Long-Term Safety and Immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-Year Follow-Up from a Phase 3 Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Treatment, Randomization, and Masking
2.3. Procedures
2.4. Outcomes
2.5. Analysis Populations
2.6. Statistics
3. Results
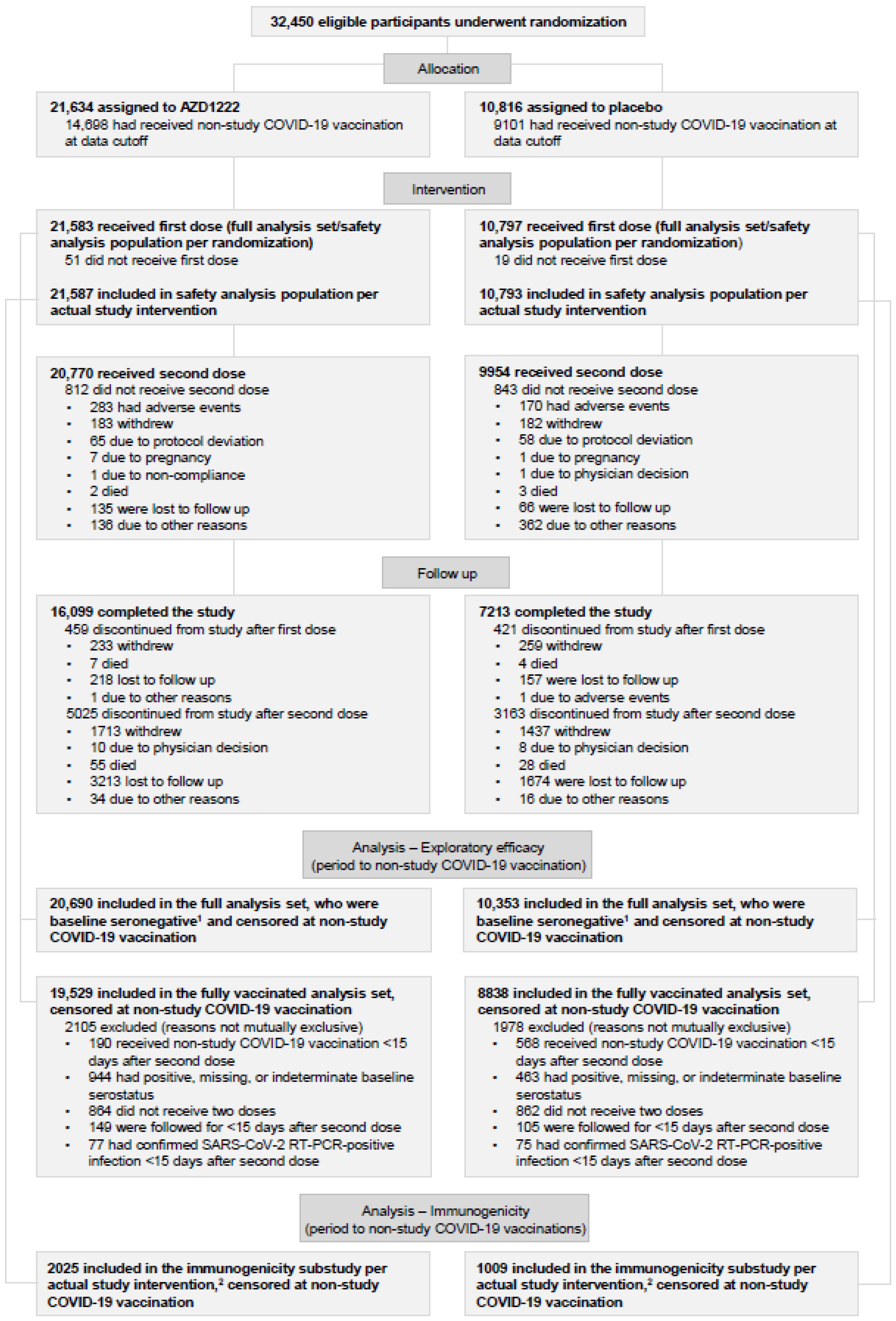
3.1. Participants
3.2. Long-Term Safety
3.3. Exploratory Efficacy
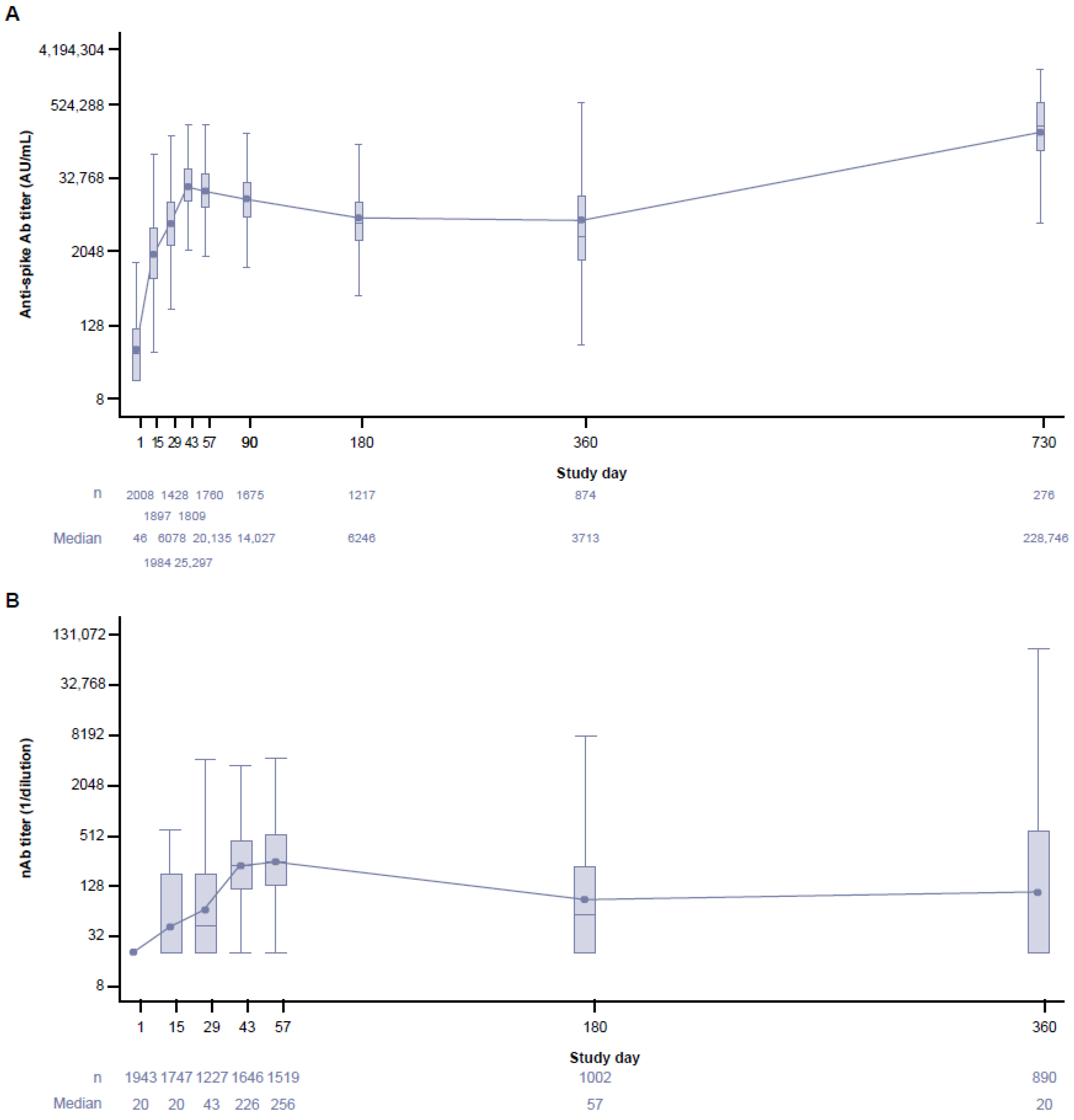
3.4. Long-Term Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Our World in Data (University of Oxford). Coronavirus (COVID-19) Vaccinations. Updated 2023. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 26 October 2023).
- US Food and Drug Administration (FDA). Emergency Use Authorization: Coronavirus Disease 2019 (COVID-19) EUA Information. Updated 2023. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#covid19euas (accessed on 26 October 2023).
- AstraZeneca PLC. AstraZeneca’s COVID-19 Vaccine Authorised for Emergency Supply in the UK. 30 December 2020. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html# (accessed on 26 October 2023).
- VIPER Group COVID-19 Vaccine Tracker Team. COVID-19 Vaccine Tracker. Approved Vaccines. Upated December 2022. Available online: https://covid19.trackvaccines.org/vaccines/approved/#vaccine-list (accessed on 19 October 2023).
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca PLC. Two Billion Doses of AstraZeneca’s COVID-19 Vaccine Supplied to Countries across the World Less than 12 Months after First Approval. 16 November 2021. Available online: https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/two-billion-doses-of-astrazenecas-covid-19-vaccine-supplied-to-countries-across-the-world-less-than-12-months-after-first-approval.html (accessed on 16 May 2023).
- European Medicines Agency EMA. European Union Risk Management Plan for Vaxzevria (ChAdOx1-S [Recombinant]). 9 February 2023. Available online: https://www.ema.europa.eu/en/documents/rmp-summary/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-risk-management-plan_en.pdf (accessed on 23 February 2024).
- World Health Organization (WHO). WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines. Updated 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccines-SAGE-Roadmap (accessed on 22 January 2024).
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Flaxman, A.; Aboagye, J.; Aley, P.K.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; Charlton, S.; Clutterbuck, E.A.; et al. Persistence of the immune response after two doses of ChAdOx1 nCov-19 (AZD1222): 1 year of follow-up of two randomized controlled trials. Clin. Exp. Immunol. 2023, 211, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Toffa, S.; Sachdeva, R.; Gallagher, E.; Groves, N.; O’Connell, A.M.; Chand, M.; Ramsay, M.; et al. COVID-19 vaccine effectiveness against the omicron (BA.2) variant in England. Lancet Infect. Dis. 2022, 22, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, Y.; Chu, M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front. Immunol. 2022, 13, 898192. [Google Scholar] [CrossRef] [PubMed]
- Joe, C.C.D.; Chopra, N.; Nestola, P.; Niemann, J.; Douglas, A.D. Rapid-response manufacturing of adenovirus-vectored vaccines. Nat. Biotechnol. 2023, 41, 314–316. [Google Scholar] [CrossRef]
- Sobieszczyk, M.E.; Maaske, J.; Falsey, A.R.; Sproule, S.; Robb, M.L.; Frenck, R.W., Jr.; Tieu, H.V.; Mayer, K.H.; Corey, L.; Neuzil, K.M.; et al. Durability of protection and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine over 6 months. J. Clin. Investig. 2022, 132, e160565. [Google Scholar] [CrossRef]
- Aksyuk, A.A.; Bansal, H.; Wilkins, D.; Stanley, A.M.; Sproule, S.; Maaske, J.; Sanikommui, S.; Hartman, W.R.; Sobieszczyk, M.E.; Falsey, A.R.; et al. AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection. Cell Rep. Med. 2023, 4, 100882. [Google Scholar] [CrossRef]
- Maaske, J.; Sproule, S.; Falsey, A.R.; Sobieszczyk, M.E.; Luetkemeyer, A.F.; Paulsen, G.C.; Riddler, S.A.; Robb, M.L.; Rolle, C.P.; Sha, B.E.; et al. Robust humoral and cellular recall responses to AZD1222 attenuate breakthrough SARS-CoV-2 infection compared to unvaccinated. Front. Immunol. 2022, 13, 1062067. [Google Scholar] [CrossRef]
- Our World in Data (University of Oxford). Coronavirus (COVID-19) Cases. Updated 2023. Available online: https://ourworldindata.org/covid-cases (accessed on 19 October 2023).
- Our World in Data (University of Oxford). SARS-CoV-2 Variants in Analyzed Sequences, United States, Chile and Peru. Available online: https://ourworldindata.org/grapher/covid-variants-area?stackMode=absolute&time=2021-07-05..2023-03-13&country=~CHL (accessed on 19 October 2023).
- Lambrou, A.S.; Shirk, P.; Steele, M.K.; Paul, P.; Paden, C.R.; Cadwell, B.; Reese, H.E.; Aoki, Y.; Hassell, N.; Zheng, X.Y.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants—United States, June 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Auti, A.M.; Di Domenico, M.; Boccellino, M. Advances in the Omicron variant development. J. Intern. Med. 2022, 292, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, D.; Aksyuk, A.A.; Ruzin, A.; Tuffy, K.M.; Green, T.; Greway, R.; Fikes, B.; Bonhomme, C.J.; Esser, M.T.; Kelly, E.J. Validation and performance of a multiplex serology assay to quantify antibody responses following SARS-CoV-2 infection or vaccination. Clin. Transl. Immunol. 2022, 11, e1385. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA Expands Eligibility for COVID-19 Vaccine Boosters. Updated 19 November 2021. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters (accessed on 26 October 2023).
- US Food and Drug Administration (FDA). FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations. Updated 22 September 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations (accessed on 23 September 2021).
- Kiazand, A.; Luther, R.; Marlind Wurtele, J.; Southall, N.; Domalik, D.; Ysander, M. Pandemic vaccines: A formidable challenge for pharmacovigilance. Nat. Rev. Drug Discov. 2023, 22, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Laffan, M.A.; Rees, S.; Yadavalli, M.; Ferstenberg, L.B.; Kumar Shankar, N.; Medin, J.; Foskett, N.; Arnold, M.; Gomes da Silva, H.; Bhuyan, P.; et al. Thrombosis with thrombocytopenia after AZD1222 (ChAdOx1 nCov-19) vaccination: Case characteristics and associations. Vaccine 2022, 40, 5585–5593. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Update on Global COVID-19 Vaccination. 5 January 2023. Available online: https://apps.who.int/gb/COVID-19/pdf_files/2023/05_01/Item1.pdf (accessed on 9 February 2024).
- Ramasamy, M.N.; Kelly, E.J.; Seegobin, S.; Dargan, P.I.; Payne, R.; Libri, V.; Adam, M.; Aley, P.K.; Martinez-Alier, N.; Church, A.; et al. Immunogenicity and safety of AZD2816, a beta variant COVID-19 vaccine, and AZD1222 (ChAdOx1 nCoV-19) as third-dose boosters for previously vaccinated adults: A multicentre, randomised, partially double-blinded, phase 2/3 non-inferiority immunobridging study in the UK and Poland. Lancet Microbe 2023, 4, e863–e874. [Google Scholar] [PubMed]
- Munro, A.P.S.; Feng, S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): A multicentre, blinded, phase 2, randomised trial. Lancet Infect. Dis. 2022, 22, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradnik, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e415. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical deatures of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, P.; Medin, J.; da Silva, H.G.; Yadavalli, M.; Shankar, N.K.; Mullerova, H.; Arnold, M.; Nord, M. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: A global safety database analysis. Lancet 2021, 398, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Sachdeva, R.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nat. Commun. 2022, 13, 7688. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Ihekweazu, C.; Rees, H.; Pollard, A.J. Decoupling of omicron variant infections and severe COVID-19. Lancet 2022, 399, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Kwatra, G.; Myers, J.E.; Jassat, W.; Dhar, N.; Mukendi, C.K.; Nana, A.J.; Blumberg, L.; Welch, R.; Ngorima-Mabhena, N.; et al. Population immunity and Covid-19 severity with Omicron variant in South Africa. N. Engl. J. Med. 2022, 386, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Thietart, S.; Rozes, A.; Tubach, F.; Marot, S.; Marcelin, A.G.; Raux, M.; Vallet, H.; Riou, B.; Boddaert, J.; Zerah, L. In-hospital mortality of older patients with COVID-19 throughout the epidemic waves in the great Paris area: A multicenter cohort study. BMC Geriatr. 2023, 23, 573. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, V.; Mitra, S.; Rafique, I.; Sharma, A.; Dhakad, M.S.; Saxena, S.; Kapoor, S.; Kumar, S. Is Omicron really mild?—Comparative analysis of comorbidities and disease outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants. Indian J. Med. Microbiol. 2023, 45, 100391. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Febriani, Y.; Ouakki, M.; Setayeshgar, S.; El Adam, S.; Zou, M.; Talbot, D.; Prystajecky, N.; Tyson, J.R.; Gilca, R.; et al. Two-dose severe acute respiratory syndrome coronavirus 2 vaccine effectiveness with mixed schedules and extended dosing intervals: Test-negative design studies from British Columbia and Quebec, Canada. Clin. Infect. Dis. 2022, 75, 1980–1992. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Nascimento, M.C.; Asano, M.; Hirata, H.; Itoh, Y.; Kelly, E.J.; Matsui, A.; Olsson, U.; Shoemaker, K.; Green, J. One year safety and immunogenicity of AZD1222 (ChAdOx1 nCoV-19): Final analysis of a randomized, placebo-controlled phase 1/2 trial in Japan. Vaccine 2023, 41, 4199–4205. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.K.; Moore, Z.; Zeng, D. Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Ikewaki, N.; Kurosawa, G.; Levy, G.A.; Preethy, S.; Abraham, S.J.K. Antibody dependent disease enhancement (ADE) after COVID-19 vaccination and beta glucans as a safer strategy in management. Vaccine 2023, 41, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: A test-negative case-control study. Lancet Infect. Dis. 2023, 23, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Xie, F.; Takhar, H.; Ogun, O.A.; Simmons, S.; et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: A test-negative case-control study. Lancet Respir. Med. 2023, 11, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Harman, K.; Lunt, R.J.; Andrews, N.; Groves, N.; Abdul Aziz, N.; Hope, R.; Stowe, J.; Chand, M.; Ramsay, M.; et al. Vaccine effectiveness against hospitalisation estimated using a test-negative case-control study design, and comparative odds of hospital admission and severe outcomes with COVID-19 sub-lineages BQ.1, CH.1.1. and XBB.1.5 in England. Lancet Reg. Health Eur. 2023, 35, 100755. [Google Scholar] [CrossRef] [PubMed]
- Meeraus, W.; Stuurman, A.L.; Durukal, I.; Conde-Sousa, E.; Lee, A.; Maria, A.S.; Furtado, B.E.; Ouwens, M.; Gray, C.M.; Valverde, D.A.; et al. COVID-19 vaccine booster doses provide increased protection against COVID-19 hospitalization compared with previously vaccinated individuals: Interim findings from the REFORCO-Brazil real-world effectiveness study during Delta and Omicron. Vaccine 2023, 41, 6366–6378. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Weber, Z.A.; Reese, S.E.; Payne, A.B.; Gaglani, M.; Adams, K.; Kharbanda, A.B.; Natarajan, K.; DeSilva, M.B.; Dascomb, K.; et al. Estimates of Bivalent mRNA Vaccine Durability in Preventing COVID-19-Associated Hospitalization and Critical Illness Among Adults with and Without Immunocompromising Conditions—VISION Network, September 2022-April 2023. Am. J. Transpl. 2023, 23, 1062–1076. [Google Scholar] [CrossRef]
- Lin, D.Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Follmann, D.; Neuzil, K.M.; August, A.; Clouting, H.; Fortier, G.; Deng, W.; Han, S.; et al. Phase 3 Trial of mRNA-1273 during the Delta-Variant Surge. N. Engl. J. Med. 2021, 385, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Upshur, R.E.G. The granting of emergency use designation to COVID-19 candidate vaccines: Implications for COVID-19 vaccine trials. Lancet Infect. Dis. 2021, 21, e103–e109. [Google Scholar] [CrossRef]

| AEs, Participants (%) Events/Adj. Rate | AZD1222 (Safety Population) | ||
|---|---|---|---|
| Prior to Non-Study COVID-19 Vaccination N = 21,587 Patient Years = 20,223 | After Non-Study COVID-19 Vaccination N = 14,667 Patient Years = 17,088 | Overall N = 21,587 Patient Years = 37,311 | |
| AEs with outcome of death Related AEs with outcome of death | 42 (0.2) 46/< 0.01 0 | 20 (0.1) 22/< 0.01 0 | 62 (0.3) 68/< 0.01 0 |
| AEs leading to study discontinuation 1 Related AEs leading to study discontinuation 1 | 43 (0.2) 46/< 0.01 0 | 20 (0.1) 20/< 0.01 0 | 63 (0.3) 66/< 0.01 0 |
| SAEs 2 Related SAEs 2 | 621 (2.9) 870/0.03 7 (<0.1) 9/< 0.01 | 456 (3.1) 622/0.03 0 | 1039 (4.8) 1492/0.03 7 (<0.1) 9/< 0.01 |
| MAAEs 2 Related MAAEs 2 | 4750 (22.0) 8300/0.23 107 (0.5) 176/< 0.01 | 3344 (22.8) 5660/0.20 2 (<0.1) 2/< 0.01 | 6955 (32.2) 13,960/0.19 108 (0.5) 178/< 0.01 |
| AESIs 2 Related AESIs 2 | 2516 (11.7) 2787/0.12 68 (0.3) 83/< 0.01 | 4369 (29.8) 4710/0.26 1 (<0.1) 1/< 0.01 | 6622 (30.7) 7497/0.18 68 (0.3) 84/< 0.1 |
| Time Period | AZD1222 (FVAS, Censored at Non-Study COVID-19 Vaccination) | ||
|---|---|---|---|
| n/N (%) 1 | Follow-Up Time 2 | Incidence Rate 2 | |
| ≥15 days post-second dose | 2925/19,409 (15.1) | 15.47 | 189.05 |
| ≥15 days post-second dose to <6 months post-first dose | 421/19,409 (2.2) | 6.31 | 66.72 |
| ≥6 months post-first dose | 2504/14,520 (17.2) | 9.16 | 273.28 |
| ≥1 year post-first dose | 1678/6850 (24.5) | 3.44 | 487.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoemaker, K.; Soboleva, K.; Branche, A.; Shankaran, S.; Theodore, D.A.; Bari, M.; Ezeh, V.; Green, J.; Kelly, E.; Lan, D.; et al. Long-Term Safety and Immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-Year Follow-Up from a Phase 3 Study. Vaccines 2024, 12, 883. https://doi.org/10.3390/vaccines12080883
Shoemaker K, Soboleva K, Branche A, Shankaran S, Theodore DA, Bari M, Ezeh V, Green J, Kelly E, Lan D, et al. Long-Term Safety and Immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-Year Follow-Up from a Phase 3 Study. Vaccines. 2024; 12(8):883. https://doi.org/10.3390/vaccines12080883
Chicago/Turabian StyleShoemaker, Kathryn, Karina Soboleva, Angela Branche, Shivanjali Shankaran, Deborah A. Theodore, Muhammad Bari, Victor Ezeh, Justin Green, Elizabeth Kelly, Dongmei Lan, and et al. 2024. "Long-Term Safety and Immunogenicity of AZD1222 (ChAdOx1 nCoV-19): 2-Year Follow-Up from a Phase 3 Study" Vaccines 12, no. 8: 883. https://doi.org/10.3390/vaccines12080883





