B Cells Isolated from Individuals Who Do Not Respond to the HBV Vaccine Are Characterized by Higher DNA Methylation-Estimated Aging Compared to Responders
Abstract
:1. Introduction
Aim
2. Materials and Methods
2.1. Cohort
2.2. Biological Material, DNA Extraction, Bisulfite Conversion
2.3. DNA Methylation Data Preprocessing
2.4. Epigenetic Age Estimation of DNAm-Based Biomarkers
2.5. Statistical Analysis
2.6. Distribution of Standard Deviation
3. Results
3.1. Sample Processing
3.2. Cohort Characteristics
3.3. Biological Age Estimates in the Overall Responder/Non-Responder Population
3.4. Biological Age Estimates in the Female Responder/Non-Responder Population
3.5. Biological Age Estimates in the Male Responder/Non-Responder Population
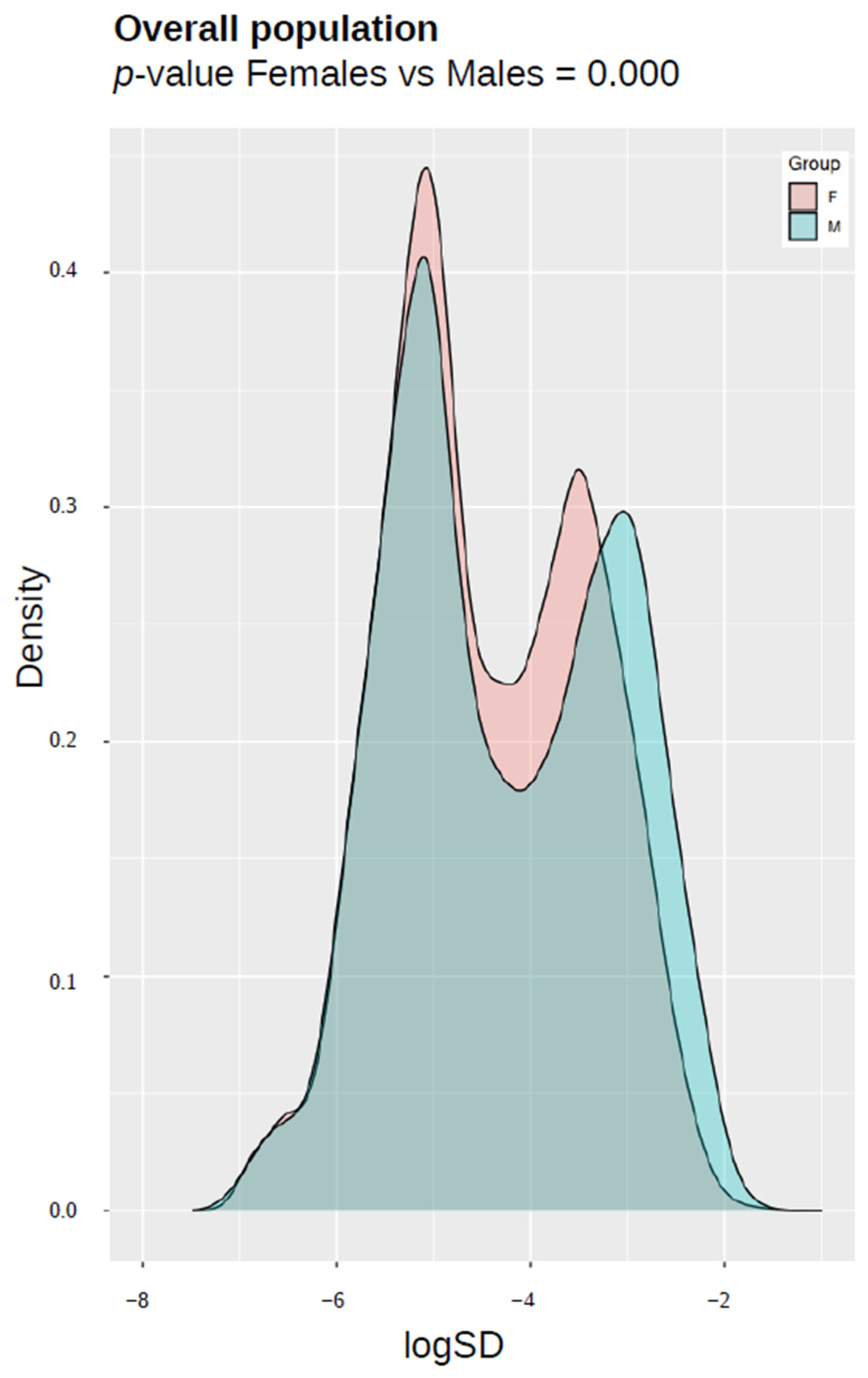
3.6. Epigenetic Entropy (Variance Estimation in DNA Methylation) in Responder/Non-Responder Population
4. Discussion
Perspectives and Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization. Fact Sheets. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 3 January 2023).
- Mahamat, G.; Kenmoe, S.; Akazong, E.W.; Ebogo-Belobo, J.T.; Mbaga, D.S.; Bowo-Ngandji, A.; Foe-Essomba, J.R.; Amougou-Atsama, M.; Monamele, C.G.; Mikangue, C.A.M.; et al. Global prevalence of hepatitis B virus serological markers among healthcare workers: A systematic review and meta-analysis. World J. Hepatol. 2021, 13, 1190–1202. [Google Scholar] [CrossRef]
- Pappas, S.C. Hepatitis B and Health Care Workers. Clin. Liver Dis. 2021, 25, 859–874. [Google Scholar] [CrossRef]
- Legge 27 Maggio 1991, n. Legge 27 Maggio 1991, n. 165: Obbligatorietà Della Vaccinazione Contro L’epatite Virale B (Law May 27, 1991, N. 165. Mandatory Vaccination against Viral Hepatitis B). Gazzetta Ufficiale N. 127, 1 June 1991. Available online: https://www.gazzettaufficiale.it/eli/id/1991/06/01/091G0201/sg (accessed on 30 January 2024).
- Meier, M.A.; Berger, C.T. A simple clinical score to identify likely hepatitis B vaccination non-responders—Data from a retrospective single center study. BMC Infect. Dis. 2020, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Jacobson, R.M. Prevention of Hepatitis B with the Hepatitis B Vaccine. N. Engl. J. Med. 2004, 351, 2832–2838. [Google Scholar] [CrossRef]
- Hall, E. Epidemiology and prevention of vaccine-preventable diseases. In Hepatitis B, 14th ed.; Public Health Foundation: Washington, DC, USA; Department of Health and Human Services, Centers for Disease Control and Prevention: Washington, DC, USA, 2021. [Google Scholar]
- Intesa Governo, Regioni e Province Autonome di Trento e di Bolzano sul “Piano Nazionale di Prevenzione Vaccinale (PNPV) 2023-2025” e sul “Calendario Nazionale Vaccinale”. Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=95963&completo=true (accessed on 20 November 2023).
- Dentico, P.; Buongiorno, R.; Volpe, A.; Zavoianni, A.; Pastore, G.; Schiraldi, O. Long term immunogenicity safety and efficacy of a recombinant hepatitis B vaccine in healthy adults. Eur. J. Epidemiol. 1992, 8, 650–655. [Google Scholar] [CrossRef]
- Hess, G.; Hingst, V.; Cseke, J.; Bock, H.L. Clemens R. Influence of vaccination schedules and host factors on antibody response fol-lowing hepatitis B vaccination. Eur. J. Clin. Microbiol. Infect. Dis. 1992, 11, 334–340. [Google Scholar] [CrossRef]
- Wood, R.C. Risk Factors for Lack of Detectable Antibody Following Hepatitis B Vaccination of Minnesota Health Care Workers. JAMA 1993, 270, 2935–2939. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, A.P.; Hoebe, C.J.; Dukers-Muijrers, N.H. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J. Clin. Virol. 2013, 58, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115, Erratum in Genome Biol. 2015, 16, 96. [Google Scholar] [CrossRef]
- Jones, M.J.; Goodman, S.J.; Kobor, M.S. DNA methylation and healthy human aging. Aging Cell 2015, 14, 924–932. [Google Scholar] [CrossRef]
- Heyn, H.; Li, N.; Ferreira, H.J.; Moran, S.; Pisano, D.G.; Gomez, A.; Diez, J.; Sanchez-Mut, J.V.; Setien, F.; Carmona, F.J.; et al. Distinct DNA methylomes of newborns and centenarians. Proc. Natl. Acad. Sci. USA 2012, 109, 10522–10527. [Google Scholar] [CrossRef]
- Talens, R.P.; Christensen, K.; Putter, H.; Willemsen, G.; Christiansen, L.; Kremer, D.; Suchiman, H.E.D.; Slagboom, P.E.; Boomsma, D.I.; Heijmans, B.T. Epigenetic variation during the adult lifespan: Cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell 2012, 11, 694–703. [Google Scholar] [CrossRef]
- Triche, T.J., Jr.; Weisenberger, D.J.; Van Den Berg, D.; Laird, P.W.; Siegmund, K.D. Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res. 2013, 41, e90. [Google Scholar] [CrossRef]
- Lu, A.T.; Seeboth, A.; Tsai, P.-C.; Sun, D.; Quach, A.; Reiner, A.P.; Kooperberg, C.; Ferrucci, L.; Hou, L.; Baccarelli, A.A.; et al. DNA methylation-based estimator of telomere length. Aging 2019, 11, 5895–5923. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Baccarelli, A.A.; Corcoran, D.L.; Gao, X.; Hannon, E.; Harrington, H.L.; Rasmussed, L.J.H.; Houts, R.; et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 2020, 9, e54870. [Google Scholar] [CrossRef] [PubMed]
- Camillo, L.P.d.L.; Lapierre, L.R.; Singh, R. A pan-tissue DNA-methylation epigenetic clock based on deep learning. npj Aging 2022, 8, 1–15. [Google Scholar] [CrossRef]
- Wielscher, M.; Mandaviya, P.R.; Kuehnel, B.; Joehanes, R.; Mustafa, R.; Robinson, O.; Zhang, Y.; Bodinier, B.; Walton, E.; Mishra, P.P.; et al. DNA methylation signature of chronic low-grade inflammation and its role in cardio-respiratory diseases. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Higgins-Chen, A.T.; Thrush, K.L.; Wang, Y.; Minteer, C.J.; Kuo, P.-L.; Wang, M.; Niimi, P.; Sturm, G.; Lin, J.; Moore, A.Z.; et al. A computational solution for bolstering reliability of epigenetic clocks: Implications for clinical trials and longitudinal tracking. Nat. Aging 2022, 2, 644–661. [Google Scholar] [CrossRef]
- Di Lello, F.A.; Martínez, A.P.; Flichman, D.M. Insights into induction of the immune response by the hepatitis B vaccine. World J. Gastroenterol. 2022, 28, 4249–4262. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Crasta, P.; Cheuvart, B.; De Ridder, M. Characterization of an age-response relationship to GSK’s recombinant hepatitis B vaccine in healthy adults: An integrated analysis. Hum. Vaccines Immunother. 2015, 11, 1725–1728. [Google Scholar] [CrossRef]
- Weinberger, B.; Haks, M.C.; de Paus, R.A.; Ottenhoff, T.H.M.; Bauer, T.; Grubeck-Loebenstein, B. Impaired Immune Response to Primary but Not to Booster Vaccination Against Hepatitis B in Older Adults. Front. Immunol. 2018, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Franceschi, C.; Blomberg, B.B.; Pirazzini, C.; Ravaioli, F.; Gentilini, D.; Di Blasio, A.M.; Garagnani, P.; Frasca, D.; Bacalini, M.G. Responders and non-responders to influenza vaccination: A DNA methylation approach on blood cells. Exp. Gerontol. 2018, 105, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Wang, S.C.; Prahash, A.; Sano, M.; Moravec, C.S.; Taffet, G.E.; Michael, L.H.; Youker, K.A.; Entman, M.L.; Schneider, M.D. Telomere attrition and Chk2 activation in human heart failure. Proc. Natl. Acad. Sci. USA 2003, 100, 5378–5383. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.J.; Winterbone, M.S.; Hughes, J.C.; Dozio, N.; Hughes, D.A. Monocyte Telomere Shortening and Oxidative DNA Damage in Type 2 Diabetes. Diabetes Care 2006, 29, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Honig, L.S.; Kang, M.S.; Schupf, N.; Lee, J.H.; Mayeux, R. Association of Shorter Leukocyte Telomere Repeat Length With Dementia and Mortality. Arch. Neurol. 2012, 69, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Watfa, G.; Dragonas, C.; Brosche, T.; Dittrich, R.; Sieber, C.C.; Alecu, C.; Benetos, A.; Nzietchueng, R. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J. Nutr. Health Aging 2011, 15, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.; de Solorzano, C.O.; Knowles, D.; Jones, A.; Chou, W.; Rodriguez, E.G.; Kuo, W.-L.; Ljung, B.-M.; Chew, K.; Myambo, K.; et al. In situ analyses of genome instability in breast cancer. Nat. Genet. 2004, 36, 984–988. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzalez-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring biological aging in humans: A quest. Aging Cell 2019, 19, e13080. [Google Scholar] [CrossRef]
- Zhang, Z.; Reynolds, S.R.; Stolrow, H.G.; Chen, J.; Christensen, B.C.; Salas, L.A. Deciphering the role of immune cell composition in epigenetic age acceleration: Insights from cell-type deconvolution applied to human blood epigenetic clocks. Aging Cell 2023, 23, e14071. [Google Scholar] [CrossRef]
- Batliwalla, F.; Damle, R.; Metz, C.; Chiorazzi, N.; Gregersen, P. Simultaneous flow cytometric analysis of cell surface markers and telomere length: Analysis of human tonsilar B cells. J. Immunol. Methods 2001, 247, 103–109. [Google Scholar] [CrossRef]
- Son, N.H.; Joyce, B.; Hieatt, A.; Chrest, F.J.; Yanovski, J.; Weng, N.-P. Stable telomere length and telomerase expression from naı̈ve to memory B-lymphocyte differentiation. Mech. Ageing Dev. 2003, 124, 427–432. [Google Scholar] [CrossRef]
- Yusipov, I.; Bacalini, M.G.; Kalyakulina, A.; Krivonosov, M.; Pirazzini, C.; Gensous, N.; Ravaioli, F.; Milazzo, M.; Giuliani, C.; Vedunova, M.; et al. Age-related DNA methylation changes are sex-specific: A comprehensive assessment. Aging 2020, 12, 24057–24080. [Google Scholar] [CrossRef]
- Saco, T.V.; Strauss, A.T.; Ledford, D.K. Hepatitis B vaccine nonresponders Possible mechanisms and solutions. Ann. Allergy Asthma Immunol. 2018, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Walayat, S.; Ahmed, Z.; Martin, D.; Puli, S.; Cashman, M.; Dhillon, S. Recent advances in vaccination of non-responders to standard dose hepatitis B virus vaccine. World J. Hepatol. 2015, 7, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Yanny, B.; Konyn, P.; Najarian, L.M.; Mitry, A.; Saab, S. Management Approaches to Hepatitis B Virus Vaccination Nonresponse. Gastroenterol. Hepatol. 2019, 15, 93–99. [Google Scholar]
- Wu, T.-W.; Chou, C.-L.; Chen, C.-F.; Wang, L.-Y. Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study. Int. J. Mol. Sci. 2023, 24, 9741. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, C.; Lai, S.; Lin, H.H.; Chu, C.; Wang, L. SNP rs7770370 in HLA-DPB1 loci as a major genetic determinant of response to booster hepatitis B vaccination: Results of a genome-wide association study. J. Gastroenterol. Hepatol. 2015, 30, 891–899. [Google Scholar] [CrossRef]
- Png, E.; Thalamuthu, A.; Ong, R.T.; Snippe, H.; Boland, G.J.; Seielstad, M. A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum. Mol. Genet. 2011, 20, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, L.; Zhang, W.; Wu, X.; Li, Y.; Yan, B.; Zhu, X.; Liu, X.; Yang, C.; Xu, J.; et al. A genome-wide association study identifies polymorphisms in the HLA-DR region associated with non-response to hepatitis B vaccination in Chinese Han populations. Hum. Mol. Genet. 2013, 23, 2210–2219. [Google Scholar] [CrossRef]
- Nishida, N.; Sugiyama, M.; Sawai, H.; Nishina, S.; Sakai, A.; Ohashi, J.; Khor, S.; Kakisaka, K.; Tsuchiura, T.; Hino, K.; et al. Key HLA-DRB1-DQB1 haplotypes and role of the BTNL2 gene for response to a hepatitis B vaccine. Hepatology 2018, 68, 848–858. [Google Scholar] [CrossRef]
- Chung, S.; Roh, E.Y.; Park, B.; Lee, Y.; Shin, S.; Yoon, J.H.; Song, E.Y. GWAS identifying HLA-DPB1 gene variants associated with responsiveness to hepatitis B virus vaccination in Koreans: Independent association of HLA-DPB1*04:02 possessing rs1042169 G - rs9277355 C - rs9277356 A. J. Viral Hepat. 2019, 26, 1318–1329. [Google Scholar] [CrossRef]
- Davila, S.; Froeling, F.E.M.; Tan, A.; Bonnard, C.; Boland, G.J.; Snippe, H.; Hibberd, M.L.; Seielstad, M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010, 11, 232–238. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Fielding, K.; Hill, A.V.; Mendy, M.; Rayco-Solon, P.; Sirugo, G.; van der Sande, M.A.; Waight, P.; Whittle, H.C.; Hall, A.J.; et al. Host Genetic Factors and Vaccine-Induced Immunity to HBV Infection: Haplotype Analysis. PLoS ONE 2010, 5, e12273. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]


| p-Value | Non-Responders (NR) | Responders (R) | |
|---|---|---|---|
| - | 30 | 41 | Total number of subjects |
| 0.1795 | 15/15 | 27/14 | Number of Females/Males |
| 0.3333 | 3.135 × 106 ± 1.705 | 2.726 × 106 ± 1.791 | Average Cell Count ± SD |
| - | 22–62 | 22–53 | Age Range (years) |
| 0.3194 | 35.92 ± 11.70 | 33. 45 ± 7.86 | Group Average Age (years) ± SD |
| Males (29) | Females (42) | |||||
|---|---|---|---|---|---|---|
| p-Value | NR | R | p-Value | NR | R | |
| - | 15 | 14 | - | 15 | 27 | Number of subjects |
| 0.2765 | 3.260 × 106 ±1.519 | 2.632 × 106 ±1.522 | 0.7083 | 3.010 × 106 ±1.918 | 2.775 × 106 ±1.951 | Average Cell Count ± SD |
| - | 23–50 | 26–49 | - | 22–62 | 22–53 | Age Range [years] |
| 0.8848 | 37.06 ±9.50 | 36.64 ±5.76 | 0.4523 | 34.80 ±13.81 | 31.80 ±8.37 | Average Age [years] ± SD |
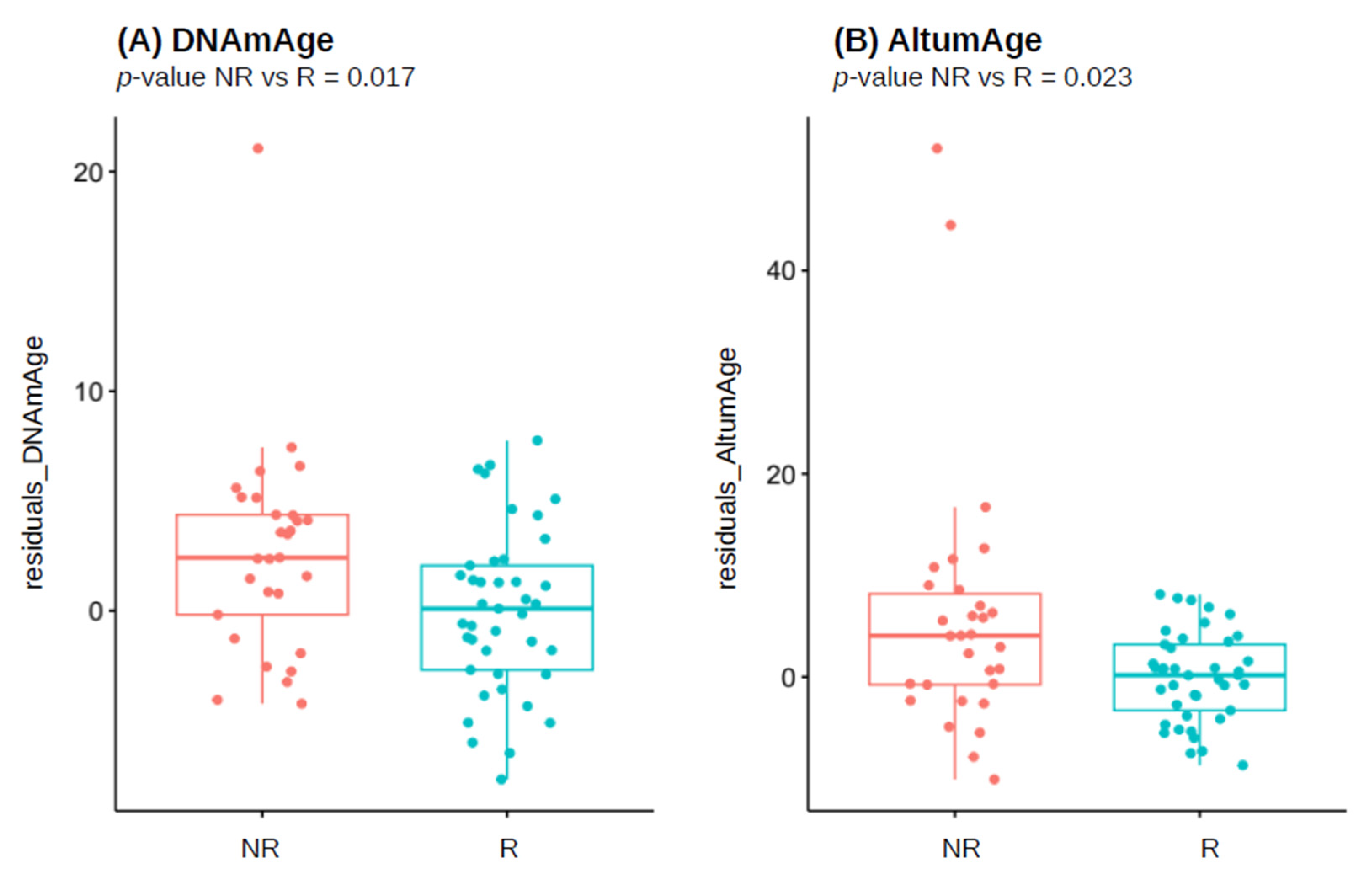
| Adjusted p-Value | p-Value | Median in Responders | Median in Non-Responders | Number of Outliers | DNAm-Based Estimate |
|---|---|---|---|---|---|
| 0.081 | 0.017 | 26.463 | 31.792 | 1 | DNAmAge |
| 0.124 | 0.053 | 7.226 | 7.253 | 2 | DNAmTL |
| 0.407 | 0.349 | 1.003 | 1.019 | 1 | DunedinPoAm |
| 0.081 | 0.023 | 20.187 | 25.153 | 0 | AltumAge |
| 0.407 | 0.318 | −28.692 | −51.208 | 1 | CRP_CpG_risk_score |
| 0.733 | 0.733 | 35.202 | 35.246 | 1 | PCHorvath1 |
| 0.249 | 0.142 | 7.449 | 7.489 | 3 | PCDNAmTL |
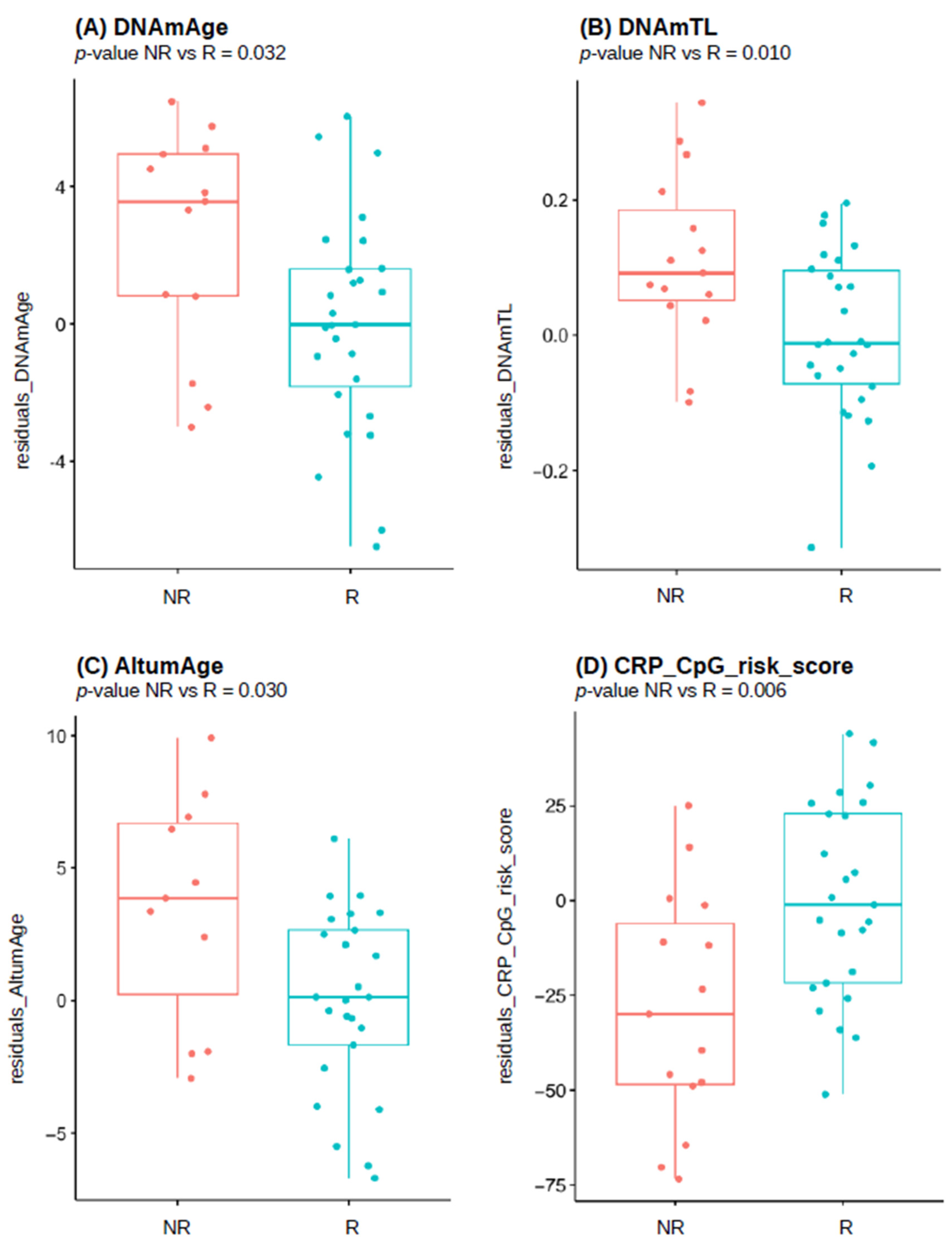
| Adjusted p-Value | p-Value | Median in Responders | Median in Non-Responders | Number of Outliers | DNAm-Based Estimate |
|---|---|---|---|---|---|
| 0.045 | 0.032 | 24.176 | 26.727 | 2 | DNAmAge |
| 0.028 | 0.010 | 7.229 | 7.272 | 1 | DNAmTL |
| 0.932 | 0.932 | 1.024 | 0.984 | 1 | DunedinPoAm |
| 0.045 | 0.030 | 17.171 | 19.833 | 6 | AltumAge |
| 0.028 | 0.006 | −29.566 | −48.746 | 2 | CRP_CpG_risk_score |
| 0.134 | 0.115 | 33.155 | 28.970 | 1 | PCHorvath1 |
| 0.028 | 0.012 | 7.457 | 7.549 | 1 | PCDNAmTL |
| Adjusted p-Value | p-Value | Median in Responders | Median in Non-Responders | Number of Outliers | DNAm-Based Estimate |
|---|---|---|---|---|---|
| 0.730 | 0.317 | 32.798 | 39.082 | 1 | DNAmAge |
| 0.906 | 0.906 | 7.225 | 7.183 | 0 | DNAmTL |
| 0.091 | 0.013 | 0.969 | 1.030 | 2 | DunedinPoAm |
| 0.424 | 0.121 | 28.108 | 36.610 | 0 | AltumAge |
| 0.730 | 0.625 | −37.239 | −56.821 | 0 | CRP_CpG_risk_score |
| 0.730 | 0.599 | 38.743 | 41.396 | 1 | PCHorvath1 |
| 0.730 | 0.626 | 7.397 | 7.396 | 1 | PCDNAmTL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, K.M.; Anticoli, S.; Salvioli, S.; Calzari, L.; Gentilini, D.; Albano, C.; Di Prinzio, R.R.; Zaffina, S.; Carsetti, R.; Ruggieri, A.; et al. B Cells Isolated from Individuals Who Do Not Respond to the HBV Vaccine Are Characterized by Higher DNA Methylation-Estimated Aging Compared to Responders. Vaccines 2024, 12, 880. https://doi.org/10.3390/vaccines12080880
Kwiatkowska KM, Anticoli S, Salvioli S, Calzari L, Gentilini D, Albano C, Di Prinzio RR, Zaffina S, Carsetti R, Ruggieri A, et al. B Cells Isolated from Individuals Who Do Not Respond to the HBV Vaccine Are Characterized by Higher DNA Methylation-Estimated Aging Compared to Responders. Vaccines. 2024; 12(8):880. https://doi.org/10.3390/vaccines12080880
Chicago/Turabian StyleKwiatkowska, Katarzyna Malgorzata, Simona Anticoli, Stefano Salvioli, Luciano Calzari, Davide Gentilini, Christian Albano, Reparata Rosa Di Prinzio, Salvatore Zaffina, Rita Carsetti, Anna Ruggieri, and et al. 2024. "B Cells Isolated from Individuals Who Do Not Respond to the HBV Vaccine Are Characterized by Higher DNA Methylation-Estimated Aging Compared to Responders" Vaccines 12, no. 8: 880. https://doi.org/10.3390/vaccines12080880






