Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Cultivation of E. chaffeensis
2.2. Animal Infections
2.3. E. chaffeensis-Specific PCR
2.4. ELISA
2.5. Preparation and Peripheral Blood Mononuclear Cells (PBMCs)
2.6. ELISPOT Assays
2.7. ELISA for IFNγ
2.8. Intracellular Cytokine Staining and Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. E. chaffeensis MLAV Vaccination
3.2. Antibody Responses Observed in MLAV-Vaccinated Dogs after Vaccination and Following Infection Challenge
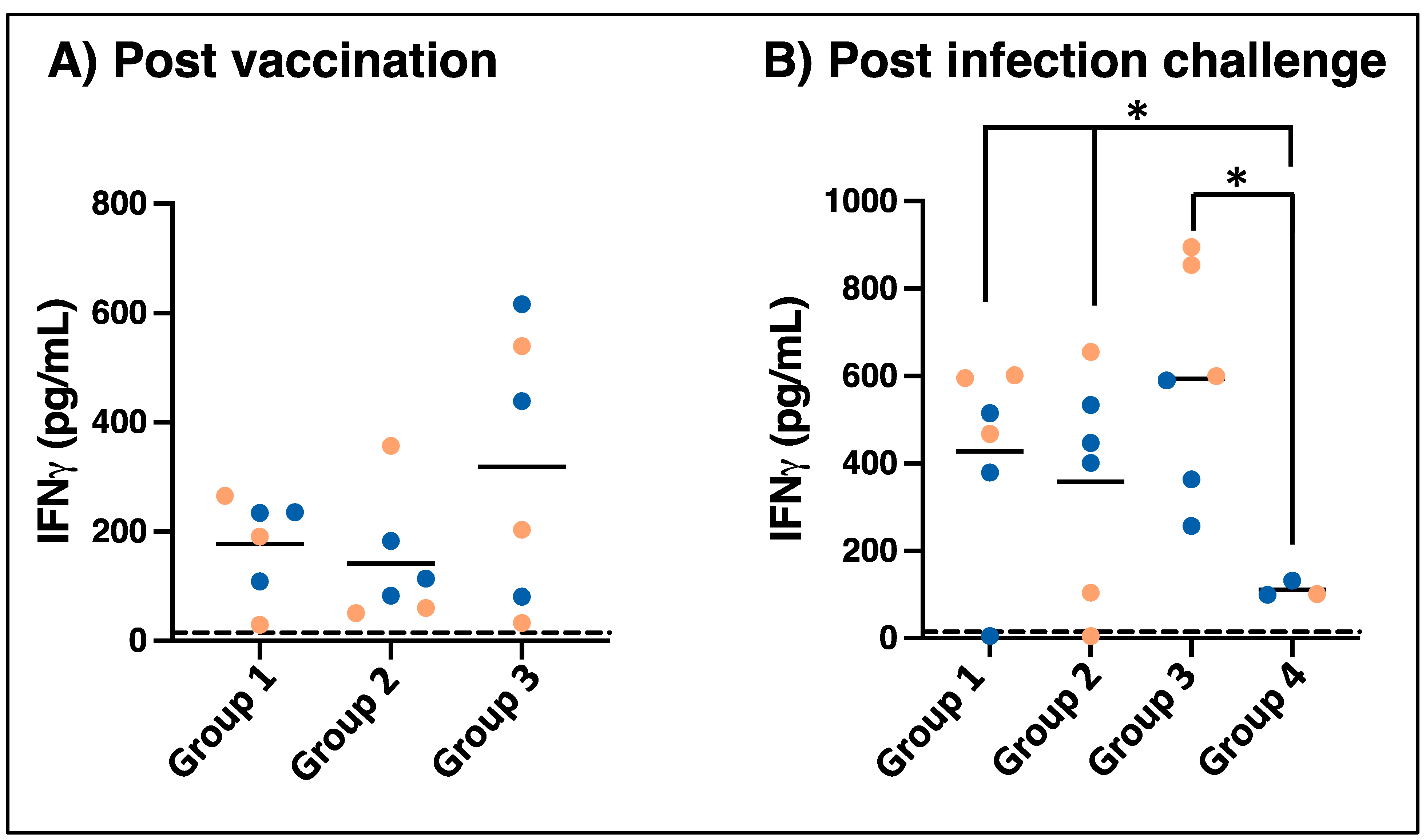
3.3. Vaccination and Wild-Type E. chaffeensis Challenge Induce Antigen-Specific Cellular Immune Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breitschwerdt, E.B.; Hegarty, B.C.; Qurollo, B.A.; Saito, T.B.; Maggi, R.G.; Blanton, L.S.; Bouyer, D.H. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit. Vectors 2014, 7, 1756–3305. [Google Scholar] [CrossRef]
- Dumler, J.S.; Sutker, W.L.; Walker, D.H. Persistent Infection with Ehrlichia chaffeensis. Clin. Infect. Dis. 1993, 17, 903–905. [Google Scholar] [CrossRef]
- Paddock, C.D.; Childs, J.E. Ehrlichia chaffeensis: A prototypical emerging pathogen. Clin. Microbiol. Rev. 2003, 16, 37–64. [Google Scholar] [CrossRef]
- Dugan, V.G.; Little, S.E.; Stallknecht, D.E.; Beall, A.D. Natural infection of domestic goats with Ehrlichia chaffeensis. J. Clin. Microbiol. 2000, 38, 448–449. [Google Scholar] [CrossRef]
- Kocan, A.A.; Levesque, G.C.; Whitworth, L.C.; Murphy, G.L.; Ewing, S.A.; Barker, R.W. Naturally occurring Ehrlichia chaffeensis infection in coyotes from Oklahoma. Emerg. Infect. Dis. 2000, 6, 477–480. [Google Scholar] [CrossRef]
- Davidson, W.R.; Lockhart, J.M.; Stallknecht, D.E.; Howerth, E.W.; Dawson, J.E.; Rechav, Y. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J. Wildl. Dis. 2001, 37, 538–546. [Google Scholar] [CrossRef]
- Dawson, J.E.; Anderson, B.E.; Fishbein, D.B.; Sanchez, J.L.; Goldsmith, C.S.; Wilson, K.H.; Duntley, C.W. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 1991, 29, 2741–2745. [Google Scholar] [CrossRef]
- Walker, D.H.; Dumler, J.S. Emergence of the ehrlichiosis as human health problems. Emerg. Infect. Dis. 1996, 2, 18–29. [Google Scholar] [CrossRef]
- Kuriakose, K.; Pettit, A.C.; Schmitz, J.; Moncayo, A.; Bloch, K.C. Assessment of risk factors and outcomes of severe ehrlichiosis infection. JAMA Netw. Open 2020, 3, e2025577. [Google Scholar] [CrossRef]
- Safdar, N.; Love, R.B.; Maki, D.G. Severe Ehrlichia chaffeensis infection in a lung transplant recipient: A review of ehrlichiosis in the immunocompromised patient. Emerg. Infect. Dis. 2002, 8, 320–323. [Google Scholar] [CrossRef]
- McQuiston, J.H.; Childs, J.E.; Chamberland, M.E.; Tabor, E. Transmission of tick-borne agents of disease by blood transfusion: A review of known and potential risks in the United States. Transfusion 2000, 40, 274–284. [Google Scholar] [CrossRef]
- Sachdev, S.H.; Joshi, V.; Cox, E.R.; Amoroso, A.; Palekar, S. Severe life-threatening Ehrlichia chaffeensis infections transmitted through solid organ transplantation. Transpl. Infect. Dis. 2014, 16, 119–124. [Google Scholar] [CrossRef]
- McBride, J.W.; Walker, D.H. Molecular and cellular pathobiology of Ehrlichia infection: Targets for new therapeutics and immunomodulation strategies. Expert. Rev. Mol. Med. 2011, 13, e3. [Google Scholar] [CrossRef]
- Dumler, J.S.; Madigan, J.E.; Pusterla, N.; Bakken, J.S. Ehrlichioses in humans: Epidemiology, clinical presentation, diagnosis, and treatment. Clin. Infect. Dis. 2007, 45 (Suppl. S1), S45–S51. [Google Scholar] [CrossRef]
- Nair, A.D.S.; Cheng, C.; Jaworski, D.C.; Willard, L.H.; Sanderson, M.W.; Ganta, R.R. Ehrlichia chaffeensis infection in the reservoir host (white-tailed deer) and in an incidental host (dog) is impacted by its prior growth in macrophage and tick cell environments. PLoS ONE 2014, 9, e109056. [Google Scholar] [CrossRef]
- Yabsley, M.J. Natural History of Ehrlichia chaffeensis: Vertebrate hosts and tick vectors from the United States and evidence for endemic transmission in other countries. Vet. Parasitol. 2010, 167, 136–148. [Google Scholar] [CrossRef]
- Ohashi, N.; Zhi, N.; Zhang, Y.; Rikihisa, Y. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 1998, 66, 132–139. [Google Scholar] [CrossRef]
- Li, J.S.; Yager, E.; Reilly, M.; Freeman, C.; Reddy, G.R.; Reilly, A.A.; Chu, F.K.; Winslow, G.M. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 2001, 166, 1855–1862. [Google Scholar] [CrossRef]
- Nyika, A.; Barbet, A.F.; Burridge, M.J.; Mahan, S.M. DNA vaccination with map1 gene followed by protein boost augments protection against challenge with Cowdria ruminantium, the agent of heartwater. Vaccine 2002, 20, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Geysen, D.; Ceesay, A.; Marcelino, I.; Alves, P.M.; Taoufik, A.; Postigo, M.; Bell-Sakyi, L.; Jongejan, F. Immunisation of sheep against heartwater in The Gambia using inactivated and attenuated Ehrlichia ruminantium vaccines. Vaccine 2007, 25, 7939–7947. [Google Scholar] [CrossRef] [PubMed]
- Rudoler, N.; Baneth, G.; Eyal, O.; van Straten, M.; Harrus, S. Evaluation of an attenuated strain of Ehrlichia canis as a vaccine for canine monocytic ehrlichiosis. Vaccine 2012, 31, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nair, A.D.S.; Alhassan, A.; Jaworski, D.C.; Liu, H.; Trinkl, K.; Hove, P.; Ganta, C.K.; Burkhardt, N.; Munderloh, U.G.; et al. Multiple Ehrlichia chaffeensis genes critical for its persistent infection in a vertebrate host are identified by random mutagenesis coupled with in vivo infection assessment. Infect. Immun. 2020, 88, e00316-20. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.D.S.; Cheng, C.; Jaworski, D.C.; Ganta, S.; Sanderson, M.W.; Ganta, R.R. Attenuated mutants of Ehrlichia chaffeensis induce protection against wild-type infection challenge in the reservoir host and in an incidental host. Infect. Immun. 2015, 83, 2827–2835. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Nair, A.D.S.; Cheng, C.; Rusk, R.A.; Jaworski, D.C.; Ganta, R.R. Vaccination with an attenuated mutant of Ehrlichia chaffeensis induces pathogen-specific CD4+ t cell immunity and protection from tick-transmitted wild-type challenge in the canine host. PLoS ONE 2016, 11, e0148229. [Google Scholar] [CrossRef] [PubMed]
- Torres-Escobar, A.; Juárez-Rodríguez, M.D.; Ganta, R.R. Mutations in Ehrlichia chaffeensis genes ECH_0660 and ECH_0665 cause transcriptional changes in response to zinc or iron limitation. J. Bacteriol. 2021, 203, e0002721. [Google Scholar] [CrossRef] [PubMed]
- Hove, P.; Madesh, S.; Nair, A.; Jaworski, D.; Liu, H.; Ferm, J.; Kleinhenz, M.D.; Highland, M.A.; Curtis, A.K.; Coetzee, J.F.; et al. Targeted mutagenesis in Anaplasma marginale to define virulence and vaccine development against bovine anaplasmosis. PLoS Pathog. 2022, 18, e1010540. [Google Scholar] [CrossRef] [PubMed]
- Munderloh, U.G.; Jauron, S.D.; Fingerle, V.; Leitritz, L.; Hayes, S.F.; Hautman, J.M.; Nelson, C.M.; Huberty, B.W.; Kurtti, T.J.; Ahlstrand, G.G.; et al. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 1999, 37, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; McBride, J.W.; Yu, X.-J. L-selectin and E-selectin expressed on monocytes mediating Ehrlichia chaffeensis attachment onto host cells. FEMS Microbiol. Lett. 2003, 227, 303–309. [Google Scholar] [CrossRef] [PubMed]
- USDA. Animal Welfare Act and Animal Welfare Regulations; USDA: Washington, DC, USA, 2013.
- Sambrook, J.R.D. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2000. [Google Scholar]
- Flannery, B.; Andrews, N.; Feikin, D.; Patel, M.K. Commentary: Estimation of vaccine effectiveness using the screening method. Int. J. Epidemiol. 2022, 52, 19–21. [Google Scholar] [CrossRef]
- Rothe, K.; Bismarck, D.; Büttner, M.; Alber, G.; von Buttlar, H. Canine peripheral blood CD4(+)CD8(+) double-positive T-cell subpopulations exhibit distinct T-cell phenotypes and effector functions. Vet. Immunol. Immunopathol. 2017, 185, 48–56. [Google Scholar] [CrossRef]
- Drexler, N.A.; Dahlgren, F.S.; Heitman, K.N.; Massung, R.F.; Paddock, C.D.; Behravesh, C.B. National Surveillance of Spotted Fever Group Rickettsioses in the United States, 2008–2012. Am. J. Trop. Med. Hyg. 2016, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Eriks, I.S.; Stiller, D.; Palmer, G.H. Impact of persistent Anaplasma marginale rickettsemia on tick infection and transmission. J. Clin. Microbiol. 1993, 31, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Graça, T.; Paradiso, L.; Broschat, S.L.; Noh, S.M.; Palmer, G.H. Primary structural variation in Anaplasma marginale Msp2 efficiently generates immune escape variants. Infect. Immun. 2015, 83, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Soong, L.; McBride, J.W.; Valbuena, G.; Olano, J.P.; Feng, H.M.; Walker, D.H. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 2004, 172, 1786–1800. [Google Scholar] [CrossRef] [PubMed]
- Bitsaktsis, C.; Huntington, J.; Winslow, G. Production of IFN-gamma by CD4 T cells is essential for resolving Ehrlichia infection. J. Immunol. 2004, 172, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- van Schaik, E.J.; Fratzke, A.P.; Gregory, A.E.; Dumaine, J.E.; Samuel, J.E. Vaccine development: Obligate intracellular bacteria new tools, old pathogens: The current state of vaccines against obligate intracellular bacteria. Front. Cell. Infect. Microbiol. 2024, 14, 1282183. [Google Scholar] [CrossRef] [PubMed]
- Budachetri, K.; Lin, M.; Chien, R.C.; Zhang, W.; Brock, G.N.; Rikihisa, Y. Efficacy and immune correlates of OMP-1B and VirB2-4 vaccines for protection of dogs from tick transmission of Ehrlichia chaffeensis. mBio 2022, 13, e0214022. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.I.; Westerhof, L.M.; MacLeod, M.K.L. The roles of resident, central and effector memory CD4 T-cells in protective immunity following infection or vaccination. Immunology 2018, 154, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Hill, A.V.S. Vaccines against intracellular infections requiring cellular immunity. Nature 2000, 406, 793–798. [Google Scholar] [CrossRef]
- Li, F.; Dang, W.; Du, Y.; Xu, X.; He, P.; Zhou, Y.; Zhu, B. Tuberculosis Vaccines and T Cell Immune Memory. Vaccines 2024, 12, 483. [Google Scholar] [CrossRef]
- Depew, C.E.; McSorley, S.J. The role of tissue resident memory CD4 T cells in Salmonella infection: Implications for future vaccines. Vaccine 2023, 41, 6426–6433. [Google Scholar] [CrossRef] [PubMed]
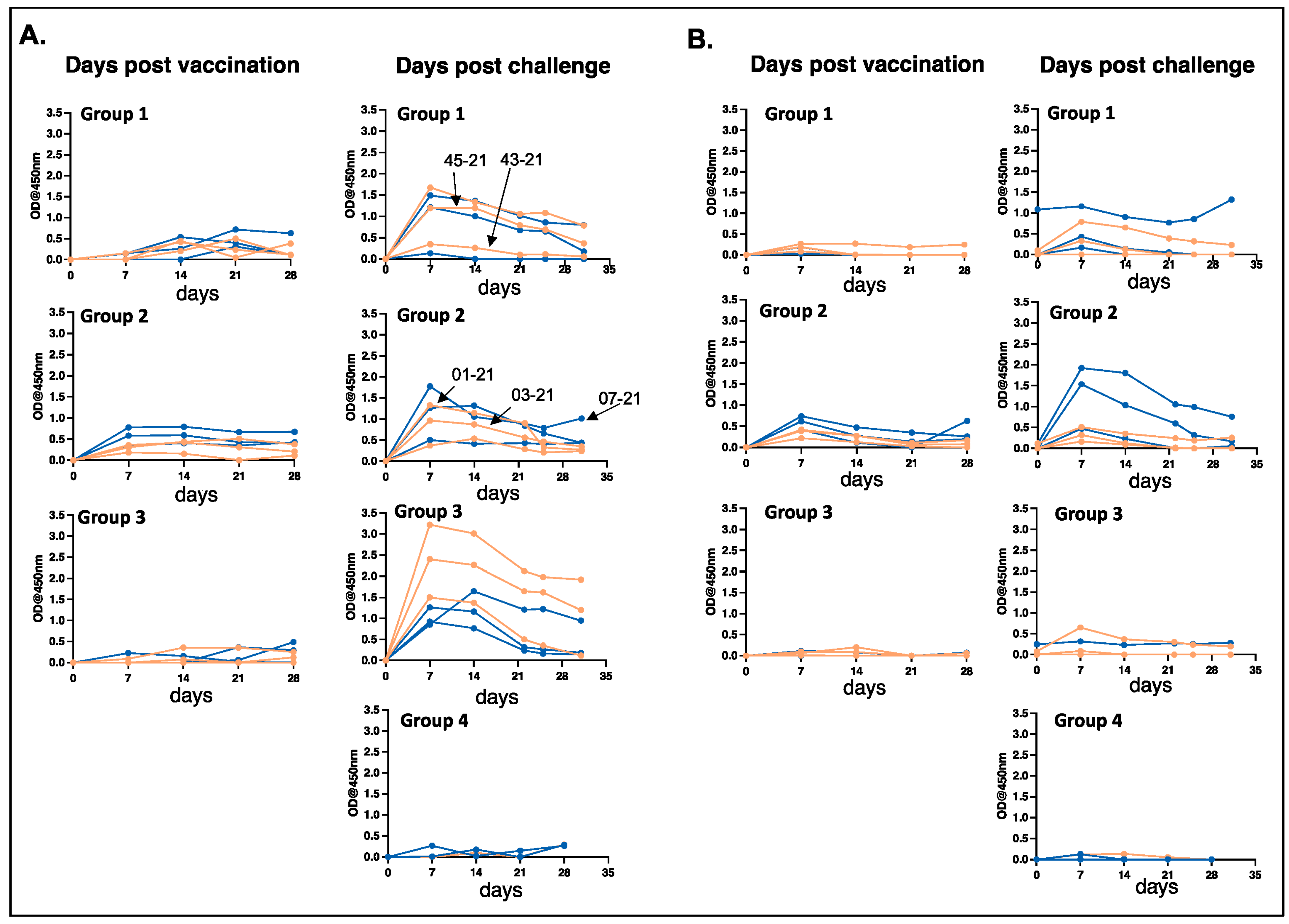


| Animal Groups | Females + Males | Challenge Time (Months Post Vaccination) |
|---|---|---|
| Group 1 | 3 + 3 | 4 months |
| Group 2 | 3 + 3 | 8 months |
| Group 3 | 3 + 3 | 12 months |
| Group 4 | 1 + 2 | Unvaccinated |
| Group 1—4 Months Post-Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|
| Animal #s | Day 0 | Day 2 | Day 7 | Day 12 | Day 14 | Day 19 | Day 22 | Day 29 |
| 43-21(F) | - | - | - | - | - | - | - | - |
| 45-21(F) | - | - | - | - | - | - | - | - |
| 47-21(F) | - | - | - | - | - | - | - | - |
| 49-21(M) | - | - | - | - | - | - | - | - |
| 50-21(M) | - | - | - | - | - | - | - | - |
| 51-21(M) | - | - | - | - | - | - | - | - |
| Group 2—8 Months Post-Vaccination | ||||||||
| Animal #s | Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | Day 17 | Day 21 | Day 28 |
| 01-21(F) | - | - | - | - | - | - | - | - |
| 02-21(F) | - | - | - | - | - | - | - | - |
| 03-21(F) | - | - | - | - | - | - | - | - |
| 07-21(M) | - | - | - | - | - | - | - | - |
| 08-21(M) | - | - | - | - | - | - | - | - |
| 09-21(M) | - | - | - | - | - | - | - | - |
| Group 3—12 Months Post-Vaccination | ||||||||
| Animal #s | Day 0 | Day 3 | Day 7 | Day 10 | Day 14 | Day 17 | Day 22 | Day 28 |
| 01-20(F) | - | - | - | - | - | - | - | - |
| 02-20(F) | - | - | - | - | - | - | - | - |
| 06-20(F) | - | - | - | - | - | - | - | - |
| 07-20(M) | - | - | - | - | - | - | - | - |
| 09-20(M) | - | - | - | - | - | - | - | - |
| 11-20(M) | - | - | - | - | - | - | - | - |
| Group 1—4 Months Post-Vaccination Challenge | ||||||||
|---|---|---|---|---|---|---|---|---|
| Animal #s | Day 3 | Day 7 | Day 10 | Day 14 | Day 18 | Day 22 | Day 25 | Day 31/32 |
| 43-21(F) | - | + | - | - | - | - | - | - |
| 45-21(F) | - | - | + | + | - | - | - | - |
| 47-21(F) | - | - | - | - | - | - | - | - |
| 49-21(M) | - | - | - | - | - | - | - | - |
| 50-21(M) | - | - | - | - | - | - | - | - |
| 51-21(M) | - | - | - | - | - | - | - | - |
| Group 2—8 Months Post-Vaccination Challenge | ||||||||
| Animal #s | Day 4 | Day 7 | Day 11 | Day 14 | Day 18 | Day 22 | Day 25 | Day 31/32 |
| 01-21(F) | - | - | - | + | + | - | - | - |
| 02-21(F) | - | - | - | - | - | - | - | - |
| 03-21(F) | - | + | - | - | - | - | - | - |
| 07-21(M) | - | - | - | + | - | + | - | - |
| 08-21(M) | - | - | - | - | - | - | - | - |
| 09-21(M) | - | - | - | - | - | - | - | - |
| Group 3—12 Months Post-Vaccination Challenge | ||||||||
| Animal #s | Day 4 | Day 7 | Day 11 | Day 14 | Day 18 | Day 22 | Day 25 | Day 31/32 |
| 01-20(F) | - | - | - | - | - | - | - | - |
| 02-20(F) | - | - | - | - | - | - | - | - |
| 06-20(F) | - | - | - | - | - | - | - | - |
| 07-20(M) | - | - | - | - | - | - | - | - |
| 09-20(M) | - | - | - | - | - | - | - | - |
| 11-20(M) | - | - | - | - | - | - | - | - |
| Animal #s | Day 4 | Day 7 | Day 11 | Day 14 | Day 18 | Day 22 | Day 24 | Day 30 |
|---|---|---|---|---|---|---|---|---|
| 56-21(M) | + | + | + | + | + | - | - | - |
| 57-21(M) | - | - | - | + | + | + | - | + |
| 60-21(F) | - | + | + | + | - | - | - | - |
| Vaccine/Infection | Group #s | Dogs Positive/Assessed | Frequency of Positives |
|---|---|---|---|
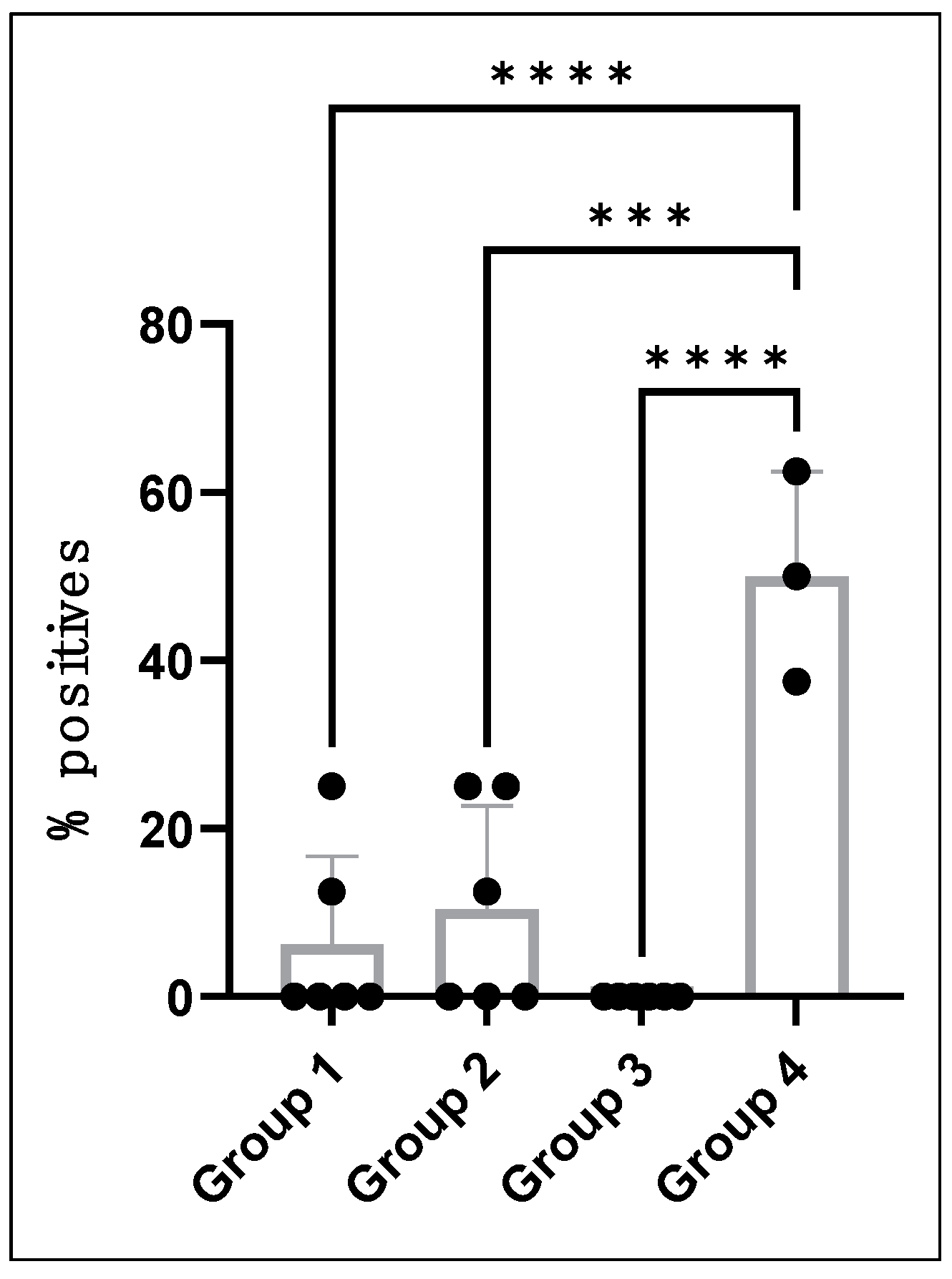
| Vaccinated and infected | 1, 2, and 3 | 5/18 (28%) | 8/144 (5.5%) |
| Unvaccinated and infected | 4 | 3/3 (100%) | 12/24 (50%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madesh, S.; McGill, J.; Jaworski, D.C.; Ferm, J.; Liu, H.; Fitzwater, S.; Hove, P.; Ferm, D.; Nair, A.; Knox, C.A.; et al. Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine. Vaccines 2024, 12, 903. https://doi.org/10.3390/vaccines12080903
Madesh S, McGill J, Jaworski DC, Ferm J, Liu H, Fitzwater S, Hove P, Ferm D, Nair A, Knox CA, et al. Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine. Vaccines. 2024; 12(8):903. https://doi.org/10.3390/vaccines12080903
Chicago/Turabian StyleMadesh, Swetha, Jodi McGill, Deborah C. Jaworski, Jonathan Ferm, Huitao Liu, Shawna Fitzwater, Paidashe Hove, Dominica Ferm, Arathy Nair, Cheyenne A. Knox, and et al. 2024. "Long-Term Protective Immunity against Ehrlichia chaffeensis Infection Induced by a Genetically Modified Live Vaccine" Vaccines 12, no. 8: 903. https://doi.org/10.3390/vaccines12080903








