ TCM | 138.12 (178) | 141.81 (229) | 0.860 | 34.25 (30.95) | 27.4 (22.10) | 0.442 |
| CD8+ TEM | 37.03 (22) | 18.47 (32) | 0.092 | 7.2 (4.15) | 3.75 (4.9) | 0.054 |
| CD8+ TEMRA | 80.43 (202) | 92.29 (120) | 0.984 | 12.75 (29.75) | 19.4 (30.30) | 0.896 |
| CD3+CD4+CD28+ T-cells | 774.71 (462) | 840.86 (389) | 0.512 | 87.8 (14.12) | 93.7 (7.72) | 0.084 |
| CD3+CD4+CD28− T-cells | 118 (181) | 52.83 (162) | 0.180 | 11.75 (14.85) | 5.25 (9.15) | 0.050 |
| CD3+CD8+CD28+ T-cells | 223.53 (186) | 212.18 (268) | 0.925 | 37.15 (16.05) | 42.45 (30.10) | 0.760 |
| CD3+CD8+CD28− T-cells | 330.91 (221) | 275.31 (399) | 0.488 | 63.6 (14.65) | 58.70 (29.72) | 0.679 |
| CD3+PD1+ T-cells | 44.72 (63) | 30.28 (46) | 0.152 | 2.8 (5.4) | 2.15 (2) | 0.231 |
| Cellular Subpopulation | Cell Concentrations (Cells/µL) at T2 MED.(IQR) | Cell Percentages (%) at T2 MED.(IQR) | ||||
|---|---|---|---|---|---|---|
| Responders at Both T1 and T2 | Non Responders at T1—Responders at T2 | p Value | Responders at Both T1 and T2 | Non Responders at T1—Responders at T2 | p Value | |
| Naïve CD4+ cells | 138.80 (213) | 202.77 (293) | 0.280 | 18.35 (21.28) | 25.10 (26.63) | 0.316 |
| CD4+ TCM | 465.76 (467) | 542.61 (317) | 0.909 | 68.90 (31.45) | 58.35 (31.20) | 0.799 |
| CD4+ TEM | 42.2 (59) | 33.11 (46) | 0.537 | 5.35 (4.90) | 4.45 (4.72) | 0.468 |
| CD4+ TEMRA | 43.35 (83) | 57.94 (75) | 0.423 | 4.40 (7.62) | 7.25 (9.95) | 0.444 |
| Naïve CD8+ T-cells | 144.55 (129) | 134.04 (259) | 0.909 | 29 (18.27) | 31.65 (26.78) | 0.421 |
| CD8+ TCM | 132.44 (144) | 158.31 (167) | 0.945 | 31.7 (26.78) | 26.7 (30.10) | 0.769 |
| CD8+ TEM | 61.7 (60) | 30.05 (28) | 0.059 | 10.60 (8.45) | 8.9 (7.9) | 0.316 |
| CD8+ TEMRA | 177.5 (192) | 108.23 (94) | 0.347 | 22.05 (34.28) | 22.10 (23.55) | 0.953 |
| CD3+CD4+CD28+ T-cells | 795.42 (495) | 743.56 (464) | 0.723 | 92.75 (6.92) | 95.85 (6.77) | 0.035 |
| CD3+CD4+CD28− T-cells | 66.18 (51) | 46.67 (79) | 0.200 | 7.10 (6.50) | 4.65 (6.13) | 0.055 |
| CD3+CD8+CD28+ T-cells | 198.18 (171) | 245.69 (184) | 0.465 | 36.15 (29.08) | 49.25 (31.07) | 0.321 |
| CD3+CD8+CD28− T-cells | 320.73 (287) | 210.79 (239) | 0.723 | 64.65 (29.55) | 50.15 (30.78) | 0.358 |
| CD3+PD1+ T-cells | 38.86 (50) | 31.73 (28) | 0.950 | 2.45 (2.8) | 2.4 (2) | 0.722 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stai, S.; Lioulios, G.; Xochelli, A.; Papadopoulou, A.; Yannaki, E.; Kasimatis, E.; Christodoulou, M.; Moysidou, E.; Samali, M.; Testa, T.; et al. Vaccination with Tozimameran Induces T-Cell Activation, but Not Senescent or Exhaustive Alterations, in Kidney Transplant Recipients. Vaccines 2024, 12, 877. https://doi.org/10.3390/vaccines12080877
Stai S, Lioulios G, Xochelli A, Papadopoulou A, Yannaki E, Kasimatis E, Christodoulou M, Moysidou E, Samali M, Testa T, et al. Vaccination with Tozimameran Induces T-Cell Activation, but Not Senescent or Exhaustive Alterations, in Kidney Transplant Recipients. Vaccines. 2024; 12(8):877. https://doi.org/10.3390/vaccines12080877
Chicago/Turabian StyleStai, Stamatia, Georgios Lioulios, Aliki Xochelli, Anastasia Papadopoulou, Evangelia Yannaki, Efstratios Kasimatis, Michalis Christodoulou, Eleni Moysidou, Margarita Samali, Theodolinda Testa, and et al. 2024. "Vaccination with Tozimameran Induces T-Cell Activation, but Not Senescent or Exhaustive Alterations, in Kidney Transplant Recipients" Vaccines 12, no. 8: 877. https://doi.org/10.3390/vaccines12080877






